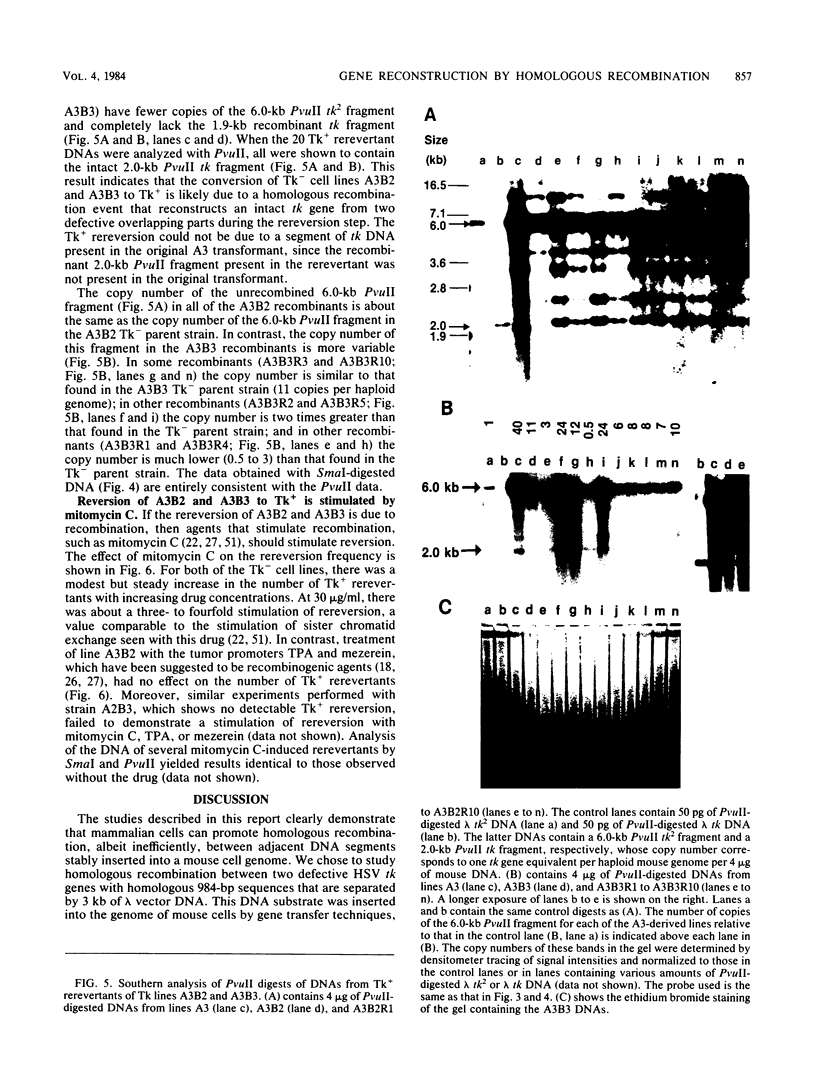
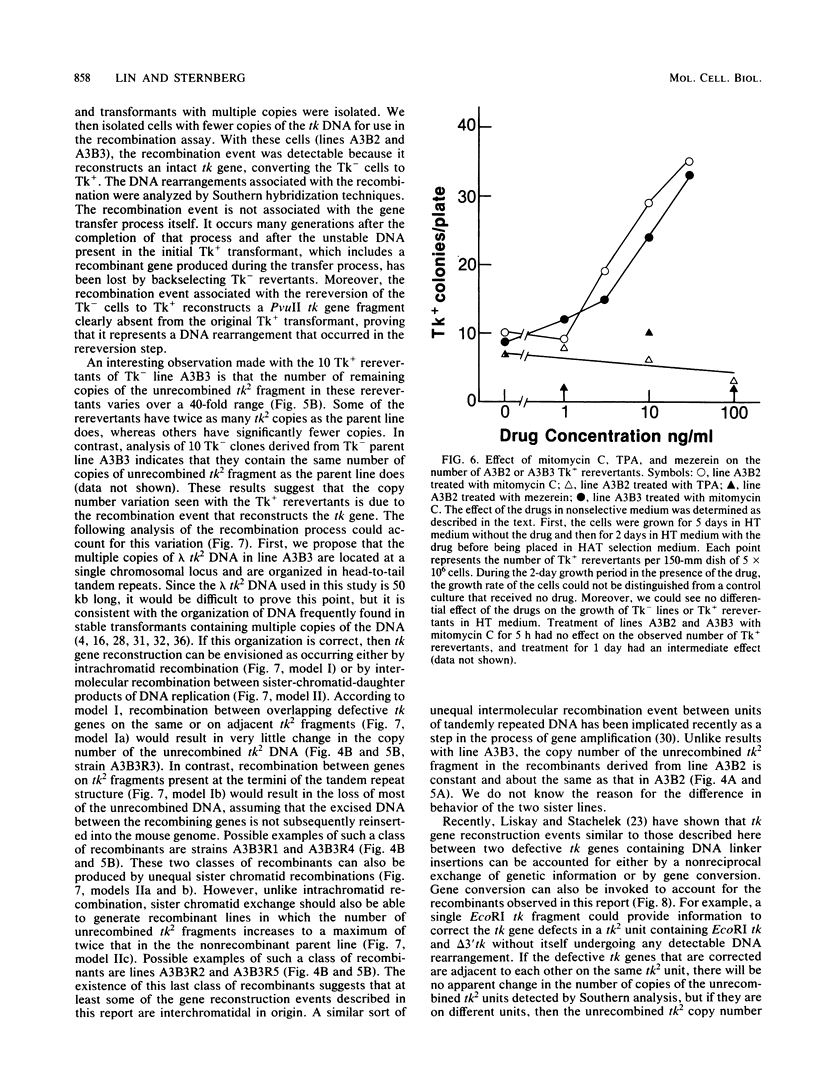
Abstract
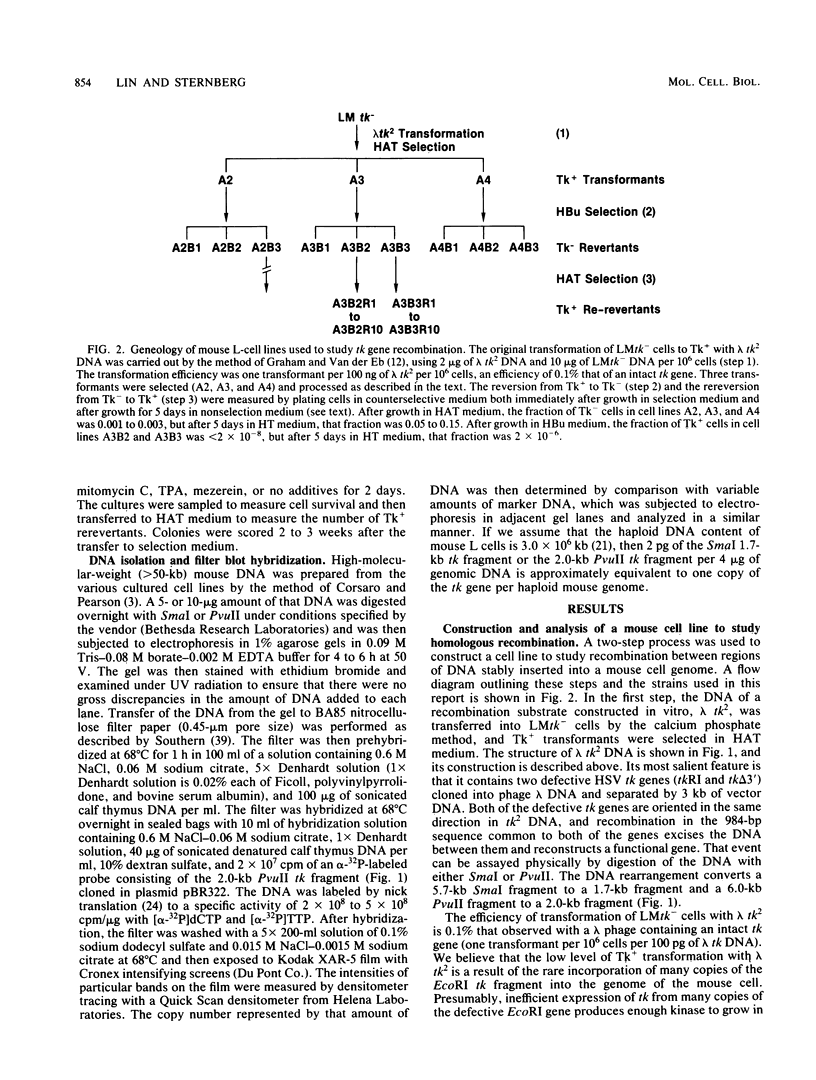
We have constructed a substrate to study homologous recombination between adjacent segments of chromosomal DNA. This substrate, designated lambda tk2 , consists of one completely defective and one partially defective herpes simplex virus thymidine kinase (tk) gene cloned in bacteriophage lambda DNA. The two genes have homologous 984-base-pair sequences and are separated by 3 kilobases of largely vector DNA. When lambda tk2 DNA was transferred into mouse LMtk- cells by the calcium phosphate method, rare TK+ transformants were obtained that contained many (greater than 40) copies of the unrecombined DNA. Tk- revertants, which had lost most of the copies of unrecombined DNA, were isolated from these TK+-transformed lines. Two of these Tk- lines were further studied by analysis of their reversion back to the Tk+ phenotype. They generated ca. 200 Tk+ revertants per 10(8) cells after growth in nonselecting medium for 5 days. All of these Tk+ revertants have an intact tk gene reconstructed by homologous recombination; they also retain various amounts of unrecombined lambda tk2 DNA. Southern blot analysis suggested that at least some of the recombination events involve unequal sister chromatid exchanges. We also tested three agents, mitomycin C, 12-O-tetradecanoyl-phorbol-13-acetate, and mezerein, that are thought to stimulate recombination to determine whether they affect the reversion from Tk- to Tk+. Only mitomycin C increased the number of Tk+ revertants.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christy B., Scangos G. Expression of transferred thymidine kinase genes is controlled by methylation. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6299–6303. doi: 10.1073/pnas.79.20.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsaro C. M., Pearson M. L. Enhancing the efficiency of DNA-mediated gene transfer in mammalian cells. Somatic Cell Genet. 1981 Sep;7(5):603–616. doi: 10.1007/BF01549662. [DOI] [PubMed] [Google Scholar]
- Crouse G. F., McEwan R. N., Pearson M. L. Expression and amplification of engineered mouse dihydrofolate reductase minigenes. Mol Cell Biol. 1983 Feb;3(2):257–266. doi: 10.1128/mcb.3.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta U. B., Summers W. C. Genetic recombination of herpes simplex virus, the role of the host cell and UV-irradiation of the virus. Mol Gen Genet. 1980;178(3):617–623. doi: 10.1007/BF00337869. [DOI] [PubMed] [Google Scholar]
- Davies R. L., Fuhrer-Krusi S., Kucherlapati R. S. Modulation of transfected gene expression mediated by changes in chromatin structure. Cell. 1982 Dec;31(3 Pt 2):521–529. doi: 10.1016/0092-8674(82)90308-7. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Vande Woude G. F., Wagner M., Smiley J. R., Summers W. C. Construction and characterization of a recombinant plasmid encoding the gene for the thymidine kinase of Herpes simplex type 1 virus. Gene. 1979 Nov;7(3-4):335–342. doi: 10.1016/0378-1119(79)90052-0. [DOI] [PubMed] [Google Scholar]
- Folger K. R., Wong E. A., Wahl G., Capecchi M. R. Patterns of integration of DNA microinjected into cultured mammalian cells: evidence for homologous recombination between injected plasmid DNA molecules. Mol Cell Biol. 1982 Nov;2(11):1372–1387. doi: 10.1128/mcb.2.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huttner K. M., Ruddle F. H. Study of mitomycin C-induced chromosomal exchange. Chromosoma. 1976 Jun 30;56(1):1–13. doi: 10.1007/BF00293724. [DOI] [PubMed] [Google Scholar]
- Huttner K. M., Scangos G. A., Ruddle F. H. DNA-mediated gene transfer of a circular plasmid into murine cells. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5820–5824. doi: 10.1073/pnas.76.11.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella A. R., Radman M. Tumor promoter induces sister chromatid exchanges: relevance to mechanisms of carcinogenesis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6149–6153. doi: 10.1073/pnas.75.12.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer P. J., Bowman A. H., Huberman M. H., Sanders-Haigh L., Killos L., Anderson W. F. Recovery of recombinant bacterial plasmids from E. coli transformed with DNA from microinjected mouse cells. Nucleic Acids Res. 1981 Nov 25;9(22):6199–6217. doi: 10.1093/nar/9.22.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn E. M. Localization by Q-banding of mitotic chiasmata in cases of Bloom's syndrome. Chromosoma. 1976 Aug 4;57(1):1–11. doi: 10.1007/BF00292945. [DOI] [PubMed] [Google Scholar]
- Laird C. D. Chromatid structure: relationship between DNA content and nucleotide sequence diversity. Chromosoma. 1971 Mar 16;32(4):378–406. doi: 10.1007/BF00285251. [DOI] [PubMed] [Google Scholar]
- Latt S. A. Sister chromatid exchange formation. Annu Rev Genet. 1981;15:11–55. doi: 10.1146/annurev.ge.15.120181.000303. [DOI] [PubMed] [Google Scholar]
- Liskay R. M., Stachelek J. L. Evidence for intrachromosomal gene conversion in cultured mouse cells. Cell. 1983 Nov;35(1):157–165. doi: 10.1016/0092-8674(83)90218-0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Nagasawa H., Little J. B. Effect of tumor promoters, protease inhibitors, and repair processes on x-ray-induced sister chromatid exchanges in mouse cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1943–1947. doi: 10.1073/pnas.76.4.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry J. M., Parry E. M., Barrett J. C. Tumour promoters induce mitotic aneuploidy in yeast. Nature. 1981 Nov 19;294(5838):263–265. doi: 10.1038/294263a0. [DOI] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980 Nov;22(1 Pt 1):309–317. doi: 10.1016/0092-8674(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., Buck L. B., Axel R. A structure for amplified DNA. Cell. 1983 May;33(1):53–63. doi: 10.1016/0092-8674(83)90334-3. [DOI] [PubMed] [Google Scholar]
- Robins D. M., Axel R., Henderson A. S. Chromosome structure and DNA sequence alterations associated with mutation of transformed genes. J Mol Appl Genet. 1981;1(3):191–203. [PubMed] [Google Scholar]
- Robins D. M., Ripley S., Henderson A. S., Axel R. Transforming DNA integrates into the host chromosome. Cell. 1981 Jan;23(1):29–39. doi: 10.1016/0092-8674(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Rosenstraus M. J., Chasin L. A. Separation of linked markers in Chinese hamster cell hybrids: mitotic recombination is not involved. Genetics. 1978 Dec;90(4):735–760. doi: 10.1093/genetics/90.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Scangos G. A., Huttner K. M., Juricek D. K., Ruddle F. H. Deoxyribonucleic acid-mediated gene transfer in mammalian cells: molecular analysis of unstable transformants and their progression to stability. Mol Cell Biol. 1981 Feb;1(2):111–120. doi: 10.1128/mcb.1.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scangos G., Ruddle F. H. Mechanisms and applications of DNA-mediated gene transfer in mammalian cells - a review. Gene. 1981 Jun-Jul;14(1-2):1–10. doi: 10.1016/0378-1119(81)90143-8. [DOI] [PubMed] [Google Scholar]
- Shapira G., Stachelek J. L., Letsou A., Soodak L. K., Liskay R. M. Novel use of synthetic oligonucleotide insertion mutants for the study of homologous recombination in mammalian cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4827–4831. doi: 10.1073/pnas.80.15.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J., Scangos G. Recombination during gene transfer into mouse cells can restore the function of deleted genes. Science. 1983 Jan 14;219(4581):174–176. doi: 10.1126/science.6294829. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stein R., Razin A., Cedar H. In vitro methylation of the hamster adenine phosphoribosyltransferase gene inhibits its expression in mouse L cells. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3418–3422. doi: 10.1073/pnas.79.11.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Subramani S., Berg P. Homologous and nonhomologous recombination in monkey cells. Mol Cell Biol. 1983 Jun;3(6):1040–1052. doi: 10.1128/mcb.3.6.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W. The double-strand-break repair model for recombination. Cell. 1983 May;33(1):25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Baker R. M., Carrano A. V., Brookman K. W. Failure of the phorbol ester 12-O-tetradecanoylphorbol-13-acetate to enhance sister chromatid exchange, mitotic segregation, or expression of mutations in Chinese hamster cells. Cancer Res. 1980 Sep;40(9):3245–3251. [PubMed] [Google Scholar]
- Varshavsky A. Phorbol ester dramatically increases incidence of methotrexate-resistant mouse cells: possible mechanisms and relevance to tumor promotion. Cell. 1981 Aug;25(2):561–572. doi: 10.1016/0092-8674(81)90074-x. [DOI] [PubMed] [Google Scholar]
- Vogel T. Recombination between endogenous and exogenous simian virus 40 genes. III. Rescue of SV40 tsA and tsBC mutants by passage in permissive transformed monkey lines. Virology. 1980 Jul 15;104(1):73–83. doi: 10.1016/0042-6822(80)90366-9. [DOI] [PubMed] [Google Scholar]
- Wagner M. J., Sharp J. A., Summers W. C. Nucleotide sequence of the thymidine kinase gene of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1441–1445. doi: 10.1073/pnas.78.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake C. T., Wilson J. H. Simian virus 40 recombinants are produced at high frequency during infection with genetically mixed oligomeric DNA. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2876–2880. doi: 10.1073/pnas.76.6.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Levy D., Perucho M. The somatic replication of DNA methylation. Cell. 1981 Apr;24(1):33–40. doi: 10.1016/0092-8674(81)90498-0. [DOI] [PubMed] [Google Scholar]
- Wolff S. Sister chromatid exchange. Annu Rev Genet. 1977;11:183–201. doi: 10.1146/annurev.ge.11.120177.001151. [DOI] [PubMed] [Google Scholar]
- Young C. S., Silverstein S. J. The kinetics of adenovirus recombination in homotypic and heterotypic genetic crosses. Virology. 1980 Mar;101(2):503–515. doi: 10.1016/0042-6822(80)90464-x. [DOI] [PubMed] [Google Scholar]
- de Saint Vincent B. R., Wahl G. M. Homologous recombination in mammalian cells mediates formation of a functional gene from two overlapping gene fragments. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2002–2006. doi: 10.1073/pnas.80.7.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]