Abstract
Objective
The objective of this study was to investigate the association between chronic back pain and urinary incontinence in women.
Study Design
This study was a cross-sectional, observational study.
Background
There are numerous factors associated with the development of back pain, yet little consideration has been given to the pelvic floor musculature and dysfunction of this musculature which may also cause urinary incontinence. Currently, limited research exists evaluating the relationship between back pain and urinary incontinence.
Methods and Measures
Data from a sample of 2,341 women from the Kentucky Women’s Health Registry were used for analysis. The primary variables of interest were self-reported chronic back pain (CBP) and stress urinary incontinence (SUI), with stress urinary incontinence serving as the primary dependent variable. Simple comparisons were performed using chi-square tests and two-sample t-tests, and multivariable associations were assessed using binary logistic regression.
Results
Reports of stress urinary incontinence were higher in women reporting CBP than those not reporting CBP (49.0% vs. 35.2%, p<0.01). After controlling for potential confounders, the adjusted SUI odds ratio for CBP versus not was 1.44 (95% CI 1.11, 1.86).
Conclusion
Women who report CBP have an increased odds of having SUI. Therefore, clinicians must consider this association and the relationship of relevant trunk muscles, including pelvic floor musculature, in patients presenting with CBP and/or UI.
Introduction
Back pain is a highly prevalent musculoskeletal condition. Specifically, low back pain (LBP) is the most common type of pain reported by U.S. adults, with one in four adults reporting the experience of LBP in the past 3 months.1,2 Furthermore, a reported 70-85% of adults will experience an episode of LBP at some point in their lifetime.3-7
Although it is generally believed that most cases of acute LBP tend to resolve within a relatively short timeframe, some individuals go on to develop chronic back pain.8 Data from a recent systematic review reveals between 44-78% of individuals experience a relapse of LBP and between 42-75% of individuals still report LBP after 12 months.9 Furthermore, Freburger et al report a recent increase in both the prevalence of chronic LBP as well as the number of individuals that seek care from a healthcare provider for their chronic LBP.10
Low back pain is one of the most common diagnoses treated by physical therapists.11 Additionally, despite advances in diagnosis and treatment, physical therapy is one of the largest direct cost components for the treatment of LBP.12 Therefore, appropriate clinical management of chronic LBP is important. Chronic low back pain represents a clinical challenge because it tends to not improve over time and is a significant economic burden on individuals and society.8,12-14 Furthermore, majority of patients with back pain have non-specific low back pain which is not attributable to a known, specific pathology.14,15 The exact mechanism for the development of back pain can be multifactorial and may not always be clearly understood. This complicates the physical therapy management of back pain due to the inability to find a specific anatomic cause of pain.
One factor the physical therapist may consider when determining the origin of back pain is dysfunction in relevant trunk musculature. Trunk control is reliant on the function and coordination of muscles in the abdomino-pelvic cavity and dysfunction of this musculature may lead to pain and disability. Previous research has focused on the contribution of “traditional trunk musculature” to provide trunk stability, particularly, the rectus abdominis, transversus abdominis, and multifidus.16-19 Recent research has focused on the role of the pelvic floor muscles (PFMs) to aid spinal stability.20-22 In addition, the role of the pelvic floor muscles in promoting continence is well documented.23-25 Given the PFMs dual role, it is logical to hypothesize a relationship between continence status and the presence of back pain.
Several studies have shown an association between back pain and UI.26-29 Finkelstein et al reported a strong association between “back problems” and UI in both men and women.26 A cross-sectional study of women only by Smith et al found a relationship between continence disorders and back pain “in the past 12 months.”27 In addition, Kim et al found women with greater UI severity also have a higher perceived severity of LBP and LBP perceived disability.28 Lastly, Eliasson et al surveyed women who were receiving physical therapy for LBP and reported 78% of these women also reported UI.29
Although studies have shown a relationship between LBP and UI, the definition of back pain across studies varied. No study has specifically used the terminology ‘chronic back pain’ and investigated the association between chronic back pain (CBP) and UI. Furthermore, no known study has been conducted in the United States to determine the association between back pain and UI in women. Therefore, the primary aim of this study is to determine whether there is an association between CBP and UI in women.
Methods and Measures
Study Sample
This study is a cross-sectional analysis of data from the Kentucky Women’s Health Registry (KWHR). The KWHR is a state-wide database that uses a postal survey and internet correspondence to collect annual self-reported data on physical and mental well-being, health behaviors, diagnoses, symptoms and social factors reported from Kentucky women aged 18 and older. The KWHR includes data from 13,328 women participating in the survey from March 2006 (start of registry) to March 2010. The KWHR received its first institutional review board approval on May 11, 2005, IRB number 05-0387-P3G, and has had continued renewals with the most recent renewal date of July 12, 2012.
Since the KWHR is a longitudinal registry, women who reported CBP were identified and only data from their most recent survey responses that included CBP were used. Data from the most recent survey were selected from women without a history of CBP. Data from women with a self-reported history of cancer, neurological disorders, bone disorders, pelvic disease, or history of joint, spine and/or disc surgery were excluded. Data from a total of 2,418 women were included in study analyses (Figure 1). Of the original sample of 2,803 women, continence status, back pain status, and other demographic data needed for study analyses were missing from 27, 17, and 341 women, respectively.
Figure 1. Flowchart of subject selection for analyzable dataset.
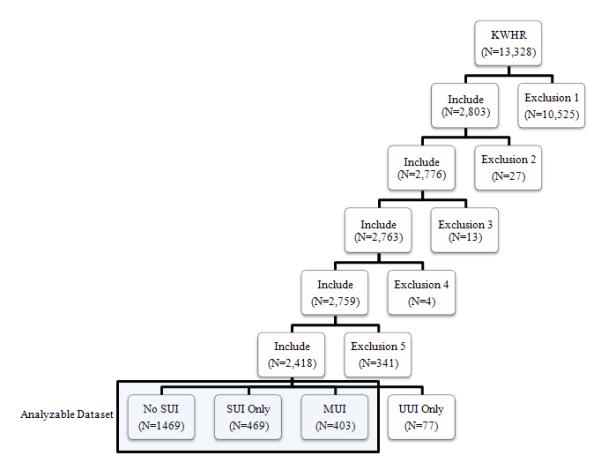
Note: KWHR = Kentucky Women’s Health Registry; SUI = stress urinary incontinence, MUI = mixed urinary incontinence; UUI = urge incontinence only. Exclusion 1 includes patients with a self-reported history of cancer, neurological disorders, bone disorders, pelvic disease or history of joint, spine and/or disc surgery. Exclusion 2 includes patients with missing data for chronic back pain. Exclusion 3 includes patients with missing data for UI. Exclusion 4 includes patients with missing data for type of UI (SUI or UUI). Exclusion 5 includes patients with missing data for any of the other variables included in the analysis. The final analyzable dataset included women who either reported No SUI, SUI only, or MUI (SUI and UUI). Women who reported UUI only were excluded from analyses.
Variables and Categorization
The primary variables of interest were self-reported urinary incontinence (UI) and chronic back pain (CBP). Urinary incontinence was collected as a binary variable and served as the dependent variable. Incontinence was defined in relation to the definitions of the International Continence Society.30 First, a general question on UI was asked of participants. Urinary incontinence was defined for participants based on a positive response to the question “Do you leak urine (or) water when you didn’t want to?” UI was further classified by type. Stress urinary incontinence (SUI) was defined as a positive response to the question “Do you leak urine (or) water when you cough, sneeze or exercise?” Urge urinary incontinence (UUI) was defined as a positive response to the question “Do you ever leak urine (or) water on the way to use the bathroom?” Mixed urinary incontinence (MUI), the complaint of involuntary leakage associated with urgency and also with exertion, effort, sneezing or coughing, was determined if participants were categorized as having both SUI and UUI. A total of 949 women indicated urinary incontinence, but most of these women (91.9%) reported SUI, leaving only 77 participants who indicated UUI alone. Given the small number of participants reporting UUI but not SUI, these participants were removed, providing an analyzable set of 2,341 women; all 872 women reporting UI also reported SUI (Figure 1).
Chronic back pain was also categorized as a binary variable and served as the independent variable. Participants were categorized as reporting CBP if they endorsed: 1) the “back” response to the question “Which of the following body areas are chronically painful” and /or 2) responded with “chronic back pain” to the question, “Do you have any of the following musculoskeletal disorders?”
Potential confounding variables were also investigated. These variables included obesity, physical activity, health status, parity, vaginal birth, asthma, educational level, age, and race(Table 1).
Table 1.
Definition of confounding variables
| Confounding Variable | Category Levels |
|---|---|
| Obesity | BMI ≥30 kg/m2
BMI < 30 kg/m2 |
| Physical activity | Sedentary Moderate Very Active |
| General health status | Excellent/Very Good Good/Fair/Poor |
| Parity | 0 births 1 birth 2+ births |
| Vaginal birth (ever) | Yes No |
| Asthma (ever) | Yes No |
| Educational attainment | Less than college degree College degree or higher |
| Race | White Non-White |
Note: All variables are based on self-reported responses
Statistical analysis
Descriptive statistics, means and standard deviations for continuous data and percentages for categorical data, were computed. Participants were described according to CBP and UI. Groups were compared using two-sample t-tests for continuous variables and chi-square tests for categorical variables. Binary logistic regression was used to investigate the relationship between CBP and SUI, while controlling for potential confounding variables. As an additional, subset analysis, binary logistic regression was also used to investigate the relationship of SUI alone and SUI with UUI (MUI). Potential confounders were identified using published studies31-33 and when bivariate comparisons suggested a relationship with CBP and UI. Potential confounders included race, education, obesity, sedentary lifestyle, self-report of asthma, parity and delivery type. Adjusted odds ratios obtained from the logistic regression are reported with 95% confidence intervals. P-values less than 0.05 are considered statistically significant for all statistical tests. All analyses were performed using SAS v9.2.
Results
Characteristics of participants (n = 2,341) based on CBP status are presented in Table 2. Table 3 includes participant characteristics based on continence status. The women in this sample are predominantly white (93.1%) and college educated (62.8%). The sample represents a healthy population of women with 69.8% reporting excellent/very good health, 82.6% indicating at least moderate activity levels and only 29.5% with BMI levels in the obese category.
Table 2.
Characteristics of participants by chronic back pain (CBP)
| Variables |
No CBP
(N=1998) |
CBP
(N = 343) |
Total
(N = 2341) |
P-value 1 |
|---|---|---|---|---|
| Age Mean(SD) | 41.90(14.42) | 43.63(14.35) | 42.15(14.42) | 0.039 |
| White Race | 1863 (93.2%) | 317 (92.4%) | 2180 (93.1%) | 0.578 |
| College Education | 1281 (64.1%) | 188 (54.8%) | 1469 (62.8%) | 0.001 |
| Perceived Health | <0.001 | |||
| Excellent/Very good | 1464 (73.3%) | 169 (49.3%) | 1633 (69.8%) | |
| Good | 460 (23.0%) | 117 (34.1%) | 577 (24.6%) | |
| Fair/Poor | 74 (3.7%) | 57 (16.6%) | 131 (5.6%) | |
| Activity Level | <0.001 | |||
| Sedentary | 312 (15.6%) | 95 (27.7%) | 407 (17.4%) | |
| Moderately active | 1271 (63.6%) | 207 (60.3%) | 1478 (63.1%) | |
| Very active | 415 (20.8%) | 41 (12.0%) | 456 (19.5%) | |
| Obese | 547 (27.4%) | 144 (42.0%) | 691 (29.5%) | <0.001 |
| Parity | 0.501 | |||
| No live birth child | 679 (34.0%) | 109 (31.8%) | 788 (33.7%) | |
| One live birth child | 374 (18.7%) | 60 (17.5%) | 434 (18.5%) | |
| 2 or more live births | 945 (47.3%) | 174 (50.7%) | 1119 (47.8%) | |
| Delivery Type | 0.039 | |||
| No vaginal births | 959 (48.0%) | 144 (42.0%) | 1103 (47.1%) | |
| Ever vaginal birth | 1039 (52.0%) | 199 (58.0%) | 1238 (52.9%) | |
| Asthma | 263 (13.2%) | 47 (13.7%) | 310 (13.2%) | 0.785 |
| SUI | 704 (35.2%) | 168 (49.0%) | 872 (37.2%) | <0.001 |
| SUI Only 2 | 389 (55.3%) | 80 (47.6%) | 469 (53.8%) | |
| MUI 2 | 315 (44.7%) | 88 (52.4%) | 403 (46.2%) |
P-values are obtained using two-sample t-test for continuous variables and chi-square test of independence for categorical variables
Denominator for percentages is based on the number reporting SUI
Table 3.
Characteristics of participants by stress urinary incontinence (SUI)
| Variables |
No SUI
(N = 1469 ) |
SUI
(N =872) |
Total
(N = 2341) |
P-value |
|---|---|---|---|---|
| Age Mean(SD) | 38.84(14.07) | 47.72(13.25) | 42.15(14.42) | <0.001 |
| White Race | 1354 (92.2%) | 826 (94.7%) | 2180 (93.1%) | 0.002 |
| College Education | 964 (65.6%) | 505 (57.9%) | 1469 (62.8%) | 0.001 |
| Perceived Health | <0.001 | |||
| Excellent/Very good | 1086 (73.9%) | 547 (62.7%) | 1633 (69.8%) | |
| Good | 324 (22.1%) | 253 (29.0%) | 577 (24.6%) | |
| Fair/Poor | 59 (4.0%) | 72 (8.3%) | 131 (5.6%) | |
| Activity Level | <0.001 | |||
| Sedentary | 223 (15.2%) | 184 (21.1%) | 407 (17.4%) | |
| Moderately active | 940 (64.0%) | 538 (61.7%) | 1478 (63.1%) | |
| Very active | 306 (20.8%) | 150 (17.2%) | 456 (19.5%) | |
| Obese | 350 (23.8%) | 341 (39.1%) | 691 (29.5%) | <0.001 |
| Parity | <0.001 | |||
| No live birth child | 644 (43.8%) | 144 (16.5%) | 788 (33.7%) | |
| One live birth child | 263 (17.9%) | 171 (19.6%) | 434 (18.5%) | |
| 2 or more live births | 562 (38.3%) | 557 (63.9%) | 1119 (47.8%) | |
| Delivery Type | <0.001 | |||
| No vaginal births | 850 (57.9%) | 253 (29.0%) | 1103 (47.1%) | |
| Ever vaginal birth | 619 (42.1%) | 619 (71.0%) | 1238 (52.9%) | |
| Asthma | 183 (12.5%) | 127 (14.6%) | 310 (13.2%) | 0.150 |
| Chronic Back Pain | 175 (11.9%) | 168 (19.3%) | 343 (14.7%) | <0.001 |
Note: P-values are obtained using two-sample t-test for continuous variables and chisquare test of independence for categorical variables
Of the 2,341 women in the sample, 872 (37.2%) reported SUI. Of these women, 469 (53.8%) reported SUI alone and 403 (46.2%) reported SUI with urge urinary incontinence (MUI). Chronic back pain was reported in 343 (14.7%) participants.
When compared with women without CBP (n = 1998), those who self-reported CBP (n = 343) were less likely to have college degrees (54.8% vs. 64.1%, p=0.001), were more likely to be obese (42.0% vs. 27.4%, p<0.001), indicated lower rates of excellent/very good perceived health (49.3% vs. 73.3%, p<0.001), and were more likely to lead a sedentary life style (27.7% vs. 15.6%, p<0.001). Reports of stress urinary incontinence were higher in women reporting CBP (49.0% vs. 35.2%, p<0.001).
Compared with women who did not indicate SUI, those who self-reported SUI were less likely to have college degrees (57.9% vs. 65.6%, p<0.001) and were less likely to report excellent/very good health (62.7% vs. 73.9%, p<0.001). Women reporting SUI were also more likely to report being obese (39.1% vs. 23.8%, p<0.001), and were more likely to lead a sedentary life style (21.1% vs. 15.2%, p<0.001). Most (71.0%) women in the SUI group report having had a vaginal birth, but far fewer (42.1%) report a vaginal birth in the non-SUI group (p<0.001). Chronic back pain is also reported at a higher rate in the SUI group compared to the non-SUI group (19.3% vs. 11.9%, p<0.001).
Given the differences between those reporting CBP or not, as well as the differences between those reporting SUI or not, a multiple logistic regression was used to investigate the relationship between CBP and SUI, while accounting for potential confounding variables. Potential confounding variables included white race, age, education, perceived health status, activity level, obesity, parity, vaginal birth and asthma (Table 4). After controlling for potential confounding, the odds of SUI increased by 44% for women with CBP compared to those not reporting CBP, ([aOR]=1.44, 95% CI 1.11-1.86). Also of note were increased odds of SUI for women who were obese, had asthma, and had less than ‘excellent/very good’ perceived health, had one or more live births, and had a vaginal birth. Further partitioning the self-report of SUI into SUI alone and SUI with UUI (MUI), demonstrated that women with CBP had increased odds of SUI alone ([aOR]=1.35, 95% CI 0.99-1.84) and MUI ([aOR]=1.46, 95% CI 1.05-2.05), although only the MUI analysis achieved significance at the 0.05 level (Table 4).
Table 4.
Adjusted odd ratios for reporting SUI
| SUI (yes/no) (Model I) |
SUI only (Model II) |
MUI only (Model III) |
|
|---|---|---|---|
| Chronic back pain | |||
| No | Reference | Reference | Reference |
| Yes | 1.44 (1.11,1.86 ) p=0.006 |
1.35 (0.99,1.84 ) p=0.063 |
1.46 (1.05,2.05 ) p=0.027 |
| Perceived Health | |||
| Excellent/ Very Good | Reference | Reference | Reference |
| Good | 1.28 (1.02,1.61 ) p=0.0320 |
1.12 (0.85,1.47 ) p=0.415 |
1.46 (1.08,1.96 ) p=0.013 |
| Fair / Poor | 1.66 (1.10,2.52 ) p=0.017 |
1.06 (0.61,1.84 ) p=0.848 |
2.51 (1.53,4.12 ) p<0.001 |
| Activity level | |||
| Very active | Reference | Reference | Reference |
| Moderately active | 1.04 (0.81,1.33 ) p=0.7742 |
1.13 (0.84,1.52 ) p=0.426 |
0.93 (0.67,1.30 ) p=0.663 |
| Sedentary | 1.35 (0.98,1.87 ) p=0.064 |
1.32 (0.89,1.95 ) p=0.163 |
1.47 (0.97,2.23 ) p=0.070 |
| Obese | |||
| No | Reference | Reference | Reference |
| Yes | 1.77 (1.43,2.20 ) p<0.001 |
1.62 (1.25,2.10 ) p<0.001 |
1.97 (1.49,2.61 ) p<0.001 |
| Parity | |||
| No live birth child | Reference | Reference | Reference |
| One live birth child | 1.63 (1.16,2.28 ) p=0.005 |
1.74 (1.14,2.66 ) p=0.010 |
1.52 (0.96,2.41 ) p=0.074 |
| 2 or more live births | 1.88 (1.35,2.62 ) p<0.001 |
2.02 (1.32,3.08 ) p=0.001 |
1.87 (1.20,2.91 ) p=0.006 |
| Vaginal birth | |||
| No | Reference | Reference | Reference |
| Yes | 1.77 (1.34,2.33 ) p<0.001 |
2.12 (1.50,2.99 ) p<0.001 |
1.34 (0.93,1.94 ) p=0.115 |
| Asthma | |||
| No | Reference | Reference | Reference |
| Yes | 1.46 (1.11,1.92 ) p=0.006 |
1.55 (1.13,2.12 ) p=0.007 |
1.27 (0.88,1.86 ) p=0.207 |
Note: Model I includes all women in the analyzable dataset (n = 2,341); 872 reported SUI. Of the women reporting SUI, 403 reported SUI and UUI (MUI) and 469 reported SUI only. Model II excludes women classified as MUI (n =403), resulting in a dependent variable of SUI only. Model III excludes women who reported SUI only (n = 469), resulting in a dependent variable of MUI only. Results are presented as adjusted odds ratio estimate (95% confidence interval). Odds ratios are adjusted for all variables and education, race, and age.
Comment
The aim of our study was to establish the association between chronic back pain and urinary incontinence in women. Our data indicate that women who report CBP have significantly greater odds of SUI. This study also identified other confounding factors associated with CBP, including obesity, physical activity, health status, and education level. These results are similar to other reported risk factors for back pain.1,2,27,34,35 Obesity, in addition to being associated with CBP, was also associated with increased odds of SUI. Lastly, a greater odds of SUI was found in women reporting more than one child birth and women reporting vaginal births. It has been reported by Allen et al. that vaginal childbirth could cause damage to the pelvic floor muscles and their nerve supply.36 This could therefore lead to pelvic floor muscle dysfunction such as incontinence.
The participants in the KWHR represent a healthy population with “excellent/good” perceived health reported more frequently than “poor” health. However, there is still a considerable disparity in the reporting of ‘excellent/very good’ perceived health between those with (49.3%) and without (73.3%) CBP and those reporting SUI (62.7%) or not (73.9%). Furthermore, rates of reported CBP (14.7%) and SUI (37.2%) are similar to prevalence rates reported in the literature. The prevalence rates of UI have a wide variance from 5-69%, but most studies report some degree of UI in 25-45% of women.37,38 Chronic back pain has prevalence rates reported to be 10-23% in the population.7,10 However, because our sample included a select group of women that excluded those with cancer, neurological disorders, bone disorders, pelvic disease, or history of joint, spine, and/or disc surgery, the rate of CBP and SUI are actually lower in the analysis set than in the larger KWHR sample.
This study is the first known study in the United States to investigate the relationship of CBP and SUI. However, this study has several limitations. First, the variables in our study are self-reported and not medically confirmed. Confirmation of a true medical diagnosis of CBP or UI would require medical consultation. Despite this, we believe the participants were able to respond appropriately given the language of the questions. For example, participants were not specifically asked if they had UI, UUI, or SUI but were rather asked to answer questions based on the symptomology, such as, “Do you leak urine (or) water when you cough, sneeze, or exercise?” Also, responses provided to the survey may be subject to recall bias. To minimize this possible problem, the questions for CBP and UI were asked in the present verb tense, such as “Do you have%#x2026;” versus asking the participant to reflect on whether or not they had a symptom of UI or CBP over a prior time period, such as “In the past 12 months have you had%#x2026;” Moreover, the complete association of CBP and each type of UI could not be fully explored since so few participants indicated UUI without also indicating SUI. To prevent misclassification bias, the small number reporting UUI alone (n = 77) were excluded and the analysis focused on those reporting SUI versus not. Additional analyses were also conducted to investigate the CBP relationship to SUI alone and to SUI with UUI (MUI). Similar increased odds of SUI alone and MUI were observed for those reporting CBP, although only MUI achieved statistical significance. Another potential limitation is that the frequency and severity of SUI were not collected for investigation nor were the location and severity of CBP obtained as items in the KWHR. Additionally, the cross-sectional nature of the current study prevents firm conclusions from being drawn regarding the causality of the relationship between CBP and UI. Finally, generalizations of results are limited because participants are only recruited from one state and the aim of the KWHR database may have attracted women with higher interest in health issues, compared to the general population, and possibly women with higher levels of education. Participants knew information volunteered for the KWHR was specifically intended for women’s health research making selection bias a possibility. However, the lack of comparable national or other state data on the relationship between CBP and UI raises the significance of these findings.
Our data are consistent with previous data that suggest incontinence may be associated with back pain. Particularly, this study demonstrates a significant association between SUI and CBP. An increased rate of urinary incontinence in women with back pain is consistent with the previous findings of Smith and colleagues based on their survey of Australian women.27 Our findings are also consistent with data from Finkelstein et al that determined an association with ‘back problems’ and UI in the survey of Canadians.26 However, neither of these studies differentiated between the type of incontinence, either stress or urge incontinence. Our data suggest that women reporting UI indicate SUI more often, and that rates of SUI are increased in those also reporting CBP. Smith et al looked at the frequency of both back pain and UI in terms of ‘never,’ rarely,’ sometimes,’ or ‘often.’ 27 Another study by Kim et al assessed UI frequency and severity using a numeric scale and then classified participants’ total score for UI as mild, moderate, and severe. Kim et al also had participants rate back pain severity using a visual analog scale and functional disability using the Korean version of the Oswestry Disability Index.28 Our study did not examine the frequency or severity of back pain or UI. Another study by Eliasson et al was the only study to examine the types of UI (stress, urge, and mixed) in relation to LBP in addition to classifications of UI as ‘occasionally,’ ‘several times,’ and ‘often.’ Eliasson reported 78% of the women with LBP reported UI and of these women 72% SUI, 1% UUI, and 27% MUI.29 In our study, 51.4% of the women with CBP reported any UI and of these women 44.0% reported SUI alone, and 6.0% reported UUI alone, and 47.8% reported SUI with UUI (MUI). Similar to our study, other studies investigating the relationship between back pain and UI utilized surveys or questionnaires and a cross-sectional study design.26-29 However, Finkelstein et al26 asked participants if they had UI or ‘back problems’ diagnosed by a health professional as opposed to just self-reporting these symptoms. The difference between an anonymous survey and a medical diagnosis may be important, especially since less than 50% of women with UI discuss their symptoms with their healthcare provider.39,40 Lastly, the definitions of back pain and urinary incontinence in the literature vary and may complicate comparison of these studies.
One explanation for the increased likelihood of SUI occurring with CBP may be dysfunction of the pelvic floor muscles. It has been documented that the pelvic floor muscles play a role in urinary continence particularly during activities that result in increased abdominal pressure.24,25,41,42 It has also been demonstrated that the pelvic floor muscles play a role in spinal stability.20-22 A recent study by Arab et al43 reports a significant difference in the pelvic floor muscle function for women with and without LBP as measured by transabdominal ultrasound. Furthermore, Smith et al44 has suggested the postural function of the pelvic floor muscles is altered in women with incontinence, with a delayed response in incontinent versus continent women.44 Lastly, a longitudinal study completed on UI and back pain suggests pelvic floor dysfunction may contribute to the development of back pain.31
Conclusion
A significant association between CBP and stress UI was found. It is reasonable to conclude that it is important for all trunk muscles, including the pelvic floor muscles, to function in coordination with one another for postural control and for prevention of pain and dysfunction. Therefore, it may be possible that the management of chronic back pain includes assessment of and treatment for the pelvic floor muscles. Although uncertain, it is the concern of these authors that this may not be the common practice in the physical therapy management of back pain. Therefore, as part of the complete history, the physical therapist should consider the findings of this study when treating women with back pain and inquire about the patient’s level of continence. Because the topic of continence is often difficult to discuss, and because patients may not understand the relationship between this symptom and others, it is even more imperative for healthcare professionals, including physical therapists, to initiate discussion on this topic. Clinical assessment findings may indicate the need for further treatment interventions that may address the pelvic floor, referral to a physical therapist specialized in the treatment of incontinence, or to another health care provider. The relationship between UI and CBP identified in this study along with the importance of postural muscles in both UI and CBP provides the clinician insight into potential interventions for treatment and prevention.
Future research should focus on determining if physical therapists, particularly those treating women with back pain, ask these particular patients about their level of continence. Potential barriers may exist for a physical therapist to ask patients about continence or for patients to answer a question about their level of continence. Furthermore, it is unknown if the common physical therapy management for back pain includes assessment and treatment of the pelvic floor musculature. Reasons why physical therapists do or do not incorporate pelvic floor muscle exercises for patients with back pain should be explored. Further studies that assess the function of the pelvic floor muscles in women with and without back pain are needed. Treatment intervention studies that examine the effect of incorporating pelvic floor musculature into traditional back stabilization programs should be pursued and evaluated for differences in pain, disability, or total healthcare costs. It is unknown if incorporating pelvic floor muscle exercises into traditional rehabilitation strategies for CBP will result in less pain, disability, or better utilization of the health-care dollar. Finally, future research should also consider additional prospective longitudinal studies to investigate the predictive value of UI or CBP.
Acknowledgements
The authors acknowledge Mikal Mathies, PT, DPT and Talissa Dawson, PT, DPT for their interest in this research topic, Candace Brancato for her help with KWHR data, and thank the women who participated in the Kentucky Women’s Health Registry.
This publication was supported by grant number UL1RR033173 from the National Center for Research Resources (NCRR), funded by the Office of the Director, National Institutes of Health (NIH) and supported by the NIH Roadmap for Medical Research.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Stacey Pagorek, University of Kentucky College of Health Sciences Division of Physical Therapy.
Janice Kuperstein, University of Kentucky College of Health Sciences Division of Physical Therapy.
Jing Guo, University of Kentucky College of Public Health Department of Biostatistics.
Katie N Ballert, University of Kentucky College of Medicine Division of Urology.
Leslie J Crofford, University of Kentucky Department of Medicine Division of Rheumatology.
References
- 1.Strine TW, Hootman JM. US national prevalence and correlates of low back and neck pain among adults. Arthritis Rheum. 2007;57(4):656–665. doi: 10.1002/art.22684. [DOI] [PubMed] [Google Scholar]
- 2.Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from the US national surveys, 2002. Spine. 2006;31(23):2724–2727. doi: 10.1097/01.brs.0000244618.06877.cd. [DOI] [PubMed] [Google Scholar]
- 3.Frymoyer JW, Pope MH, Clements JH, Wilder DG, MacPherson B, Ashikaga T. Risk factors in low-back pain: an epidemiological survey. J Bone Joint Surg Am. 1983;65(2):213–218. doi: 10.2106/00004623-198365020-00010. [DOI] [PubMed] [Google Scholar]
- 4.Andersson GB. Epidemiological features of chronic low back pain. Lancet. 1999;354(9178):581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 5.Andersson GBJ. The epidemiology of spinal disorders. In: Frymoyer JW, editor. The Adult Spine: Principles and Practice. 2nd ed Lippincott-Raven; Philadelphia: 1997. pp. 93–141. [Google Scholar]
- 6.Walker BF. The prevalence of low back pain: a systemic review of the literature from 1966 to 1998. J Spinal Disord. 2000;13(3):205–217. doi: 10.1097/00002517-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Airaksinen O, Brox JI, Cedraschi C, for the COST B13 Working Group on Guidelines for Chronic Low Back Pain Chapter 4: European guidelines for the management of chronic nonspecific low back pain. Eur Spine J. 2006;15(suppl 2):S192–300. doi: 10.1007/s00586-006-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pengel LH, Herbert RD, Maher CG, Refshauge KM. Acute low back pain: systemic review of its prognosis. BMJ. 2003;327:323–325. doi: 10.1136/bmj.327.7410.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hestbaek L, Leboeuf-Yde C, Manniche C. Low back pain: what is the long-term course? a review of studies of general patient populations. Eur Spine J. 2003;12(2):149–165. doi: 10.1007/s00586-002-0508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251–258. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jette AM, Smith K, Haley SM, Davis KD. Physical therapy episodes of care for patients with low back pain. Phys Ther. 1994;74(2):101–110. doi: 10.1093/ptj/74.2.101. [DOI] [PubMed] [Google Scholar]
- 12.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8(1):8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Rizzo JA, Abbott TA, III, Berger ML. The labor productivity effects of chronic backache in the United States. Med Care. 1998;36(10):1471–1488. doi: 10.1097/00005650-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 14.van Tulder M, Koes B, Bombardier C. Low back pain. Best Pract Res Clin Rheumatol. 2002;16(5):761–775. doi: 10.1053/berh.2002.0267. [DOI] [PubMed] [Google Scholar]
- 15.Krismer M, van Tulder M, for the Low Back Pain Group of the Bone and Joint Health Strategies for Europe Project Strategies for prevention and management of musculoskeletal conditions. low back pain (non-specific) Best Pract Res Clin Rheumatol. 2007;21(1):77–91. doi: 10.1016/j.berh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. a motor control evaluation of transversus abdominis. Spine. 1996;21(22):2640–2650. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- 17.Radebold A, Cholewicki J, Panjabi MM, Patel TC. Muscle response pattern to sudden trunk loading in healthy individuals and in patients with chronic low back pain. Spine. 2000;25(8):947–954. doi: 10.1097/00007632-200004150-00009. [DOI] [PubMed] [Google Scholar]
- 18.Barr KP, Griggs M, Cadby T. Lumbar stabilization: core concepts and current literature, part 1. Am J Phys Med Rehabil. 2005;84(6):473–480. doi: 10.1097/01.phm.0000163709.70471.42. [DOI] [PubMed] [Google Scholar]
- 19.Barr KP, Griggs M, Cadby T. Lumbar stabilization: a review of core concepts and current literature, part 2. Am J Phys Med Rehabil. 2007;86(1):72–80. doi: 10.1097/01.phm.0000250566.44629.a0. [DOI] [PubMed] [Google Scholar]
- 20.Sapsford RR, Hodges PW. Contraction of the pelvic floor muscles during abdominal maneuvers. Arch Phys Med Rehabil. 2001;82(8):1081–1088. doi: 10.1053/apmr.2001.24297. [DOI] [PubMed] [Google Scholar]
- 21.Hodges PW, Sapsford R, Pengel LH. Postural and respiratory functions of the pelvic floor muscles. Neurourol Urodyn. 2007;26(3):362–371. doi: 10.1002/nau.20232. [DOI] [PubMed] [Google Scholar]
- 22.Pool-Goudzwaard A, van Dijke GH, van Gurp M, Mulder P, Snijders C, Stoeckart R. Contribution of pelvic floor muscles to stiffness of the pelvic ring. Clin Biomech (Bristol, Avon) 2004;19(6):564–571. doi: 10.1016/j.clinbiomech.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Sapsford R. Rehabilitation of the pelvic floor muscles utilizing trunk stabilization. Man Ther. 2004;9(1):3–12. doi: 10.1016/s1356-689x(03)00131-0. [DOI] [PubMed] [Google Scholar]
- 24.Dumoulin C, Hay-Smith J. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev. 2010 Jan;20(1):CD005654. doi: 10.1002/14651858.CD005654.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Price N, Dawood R, Jackson SR. Pelvic floor exercise for urinary incontinence: a systematic literature review. Maturitas. 2010;67(4):309–315. doi: 10.1016/j.maturitas.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Finkelstein MM. Medical conditions, medications, and urinary incontinence. analysis of a population-based survey. Can Fam Physician. 2002;48:96–101. [PMC free article] [PubMed] [Google Scholar]
- 27.Smith MD, Russell A, Hodges PW. Disorders of breathing and continence have a stronger association with back pain than obesity and physical activity. Aust J Physiother. 2006;52(1):11–16. doi: 10.1016/s0004-9514(06)70057-5. [DOI] [PubMed] [Google Scholar]
- 28.Kim JS, Kim SY, Oh DW, Choi JD. Correlation between the severity of female urinary incontinence and concomitant morbidities: a multi-center cross-sectional clinical study. Int Neurourol J. 2010;14(4):220–226. doi: 10.5213/inj.2010.14.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eliasson K, Elfving B, Nordgren B, Mattsson E. Urinary incontinence in women with low back pain. Man Ther. 2008;13(3):206–212. doi: 10.1016/j.math.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the international continence society. Neurourol Urodyn. 2002;21(2):167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 31.Smith M, Russell A, Hodges P. Do incontinence, breathing difficulties, and gastrointestinal symptoms increase the risk of future back pain? J Pain. 2009;10(8):876–886. doi: 10.1016/j.jpain.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell J, Mannino DM, Steinke D, Kryscio R, Crofford L, Bush H. Association of smoking and chronic pain syndromes in Kentucky women. J Pain. 2011;12(8):892–899. doi: 10.1016/j.jpain.2011.02.350. [DOI] [PubMed] [Google Scholar]
- 33.Coker A, Hopenhayn C, DeSimone CP, Bush H, Crofford L. Violence against women raises risk of cervical cancer. Journal of Women’s Health. 2009 Aug;18(8):1179–1185. doi: 10.1089/jwh.2008.1048. [DOI] [PubMed] [Google Scholar]
- 34.Rubin DI. Epidemiology and risk factors for spine pain. Neurol Clin. 2007;25(2):353–371. doi: 10.1016/j.ncl.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Hoy D, Brooks P, Blyth F, Buchbinder R. The epidemiology of low back pain. Best Pract & Res Clin Rheumatol. 2010;24(6):769–781. doi: 10.1016/j.berh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Allen RE, Hosker GL, Smith AR, Warrell DW. Pelvic floor damage and childbirth: a neurophysiological study. Br J Obstet Gynaecol. 1990 Sep;97(9):770–779. doi: 10.1111/j.1471-0528.1990.tb02570.x. [DOI] [PubMed] [Google Scholar]
- 37.Buckley BS, Lapitan MC. Epidemiology Committee of the Fourth International Consultation on Incontinence, Paris, 2008 Prevalence of urinary incontinence in men, women, and children-- current evidence: findings of the Fourth International Consultation on Incontinence. Urology. 2010;76(2):265–270. doi: 10.1016/j.urology.2009.11.078. [DOI] [PubMed] [Google Scholar]
- 38.Melville JL, Katon W, Delaney K, Newton K. Urinary incontinence in US women: a population-based study. Arch Intern Med. 2005;165(5):537–42. doi: 10.1001/archinte.165.5.537. [DOI] [PubMed] [Google Scholar]
- 39.Koch LH. Help-seeking behaviors of women with urinary incontinence: an integrative literature review. J Midwifery Womens Health. 2006;51(6):e39–44. doi: 10.1016/j.jmwh.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Kinchen KS, Burgio K, Diokno AC, Fultz NH, Bump R, Obenchain R. Factors associated with women’s decisions to seek treatment for urinary incontinence. J Womens Health (Larchmt) 2003;12(7):687–698. doi: 10.1089/154099903322404339. [DOI] [PubMed] [Google Scholar]
- 41.Deindl FM, Vodusek DB, Hesse U, Schussler B. Activity patterns of pubococcygeal muscles in nulliparous continent women. Br J Urol. 1993;72(1):46–51. doi: 10.1111/j.1464-410x.1993.tb06455.x. [DOI] [PubMed] [Google Scholar]
- 42.Peschers UM, Vodusek D, Fanger G, Schaer G, DeLancey J, Schussler B. Pelvic muscle activity in nulliparous volunteers. Neurourol Urodyn. 2001;20:269–275. doi: 10.1002/nau.1004. [DOI] [PubMed] [Google Scholar]
- 43.Arab AM, Behbahani RB, Lorestani L, Azari A. Assessment of pelvic floor muscle function in women with and without low back pain using transabdominal ultrasound. Man Ther. 2010;15(3):235–239. doi: 10.1016/j.math.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Smith MD, Coppieters MW, Hodges PW. Postural activity of the pelvic floor muscles is delayed during rapid arm movements in women with stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(8):901–911. doi: 10.1007/s00192-006-0259-7. [DOI] [PubMed] [Google Scholar]


