Abstract
A palladium-catalyzed 1,4-addition across the commodity chemical 1,3-butadiene to afford skipped polyene products is reported. Through a palladium σ→π→σ allyl isomerization, two new carbon-carbon bonds are formed with high regio- and trans stereochemical selectivity of the newly formed alkene. The utility of this method is highlighted by the successful synthesis of the ripostatin A skipped triene core.
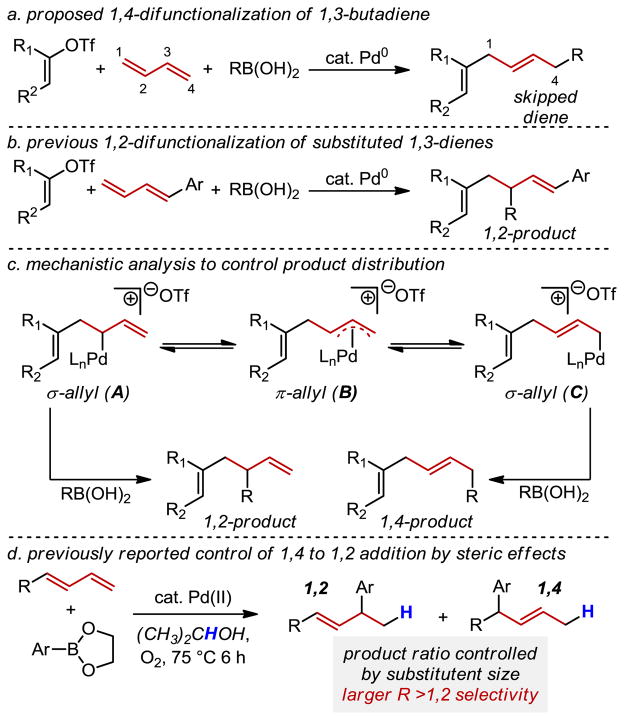
The four-carbon feedstock, 1,3-butadiene, is a product of petroleum cracking on a >10 million ton scale annually,1 which makes it an especially attractive candidate for converting into more complex building blocks for synthesis. In this regard, we envisioned using this simple and symmetric diene2 in a similar approach to that of the venerable Diels-Alder reaction by forming carbon–carbon bonds at both termini with the preservation of a single alkene in the product. In contrast to cycloadditions of 1,3-butadiene with dienophiles, we wanted to controllably form the carbon-carbon bonds using two distinct reaction partners through a 1,4-difunctionalization process (Figure 1a). This would efficiently generate significant molecular complexity from a simple feedstock. In considering possible coupling partners, a specific goal was to ultimately form skipped diene and triene products as these are common motifs in various natural products3–15 and often challenging to prepare.16–19 Herein we present the development of a Pd-catalyzed 1,4-addition of vinyl electrophiles and boronic acid derivatives across 1,3-butadiene to access these important structural motifs highlighted by the synthesis of the skipped triene core of ripostatin A.3
Figure 1.
Proposed 1,4-difunctionalizaion of 1,3-butadiene with vinyl triflates and boronic acids
To accomplish a 1,4-difunctionalization of butadiene using a three-component coupling strategy, several important selectivity issues needed to be considered.20–21 We recently reported a three-component coupling of substituted 1,3-dienes, vinyl triflates, and boronic acids wherein selective formation of the 1,2-addition product is observed (Figure 1b).21d The use of vinyl triflates is proposed to account for the high selectivity of the three-component coupling product rather than either the Heck or Suzuki products presumably due to the cationic nature of intermediates along the reaction path. The use of these and related reagents is an obvious starting point for reaction development. Achieving a 1,4-addition rather than a 1,2-addition across butadiene after oxidative addition to form A requires a σ→π→σ isomerization of the allyl complex22 (Figure 1c, A→B→C) with subsequent cross-coupling of C with a boronic acid derivative. A key previous report suggests that this isomerization is facile (Figure 1d). Specifically, a systematic study of substituent effects in the hydroarylation of terminal 1,3-dienes, which proceeds through a similar π-allyl species, reveals a linear free-energy relationship between Charton steric parameters and the logarithm of the ratio of 1,2 and 1,4-regioisomers.21e If 1,3-butadiene was employed, extrapolation of the Charton relationship would achieve >20:1 selectivity for the 1,4-addition reaction. However, the use of a Pd-Ar rather than a Pd-H species to initiate the alkene functionalization, as well as the likely requirement of different reaction conditions than those used in the hydroarylation reaction, makes the outcome of this reaction less predictable.
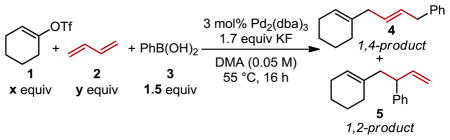
To explore the possibility of a 1,4-difunctionalization of 1,3-butadiene, vinyl triflate 1 and phenyl boronic acid 3 were chosen for reaction optimization (Table 1). The initial reaction conditions tested were those found optimal for the previously reported three-component coupling.21d While the three-component coupling products were formed in an excellent yield, the product distribution was suboptimal with a 3:1 ratio of the desired 1,4-addtion product 4 to the 1,2-addition product 5 as an inseparable mixture (entry 1). Initially, butadiene was introduced in the gaseous phase via a balloon, where the stoichiometry cannot be easily controlled. Therefore, we moved to a standard, commercial solution as the source of 1,3-butadiene (15 wt% in n-hexane). Upon this change, a significant improvement in selectivity for the 1,4- addition product is observed albeit at the cost of yield. An origin for this improvement may be inhibition of σ→π→σ isomerization when 1,3-butadiene is in substantial molar excess as it may act as a ligand on Pd. Modest concentration deviations of the diene had negligible effects.23 Further improvement of both selectivity and yield is observed upon either performing the reaction at higher overall concentration (entry 3) or adding an excess of the vinyl triflate (entry 4). Combining these changes provides an optimized procedure where a >20:1 selectivity for the 1,4-addition product and a 92:8 ratio for the desired trans alkene is observed (entry 5). It should be noted that some of the enhancement of the observed selectivity is from consumption of the undesired terminal alkene product 5 with the excess vinyl triflate. Therefore, an improvement of selectivity and ease of purification comes with a cost of overall yield. Finally, the vinyl triflate can be replaced with the more economical vinyl no-naflate,24 which produces nearly identical results (entry 6).
Table 1.
Optimization of three-component coupling with 1,3-butadiene
| ||||||
|---|---|---|---|---|---|---|
| Entry | Conc. (M) | x | y | % Yielda | 4:5b | E:Zb |
| 1 | 0.05 | 1.0 | balloon | 81 | 2.4 | 88:12 |
| 2 | 0.05 | 1.0 | 1.0c | 50 | 12.6 | 91:9 |
| 3 | 0.2 | 1.0 | 1.0c | 79 | 15.0 | 91:9 |
| 4 | 0.05 | 1.5 | 1.0c | 71 | 16.7 | 90:10 |
| 5 | 0.2 | 1.5 | 1.0c | 75 | >20 | 92:8 |
| 6d | 0.2 | 1.5 | 1.0c | 73 | 14.3 | 92:8 |
isolated yield.
Determined by 1H NMR.
15 wt% solution 2 in hexanes used.
Nonaflate used in place of triflate.
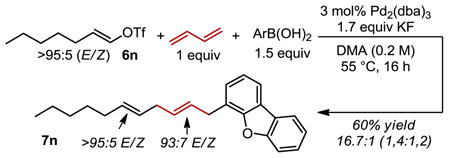
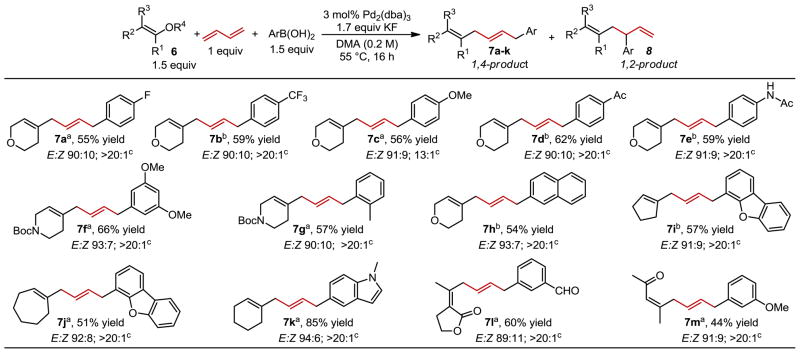
Using the optimal conditions, the scope of this 1,4-vinylarylation of 1,3-butadiene was assessed, which was initially focused on a variety of substituted arenes and heterocyclic vinyl triflates/nonaflates resulting in excellent selectivity for the 1,4-addition product. Electronic effects of the aryl boronic acid had little influence on product yields (7a–f), although a lower regioselectivity was observed for the more electron rich aryl group installed in 7c. Examining the steric influence of o-tolyl boronic acid, 7g, resulted in good selectivity and good yield. When larger arenes, including several heteroaromatic groups, were examined, the yield and selectivity of the process were not significantly influenced (7h–k). Unfortunately, boronic acids incorporating more Lewis basic heteroatoms (e.g., pyridine) fail to yield the three-component coupling product in an appreciable amount. Acyclic Z-vinyl triflates derived from β-dicarbonyls were successfully used, yielding 7l and 7m without isomerization of alkene geometry suggesting that a wide variety of skipped dienes can be accessed using this method. Finally, the use of a simple E-vinyl triflate (6n) resulted in the desired product (7n), again, without loss of stereochemical integrity originating from the vinyl triflate (Eq. 1). It should be noted that the internal alkene derived from butadiene is formed in a >8:1 ratio favoring the trans configuration in all cases, which is consistent with other difunctionalization reactions of this substrate. 20k,22c,h–j
 |
(1) |
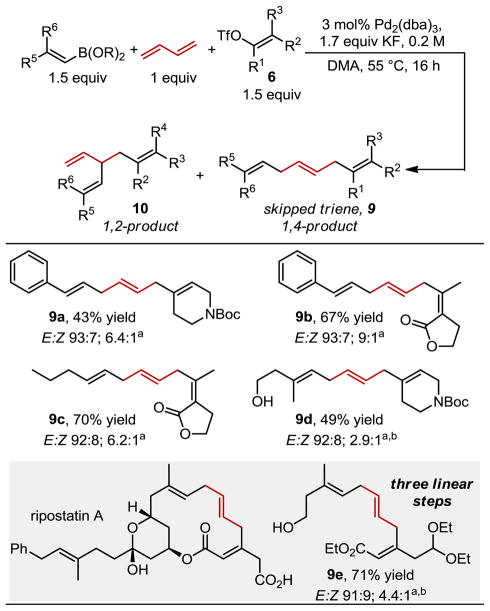
Encouraged by the successful vinylarylation three-component coupling reactions, we sought to explore a three-component vinylvinylation of 1,3-butadiene to form skipped trienes (Table 3). A styrenyl boronic acid can be coupled with a cyclic and an acyclic boronic acid with 1,3-butadiene to effectively form skipped trienes 9a and 9b in modest to good yields. A simple acyclic vinyl boronic is also an effective substrate in the synthesis of the skipped triene 9c. The use of a considerably more complex organometallic coupling partner requires the use a higher catalyst loading to prepare 9d. In this case, the pinacol boronic ester was used as it was more easily accessed (9d–9e). In these cases, the alkene geometry in both the vinyl triflate and the boronic ester remain intact. Additionally, the alkene derived from 1,3-butadiene is again formed in the trans configuration suggesting a trans σ-allyl intermediate as depicted in C in Figure 1. The selectivity for 1,4-addition relative to 1,2-addition is diminished relative to the vinylarylation process but the 1,4-addition product of the skipped dienes can be accessed by re-exposing the mixture to the reaction conditions, which selectively consumes the terminal alkene.
Table 3.
Scope of 1,4-vinylvinylation of 1,3-butadiene
|
All yields and ratios represent the average of two experiments on 0.5 mmol scale. Yields represent a mixture of stereo and regioisomers.
ratio of 9:10 and E:Z ratio determined by 1H NMR of the crude reaction mixture.
Using the pinacol boronic ester and 6 mol% Pd2(dba)3
To showcase the utility of this method, the skipped diene core of ripostatin A, a bacterial RNA-polymerase inhibitor,3 was targeted. To our delight, the desired product (9e) was formed with the appropriate display of the skipped triene core and necessary functional groups for further manipulation. This example effectively illustrates the synthetic potential of this method by incorporating a simple feedstock chemical between two relatively complex fragments.
In summary, we have developed a new method for the efficient access to skipped polyenes. This process takes advantage of the simplicity of 1,3-butadiene and the ability to selectively and independently functionalize both terminal alkenes. Additionally, the reaction proceeds with high stereochemical integrity of the alkenes formed wherein the geometry of the starting materials is relayed into the product and the alkene fashioned from 1,3-butadiene is mainly of the trans geometry. The scope of the method is highlighted by the synthesis of the skipped triene core structure of ripostatin A in only three linear steps from simple starting materials. Many mechanistic questions arise when considering substituted terminal 1,3-dienes where more highly functionalized skipped polyene products can be envisioned. Future work is focused on examining these effects and expanding the scope of coupling partners.
Supplementary Material
Table 2.
Scope of 1,4-vinylarylation of 1,3-butadiene
|
All yields and ratios represent the average of two experiments on 0.5 mmol scale. E:Z ratios determined by 1H NMR. Yields represent a mixture of stereo and regioisomers.
R4 = Tf.
R1 = Nf.
Ratio of 7:8 as determined by 1H NMR.
Acknowledgments
This work was supported by the National Institutes of Health (NIGMS RO1 GM3540).
Footnotes
Supporting Information. Experimental procedures and characterization data for new substances. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) White WC. Chem-Biol Interact. 2007;166:10. doi: 10.1016/j.cbi.2007.01.009. [DOI] [PubMed] [Google Scholar]; (b) Morrow NL. Environ Health Perspect. 1990;86:7. doi: 10.1289/ehp.90867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.For recent uses of 1,3-butadiene in precious chemical synthesis, see: Zbieg JR, Yamaguchi EL, McInturff MJ, Krische MJ. Science. 2012;336:324. doi: 10.1126/science.1219274.Arndt M, Dindaroğlu M, Schmalz H-G, Hilt G. Org Lett. 2011;13:6236. doi: 10.1021/ol202696n.
- 3.Ripostatin: Augustiniak H, Irschik H, Reichenbach H, Hoefle G. Liebigs Ann. 1996:1657.Irschik H, Augustiniak H, Gerth K, Hçfle G, Reichenbach H. J Antibiot. 1995;48:787. doi: 10.7164/antibiotics.48.787.
- 4.Piericidin: Kitaura N, Shirata K, Niwano M, Mimura M, Takahara Y. 05339156. Japanese Patent. 1993:A2.Chem Abstr. 1994;120:208590.
- 5.Lehualide: Sata N, Abinsay H, Yoshida WY, Horgen FD, Sitachitta N, Kelly M, Scheuer PJ. J Nat Prod. 2005;68:1400–1403. doi: 10.1021/np0500528.
- 6.Jerangolid: Reichenbach H, Hoefle G, Gerth K, Washausen P. DE 19607702. PCT Int Appl. 1997Gerth K, Washausen P, Hoefle G, Irschik H, Reichenbach H. J Antibiot. 1996;49:71. doi: 10.7164/antibiotics.49.71.
- 7.Actinopyrone: Hosokawa S, Yokota K, Imamura K, Suzuki Y, Kawarasaki M, Tatsuta K. Tetrahedron Lett. 2006;47:5415.Taniguchi M, Watanabe M, Nagai K, Suzumura KI, Suzuki KI, Tanaka A. J Antibiot. 2000;53:844. doi: 10.7164/antibiotics.53.844.
- 8.Ambruticin: Knauth P, Reichenbach H. J Antibiot. 2000;53:1182. doi: 10.7164/antibiotics.53.1182.Ringel SM, Greenough RC, Roemer S, Connor D, Gutt AL, Blair B, Kanter G, Von SM. J Antibiot. 1977;30:371. doi: 10.7164/antibiotics.30.371.
- 9.Halichlorine: Arimoto H, Hayakawa I, Kuramoto M, Uemura D. Tetrahedron Lett. 1998;39:861.Kuramoto M, Tong C, Yamada K, Chiba T, Hayashi Y, Uemura D. Tetrahedron Lett. 1996;37:3867.
- 10.Haterumalide: Ueda K, Hu Y. Tetrahedron Lett. 1999;40:6305.
- 11.Lobatamide: Shen R, Lin CT, Bowman EJ, Bowman BJ, Porco JA., Jr J Am Chem Soc. 2003;125:7889. doi: 10.1021/ja0352350.Shen R, Lin CT, Porco JA., Jr J Am Chem Soc. 2002;124:5650. doi: 10.1021/ja026025a.
- 12.Mycothiazole: Crews P, Kakou Y, Quinoa E. J Am Chem Soc. 1988;110:4365.
- 13.Madangamine: Kong F, Andersen RJ, Allen TM. J Am Chem Soc. 1994;116:6007.
- 14.Phorbasin: McNally M, Capon RJ. J Nat Prod. 2001;64:645. doi: 10.1021/np0005614.
- 15.Various fatty acids: Durand S, Parrain J-L, Santelli M. J Chem Soc, Perkin Trans 1. 2000:253.
- 16.Julia Olefination: Pospíšil J, Markó IE. J Am Chem Soc. 2007;129:3516. doi: 10.1021/ja0691728.Schnermann MJ, Romero A, Hwang I, Nakamaru-Ogiso E, Yagi T, Boger DL. J Am Chem Soc. 2006;128:11799. doi: 10.1021/ja0632862.
- 17.Cross-Coupling: Ye KY, He H, Liu WB, Dai LX, Helmchen G, You SL. J Am Chem Soc. 2011;133:19006. doi: 10.1021/ja2092954.Gutierrez AC, Jamison TF. Org Lett. 2011;13:6414. doi: 10.1021/ol2027015.Winter P, Vaxelaire C, Heinz C, Christmann M. Chem Commun. 2011;47:394. doi: 10.1039/c0cc02332a.Macklin TK, Micalizio GC. Nature Chem. 2010;2:638. doi: 10.1038/nchem.665.Jeso V, Micalizio GC. J Am Chem Soc. 2010;132:11422. doi: 10.1021/ja104782u.Diez PS, Micalizio GC. J Am Chem Soc. 2010;132:9576. doi: 10.1021/ja103836h.Gu Y, Snider BB. Org Lett. 2003;5:4385. doi: 10.1021/ol0356789.Thadani AN, Rawal VH. Org Lett. 2002;4:4317. doi: 10.1021/ol0269594.
- 18.Takai Olefination: Gagnepain J, Moulin E, Fürstner A. Chem Eur J. 2011;17:6964. doi: 10.1002/chem.201100178.Macklin TK, Micalizio GC. J Am Chem Soc. 2009;131:1392. doi: 10.1021/ja809491b.Schomaker JM, Borhan B. J Am Chem Soc. 2008;130:12228. doi: 10.1021/ja8043695.Liu P, Jacobsen EN. J Am Chem Soc. 2001;123:10772. doi: 10.1021/ja016893s.
- 19.Ring Closing Metathesis: Winter P, Hiller W, Christmann M. Angew Chem, Int Ed. 2012;51:3396. doi: 10.1002/anie.201108692.Tang W, Prusov EV. Angew Chem Int Ed. 2012;51:3401. doi: 10.1002/anie.201108749.Tang W, Prusov EV. Org Lett. 2012;14:4690. doi: 10.1021/ol302219x.
- 20.(a) Zhou L, Ye F, Zhang Y, Wang J. J Am Chem Soc. 2010;132:13590. doi: 10.1021/ja105762n. [DOI] [PubMed] [Google Scholar]; (b) Zhang X, Larock RC. Tetrahedron. 2010;66:4265. doi: 10.1016/j.tet.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kudirka R, Devine SKJ, Adams CS, Van Vranken DL. Angew Chem Int Ed. 2009;48:3677. doi: 10.1002/anie.200805483. [DOI] [PubMed] [Google Scholar]; (d) Shu W, Jia G, Ma S. Angew Chem Int Ed. 2009;48:2788. doi: 10.1002/anie.200805422. [DOI] [PubMed] [Google Scholar]; (e) Cheng Y-n, Duan Z, Yu L, Li Z, Zhu Y, Wu Y. Org Lett. 2008;10:901. doi: 10.1021/ol703018t. [DOI] [PubMed] [Google Scholar]; (f) Jayanth TT, Jeganmohan M, Cheng CH. Org Lett. 2005;7:2921. doi: 10.1021/ol050859r. [DOI] [PubMed] [Google Scholar]; (g) Zhou C, Larock RC. J Org Chem. 2005;70:3765. doi: 10.1021/jo048265+. [DOI] [PubMed] [Google Scholar]; (h) Oh CH, Lim YM. Tetrahedron Lett. 2003;44:267. [Google Scholar]; (i) Aftab T, Grigg R, Ladlow M, Sridharan V, Thornton-Pett M. Chem Comm. 2002:1754. doi: 10.1039/b205069b. [DOI] [PubMed] [Google Scholar]; (j) Shaulis KM, Hoskin BL, Townsend JR, Goodson FE, Incarvito CD, Rheingold AL. J Org Chem. 2002;67:5860. doi: 10.1021/jo016212b. [DOI] [PubMed] [Google Scholar]; (k) Huang TH, Chang HM, Wu MY, Cheng CH. J Org Chem. 2001;67:99. doi: 10.1021/jo010637g. [DOI] [PubMed] [Google Scholar]
- 21.(a) Saini V, Sigman MS. J Am Chem Soc. 2012;134:11372. doi: 10.1021/ja304344h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Stokes BJ, Opra SM, Sigman MS. J Am Chem Soc. 2012;134:11408. doi: 10.1021/ja305403s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Pathak TP, Sigman MS. Org Lett. 2011;13:2774. doi: 10.1021/ol200913r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Liao L, Jana R, Urkalan KB, Sigman MS. J Am Chem Soc. 2011;133:5784. doi: 10.1021/ja201358b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Liao L, Sigman MS. J Am Chem Soc. 2010;132:10209. doi: 10.1021/ja105010t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Werner EW, Urkalan KB, Sigman MS. Org Lett. 2010;12:2848. doi: 10.1021/ol1009575. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Urkalan KB, Sigman MS. Angew Chem Int Ed. 2009;48:3146. doi: 10.1002/anie.200900218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Zhang P, Brozek LA, Morken JP. J Am Chem Soc. 2010;132:10686. doi: 10.1021/ja105161f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhao B, Du H, Cui S, Shi Y. J Am Chem Soc. 2010;132:3523. doi: 10.1021/ja909459h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Denmark SE, Werner NS. J Am Chem Soc. 2008;130:16382. doi: 10.1021/ja805951j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Du H, Yuan W, Zhao B, Shi Y. J Am Chem Soc. 2007;129:7496. doi: 10.1021/ja072080d. [DOI] [PubMed] [Google Scholar]; (e) Du H, Yuan W, Zhao B, Shi Y. J Am Chem Soc. 2007;129:11688. doi: 10.1021/ja074698t. [DOI] [PubMed] [Google Scholar]; (f) Du H, Zhao B, Shi Y. J Am Chem Soc. 2007;129:762. doi: 10.1021/ja0680562. [DOI] [PubMed] [Google Scholar]; (g) Bar GgLJ, Lloyd-Jones GC, Booker-Milburn KI. J Am Chem Soc. 2005;127:7308. doi: 10.1021/ja051181d. [DOI] [PubMed] [Google Scholar]; (h) Bäckvall JE, Vaagberg JO. J Org Chem. 1988;53:5695. [Google Scholar]; (i) Bäckvall JE, Nystroem JE, Nordberg RE. J Am Chem Soc. 1985;107:3676. [Google Scholar]; (j) Bäckvall JE, Bystroem SE, Nordberg RE. J Org Chem. 1984;49:4619. [Google Scholar]
- 23.See Supporting Information for complete table of optimization data.
- 24.For a review of nonaflates, see: Högermeier J, Reißig HU. Adv Synth Catal. 2009;351:2747.Lyapkalo Ilya M, Webel M, Reißig H-U. Eur J Org Chem. 2002:1015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.