Abstract
Decreased mitochondrial oxidative metabolism is a hallmark bioenergetic characteristic of malignancy that may have an adaptive role in carcinogenesis. By stimulating proton leak, mitochondrial uncoupling proteins (UCP1-3) increase mitochondrial respiration and may thereby oppose cancer development. To test this idea, we generated a mouse model that expresses an epidermal-targeted keratin-5-UCP3 (K5-UCP3) transgene and exhibits significantly increased cutaneous mitochondrial respiration compared with wild type (FVB/N). Remarkably, we observed that mitochondrial uncoupling drove keratinocyte/epidermal differentiation both in vitro and in vivo. This increase in epidermal differentiation corresponded to the loss of markers of the quiescent bulge stem cell population, and an increase in epidermal turnover measured using a bromodeoxyuridine (BrdU)-based transit assay. Interestingly, these changes in K5-UCP3 skin were associated with a nearly complete resistance to chemically-mediated multistage skin carcinogenesis. These data suggest that targeting mitochondrial respiration is a promising novel avenue for cancer prevention and treatment.
Keywords: mitochondria, uncoupling proteins, skin, carcinogenesis, differentiation
Introduction
Metabolic reprogramming marked by increased glycolysis and decreased mitochondrial oxidative phosphorylation is a hallmark of malignancy.1,2 Whereas extensive work indicates that glycolytic upregulation promotes the growth and survival of diverse cancers,3,4 the evolution and functional importance of mitochondrial respiratory changes during carcinogenesis are less well understood. Increasing evidence indicates that mitochondrial respiration may suppress tumorigenesis.5 Induction of mitochondrial inner membrane proton leak specifically increases respiration and substrate oxidation that is uncoupled from the F1F0-ATPase. Mitochondrial uncoupling proteins (UCP1-3) are the principal mediators of proton leak in diverse tissues.6 UCP1 is expressed mainly in brown adipose tissue; however, UCP3 has a relatively broader distribution7 and appears to be present in human basal epidermis and primary keratinocytes.8
Here, we developed a novel mouse model with increased UCP3-dependent mitochondrial respiration in skin keratinocytes that exhibits extraordinary resistance to chemically mediated multistage skin carcinogenesis. Unexpectedly, the skin of these animals exhibited increased keratinocyte differentiation in vivo and in vitro, increased epidermal keratinocyte turnover and the loss of quiescent hair follicle bulge stem cells. Together, the results indicate that targeting mitochondrial respiratory uncoupling is a powerful novel strategy in cancer prevention and treatment, and warrants further investigation.
Results and Discussion
We generated hemizygous FVB/N mice expressing a respiration-inducing bovine keratin-5 promoter UCP3 (K5-UCP3) transgene in epidermal and follicular keratinocytes (Figure 1a, K5-UCP3). Isolated cutaneous mitochondria from K5-UCP3 mice exhibited increased UCP3 protein expression compared with wild type (FVB/N) skin and skeletal muscle (Figure 1b). Histochemical analyses indicated that UCP3 was properly localized in a punctate, perinuclear mitochondrial pattern in cultured K5-UCP3 primary keratinocytes (Figure 1c). Moreover, UCP3 expression in K5-UCP3 skin was found in the basal and suprabasal epidermal skin layers and the hair follicle (Figure 1d).
Figure 1.
UCP3 overexpression increases cutaneous uncoupling. (a) The construct used to generate K5-UCP3 mice. (b) Immunoblot for UCP3 and cytochrome C expression in isolated mitochondria from wild-type FVB/N (WT) whole skin & gastrocnemius muscle (skm), and K5-UCP3 skin. (c) UCP3 immunohistochemistry in primary keratinocytes. Scale bars = 10 μ. (d) Immunofluorescent staining for UCP3 expression in whole skin. Scale bars = 75 μ. (e) Oxygen consumption per gram of isolated epidermis under state 3 (no treatment) and state 4 (with addition of 1 μg/ml oligomycin) conditions. (f) Flow cytometric analysis of MitoTracker Green staining in wild type (WT) and K5-UCP3 primary keratinocytes (*P <0.001). (g) Percentage of uncoupled epidermal respiration out of total epidermal respiration, determined by oligomycin inhibited (state 4) respiration rate/untreated (state 3) respiration rate × 100% (*P = 0.019). Error bars represent means ± s.e.m.
Maximal epidermal oxygen consumption rates measured in the absence (phosphorylating state 3) or presence (non-phosphorylating state 4) of the F1F0 ATPase inhibitor oligomycin were used to test for UCP3 functionality ex vivo. Both state 3 and uncoupled state 4 epidermal respiration rates were increased in K5-UCP3 compared with wild-type mice by approximately 31% and 113%, respectively (Figure 1e). On the basis of MitoTracker Green (Grand Island, NY, USA) staining, we observed that mitochondrial mass was approximately 28% increased in K5-UCP3 keratinocytes compared with wild type (Figure 1f). To correct for differences in mitochondrial number, we compared the percentage of uncoupled per total epidermal respiration (state 4/state 3 × 100%) in epidermis ex vivo. K5-UCP3 epidermis exhibited an approximately two-fold increase in uncoupled per total respiration (Figure 1g).
During the initial characterizations of the transgenic mice, we observed that isolated K5-UCP3 keratinocytes were significantly larger than wild-type cells, suggesting that enforced UCP3 expression may induce cellular differentiation. Keratinocyte differentiation is Ca2+-dependent, marked by an increased cell size and fusion, and the appearance of cytoplasmic filamentous keratin strands.9 At low [Ca2+] (≤ 0.05 mm), cells remain small and proliferative; when cultured in 0.1–1.2 mm Ca2+, cells undergo terminal differentiation. Using light microscopy, K5-UCP3 cells grown in low (0.05 mm) Ca2+ exhibited clear differentiation phenotypes that were absent in wild-type keratinocytes (Figure 2a). Similarly, transmission electron microscopy analyses showed that the large filamentous keratin strands induced by high Ca2+ in wild-type cells were present in K5-UCP3 cells (but not wild-type cells) cultured in low Ca2+ conditions (Figure 2b). Gene expression profiling from wild-type and K5-UCP3 skin revealed a striking increase in the expression of a large cohort of differentiation genes in K5-UCP3 skin (Figure 2c). Among these, increased expression of keratin 10 protein in K5-UCP3 skin was confirmed by immunoblot (Figure 2d). Also increased in K5-UCP3 epidermis were immunoreactivities for the basal keratinocyte marker K5, along with the early and late keratinocyte differentiation markers K1, loricrin and involucrin (Figures 2d–e, high magnification), Supplementary Figure S1a (low magnification), Supplementary Figure S2 (developmental stages). Notably, the epidermis in K5-UCP3 is thicker than wild type and has an expanded layer of cells that stain positive for both K5 and K1 above the basal epidermal layer, but had decreased intensity of K5 staining. These changes appeared by 3 weeks of age (Supplementary Figure S2). Interestingly, UCP3 messenger RNA and protein levels were significantly increased in response to calcium-induced differentiation in cultured primary mouse wild type and secondary human (HaCaT) keratinocytes, respectively (Supplementary Figures S1b–c).
Figure 2.
UCP3 overexpression increases epidermal differentiation. (a) Wild type (WT) and K5-UCP3 primary keratinocyte morphology shown by light microscopy after 48 h in culture and (b) transmission electron microscopy in low (0.05 mm Ca2+) and high (1.2 mm Ca2+) calcium medium. Arrows indicate cytoplasmic keratinization (b). Scale bars = 20 μ and 2 μ (a and b) (c) Gene expression analysis of K5-UCP3 dorsal skin performed by Illumina microarray (Illumina, San Diego, CA, USA) and expressed as fold change compared with wild type. Genes shown are known stem cell or differentiation markers/regulators and significantly differ from wild type (P< 0.01). K#, keratin #; Lor, loricrin; Tgm1, transglutaminase 1; Ivl, involucrin; Sprr#, small proline rich protein #. Error bars are means ± s.e.m. (d) Immunoblot for keratin 10 and β-actin in whole tissue lysates from isolated epidermis. (e) Immunohistochemistry for basal keratinocyte marker keratin 5 (K5), and early and late keratinocyte differentiation markers keratin 1 (K1), loricrin and involucrin in adult (7-week-old) mouse epidermis. Scale bars = 20 μ.
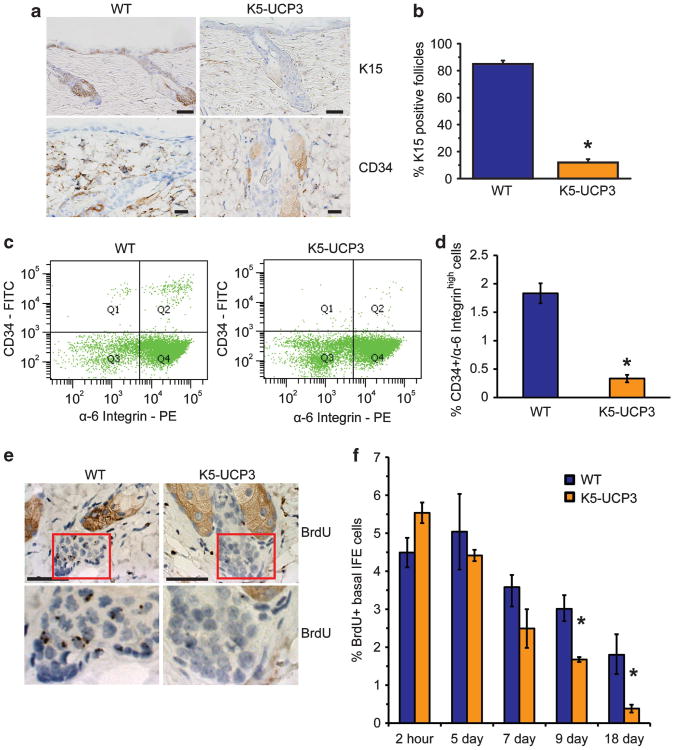
Although hotly debated, the slowly cycling (quiescent), long-lived, multipotent follicular bulge stem cells are candidate progenitors of squamous carcinomas.10,11 These cells are marked histologically by keratin 15, and the cell surface marker CD34, and by the capacity to retain labels (for example, bromodeoxyuridine (BrdU)).12 Interestingly, CD34 is both lost during differentiation and required for skin carcinogenesis.13 Remarkably, hair follicle bulge regions in adult K5-UCP3 mice exhibited dramatically decreased (∼85%) immunostaining for both K15 and CD34 (Figure 3a–b). Similarly, loss of these bulge stem cell markers was developmentally evident by 3 weeks of age (Supplementary Figures S2–3). To quantify these changes, bulge stem cell populations from primary keratinocyte isolations were labeled as the CD34+, α-6 integrinhigh population14 and analyzed by fluorescence-activated cell sorting (Figure 3c, Q2). In strong concordance with the histological data, K5-UCP3 mice exhibited an 84% decrease (0.3 vs 1.8% in wild type) in bulge stem cells ex vivo (Figure 3d). Additionally, almost no cells in the hair follicle bulge region of K5-UCP3 skin retained BrdU after an 8-week pulse-chase labeling regimen (Figure 3e), unlike wild-type bulge regions. Surprisingly, the dramatic and overlapping lack of three distinct bulge stem cell markers in the K5-UCP3 mice did not correspond to increased bulge region apoptosis compared with wild type (Supplementary Figure S4). Furthermore, no lack of functionality typically ascribed to bulge stem cells was observed in K5-UCP3 skin. Bulge stem cell depletion or ablation leads to hair follicle and sebaceous gland degeneration15 and decreased wound healing.16 K5-UCP3 mice not only had normal hair follicle number and overall morphology at 3 and 7 weeks of age (Supplementary Figures S1–2), they also exhibited enlarged sebaceous glands and healed wounds at comparable rates with wild type (Supplementary Figure S5).
Figure 3.
UCP3 overexpression decreases markers of quiescent stem cells. (a) Immunohistochemistry for keratin 15 (K15) and CD34 in follicular bulge regions of wild type (WT) and K5-UCP3 adult skin. Scale bars = 50 μ (K15) and 20 μ (CD34). (b) Quantification of K15-positive follicles (N > 250 per genotype). Error bars are means ± s.e.m; (*P<0.001). (c) Fluorescence-activated cell sorting analysis of WT and K5-UCP3 primary keratinocytes labeled with fluorescent-conjugated antibodies for CD34 and α-6 integrin. Q2 shows the CD34+, α-6 integrinhigh bulge stem cell population. (d) Quantification of fluorescence-activated cell sorting analysis shown as average percentage of cells in Q2 from three separate experiments. Error bars are means ±s.e.m; (*P = 0.0014). (e) BrdU label retention (8-week pulse-chase) in WT and K5-UCP3 skin. Bottom panels are magnified follicle bulge regions showing BrdU label-retaining cells. Scale bars = 50 μ. (f) Epidermal turnover in WT and K5-UCP3 skin, measured as retention of BrdU in basal interfollicular epidermal (IFE) cells at the indicated time points following i.p. BrdU injection. 500 basal IFE cells from each of three mice per genotype were counted at each time point. Error bars are means ± s.e.m. *indicates significantly different from WT at the same time point (P<0.05).
Taken together, the increase in expression of both basal and differentiated keratinocyte markers combined with the loss of bulge stem cell markers, but not bulge stem cell functionality, suggested the possibility that epidermal turnover was accelerated in K5-UCP3 mice. To examine this possibility, we pulse-labeled basal interfollicular epidermal keratinocytes with BrdU and quantified the labeled cells that remained in the basal layer of the epidermal cells 2 h, 5 days, 7 days, 9 days, and 18 days later. Although a slightly increased percentage of K5-UCP3 basal interfollicular epidermal cells stained positive for BrdU compared with wild type at the initial 2 h time point (P = 0.052), there was no difference between the genotypes 5 days after labeling (Figure 3f). At 9 days after BrdU treatment the trend had reversed, and at 18 days after BrdU labeling, wild-type basal epidermis retained approximately 1.8% labeled cells whereas almost no label remained in the K5-UCP3 basal interfollicular epidermal cells (∼0.4%, Figure 3f). This data indicates an increased rate of epidermal turnover and loss of labeled cells in K5-UCP3 epidermis.
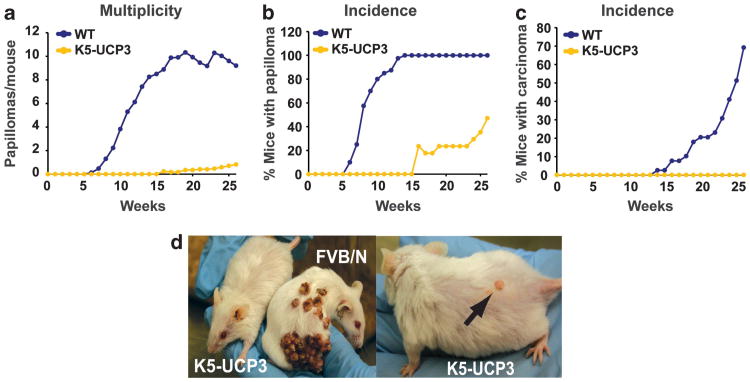
Given the significant effects of UCP3 expression on mitochondrial respiration, epidermal turnover, and the quiescent bulge stem cell niche, we reasoned that K5-UCP3 mice may be resistant to cancer development. Using a well-established two-stage chemically induced skin carcinogenesis model,17 adult wild-type and K5-UCP3 mice (N ≥ 17 per genotype) were topically administered a single dose of dimethylbenzanthracene (100 μg) followed 2 weeks later with biweekly applications of 12-O-tetradecanoyl-phorbol-13-acetate (2.5 μg) for 26 weeks. After 15 weeks of treatment, wild-type mice developed an average of nine tumors per mouse, with 100% of the animals bearing tumors (Figures 4a and b). In stark contrast, none of the K5-UCP3 mice developed any tumors by 15 weeks (Figure 4a). At 26 weeks, approximately half of the K5-UCP3 mice remained tumor-free, and those that developed tumors typically bore only one small papilloma (Figures 4b and d). Moreover, 70% of wild-type animals developed at least one carcinoma by 26 weeks, but remarkably none of the K5-UCP3 mice developed a malignancy (Figure 4c).
Figure 4.
Mitochondrial uncoupling strongly inhibits two-stage chemically induced skin carcinogenesis. (a) Tumor development in wild-type FVB/N (WT) and K5-UCP3 mice indicating total papillomas/mouse, (b) % mice bearing papillomas and (c) % mice bearing carcinomas. (d) Representative tumors in K5-UCP3 and WT mice. Arrow indicates a typical K5-UCP3 papilloma.
Several lines of evidence suggest that mitochondrial oxidative metabolism is tumor suppressive. Germline mutations in genes encoding mitochondrial fumarate hydratase18 and subunits B,19 C,20 and D21 of succinate dehydrogenase lead to mitochondrial dysfunction and increased susceptibility to hereditary renal cancer and paragangliomas, respectively. Similarly, the tumor suppressor p53 maintains murine mitochondrial respiratory functions,22 and the oncogenes hypoxia-inducible factor 1-α and myc each inhibit mitochondrial oxidative metabolism.23 Our observations strongly support a chemopreventive action of mitochondrial respiration using a novel mouse model that expresses a skin-targeted, mitochondrial respiration-inducing UCP3 transgene.
To date, only a handful of published studies in the literature deal with relationships between UCPs and cancer. Most of these are largely correlative and deal with the relatively broadly expressed close UCP3 homolog UCP2. UCP2 is associated with tumor grade in primary breast cancer and is reported to be induced in a variety of drug-resistant malignant cells and promote cell survival.24,25 On the other hand, in the only knockout mouse study we could find, UCP2-null mice show significantly increased incidence and severity of chemically induced colon carcinogenesis compared with wild type.26 We demonstrated that enforced UCP2 expression is selectively toxic to malignant compared with normal cells.27 Additionally, transgenic expression of the prototypical brown fat UCP1 in skeletal muscle decreases age-related diseases in mice, including certain types of naturally occurring hematological cancers.28 Furthermore, the work herein is the first that we know of demonstrating chemoprotective effects of UCP3 expression in a genetically modified mouse model of carcinogenesis.
The molecular mechanisms by which UCP3 expression affects cellular differentiation and prevents skin carcinogenesis in the K5-UCP3 model remain to be defined and are under intense investigation. UCP1-3 increase mitochondrial proton leak, which in turn increases electron flux across the respiratory chain and the futile oxidation of fuels (fatty acids, carboxylic acids, NADH, FADH2, etc.). Uncoupling lowers the driving force for mitochondrial reactive oxidant species (ROS) generation.29 With regard to cancer, overproduction of mitochondrial ROS from dysfunctional mitochondria is a common feature of malignancy that promotes growth and survival, and is implicated in the progression of all stages of cancer development.30 Thus, UCP3 may antagonize carcinogenesis in part by preventing procarcinogenic ROS signaling in developing tumor cells.
Fuel substrates have also emerged as novel players linking mitochondrial dysfunction to carcinogenesis. The cytoplasmic accumulation of the mitochondrial complex II substrate succinate contributes, along with ROS, to hypoxia-inducible factor 1-α stabilization even under normoxic conditions.31 Similarly, the suppression of mitochondrial substrate oxidation may lead to cataplerotic reactions that provide mitochondrial substrates necessary for the biosynthetic needs of rampant cell division to the cytoplasm. For example, the Kreb's cycle intermediate citrate is an essential signal for citrate-lyase-dependent cytoplasmic fatty acid biosynthesis, and is exported from mitochondria to the cytoplasm after sufficient accumulation (for example, from overfeeding or mitochondrial damage). Suppression of citrate lyase opposes cancer development,32 and citrate lyase activity is linked to procarcinogenic histone remodeling.33 Thus, UCP3-driven uncoupling could limit the flux of Kreb's cycle intermediates to the cytoplasm and thereby limit the biosynthetic supplies necessary for tumor development.
We also demonstrate for the first time the capacity for any uncoupling protein to promote cellular differentiation. Previous observations showing that UCP3 expression increases during skeletal myocyte differentiation34 are consistent with our results in differentiating mouse and human keratinocytes. Ca2+ is the major driver of keratinocyte differentiation in mammalian skin and keratinocyte cultures.35 Extracellular Ca2+ induces the release of endoplasmic reticulum Ca2+, and leads to the expression of a large number of differentiation regulatory genes (transglutaminases, involucrin, etc.). Surprisingly, we could find no literature dealing with relationships between mitochondrial bioenergetics and cellular Ca2+ levels in keratinocytes. However, in a variety of other contexts, including neuronal and immunological synapses36,37 and contracting muscle fibers,38 mitochondrial calcium sequestration is crucial for cell function. Additionally, the mitochondrial membrane potential drives mitochondrial Ca2+ sequestration, and mitochondrial uncoupling-induced membrane depolarization decreases sequestration.39 Thus, mitochondrial regulation of intracellular Ca2+ leading to terminal differentiation of keratinocytes may be another important bioenergetic mechanism underlying UCP3-induced cancer resistance. Consistent with this idea, though Ca2+ per se was not examined, recent preliminary reports have implicated decreased mitochondrial membrane potential and mitochondrial dysfunction as mediators of keratinocyte differentiation.40
The profound cancer resistance of K5-UCP3 mice corresponds with a sharp decrease in three biochemical markers of quiescent bulge stem cells, the potential progenitors of multistage squamous skin carcinomas.41 As mentioned, loss of bulge stem cells leads not only to skin cancer resistance but also to dramatically decreased folliculogenesis, sebaceous gland development and wound healing. However, the cancer resistance observed in K5-UCP3 mice did not correspond to any apparent defect in these bulge stem cell (bSC)-mediated functions. Thus, it is likely that the bSC of K5-UCP3 mice are present, but exist in a more differentiated, less quiescent state. This idea is supported by recent observations indicating that common adult stem cell niches, including the hair follicle, contain both quiescent and active cycling populations that function cooperatively.42 It is tempting to speculate that mitochondrial metabolism may functionally participate in the specification of stem cell fate.
Finally, we observed that UCP3 expression was lowest in undifferentiated compared with differentiated keratinocytes. Thus, expression of UCPs may be incompatible with carcinogenesis by driving the initiated stem cells to differentiate, thereby blocking promotion. Mechanistically, UCP3-induced uncoupling may lead to the accumulation of a bioenergetic messenger (for example, calcium) or metabolic process (fatty acid oxidation) that promotes differentiation while simultaneously decreasing levels of growth-stimulating ROS, citrate, etc. Future work is needed to understand the complex mechanistic relationships between mitochondrial function and carcinogenesis, especially in relation to the biology of cancer stem cells. Nonetheless, this work supports a growing body of literature, indicating that important functional links exist between mitochondrial bioenergetics and carcinogenesis, and that targeting mitochondrial function is a promising avenue for cancer prevention and treatment.
Supplementary Material
Supplemental Figure 1 UCP3 increases expression of differentiaion markers in vivo and is up-regulated in response to calcium induced differentiation in vitro. (a) Immunohistochemistry for basal keratinocyte marker keratin 5 (K5) and early and late keratinocyte differentiation markers keratin 1 (K1). loricrin, and involucrin in adult (7 week old) mouse epidermis. Scale bars = 50 microns. (b) RT-PCR for UCP3 and (32 microglobulin (β2M) mRNA expression in wild type FVB/N primary keratinocytes in response to calcium induced differentiation for 48 hours at 0.05 mM, 0.1 mM, and 1.2 mM calcium, (c) Immunoblot for hUCP3 and cytochrome C (Cyt. C) expression in isolated mitochondria from HaCat and HeLa cell lines grown in 1.2 mM calcium for 48 hours.
Supplemental Figure 2 UCP3 increases expression of keratinocyte bulge stem cell and differentiation markers across developmental stages. (a) Immunohistochemistry for bulge stem cell marker keratin 15 (K15), basal keratinocyte marker keratin 5 (K5) and early and late keratinocyte differentiation markers keratin 1 (K1), loricrin, and involucrin across developmental stages in newborn and 3 week old mouse epidermis. Scale bars = 50 microns.
Supplemental Figure 3 UCP3 increases expression of keratinocyte bulge stem cell marker CD34 across developmental stages, (a) Immunohistochemistry for stem cell marker CD34 in skin sections from newborn, 3-week, and 7-week aged mice. Arrows indicate positive immunoreactivity in the follicular bulge regions of wild type epidermis. Scale bars = 50 microns.
Supplemental Figure 4 K5-UCP3 epidermis shows no difference in bSC apoptosis following TPA treatment. TUNEL staining for apoptotic cells in skin sections from wild type FVB/N and K5-UCP3 epidermis 36 hours after application of TPA (2.5μg/200μl acetone - vehicle). Wild type FVB/N uterus was used as a positive assay control. Scale bars = 50 microns.
Supplemental Figure 5 K5-UCP3 epidermis shows no difference in bSC functionality. (a) H & E staining showing sebaceous gland morphology in wild type FVB/N (WT) and K5-UCP3 epidermis. Scale bars = 50 microns. (b) Rates of wound healing after epidermal abrasion (days). Error bars are means +/− SEM.
Acknowledgments
EMM was supported by the National Institutes of Health grant # DK089224. SMN was supported by the National Institutes of Health Toxicology Training grant T32ES07247.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 3.Dang CV, Lewis BC, Dolde C, Dang G, Shim H. Oncogenes in tumor metabolism, tumorigenesis, and apoptosis. J Bioenerg Biomembr. 1997;29:345–354. doi: 10.1023/a:1022446730452. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 5.Shakya A, Cooksey R, Cox JE, Wang V, McClain DA, Tantin D. Oct1 loss of function induces a coordinate metabolic shift that opposes tumorigenicity. Nat Cell Biol. 2009;11:320–327. doi: 10.1038/ncb1840. [DOI] [PubMed] [Google Scholar]
- 6.Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- 7.Clapham JC, Arch JR, Chapman H, Haynes A, Lister C, Moore GB, et al. Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature. 2000;406:415–418. doi: 10.1038/35019082. [DOI] [PubMed] [Google Scholar]
- 8.Mori S, Yoshizuka N, Takizawa M, Takema Y, Murase T, Tokimitsu I, et al. Expression of uncoupling proteins in human skin and skin-derived cells. J Invest Dermatol. 2008;128:1894–1900. doi: 10.1038/jid.2008.20. [DOI] [PubMed] [Google Scholar]
- 9.Watt FM, Mattey DL, Garrod DR. Calcium-induced reorganization of desmosomal components in cultured human keratinocytes. J Cell Biol. 1984;99:2211–2215. doi: 10.1083/jcb.99.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faurschou A, Haedersdal M, Poulsen T, Wulf HC. Squamous cell carcinoma induced by ultraviolet radiation originates from cells of the hair follicle in mice. Exp Dermatol. 2007;16:485–489. doi: 10.1111/j.1600-0625.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 11.Morris RJ, Tryson KA, Wu KQ. Evidence that the epidermal targets of carcinogen action are found in the interfollicular epidermis of infundibulum as well as in the hair follicles. Cancer Res. 2000;60:226–229. [PubMed] [Google Scholar]
- 12.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 13.Trempus CS, Morris RJ, Ehinger M, Elmore A, Bortner CD, Ito M, et al. Cd34 expression by hair follicle stem cells is required for skin tumor development in mice. Cancer Res. 2007;67:4173–4181. doi: 10.1158/0008-5472.CAN-06-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu WY, Morris RJ. Method for the harvest and assay of in vitro clonogenic keratinocytes stem cells from mice. Methods Mol Biol. 2005;289:79–86. doi: 10.1385/1-59259-830-7:079. [DOI] [PubMed] [Google Scholar]
- 15.Benitah SA, Frye M, Glogauer M, Watt FM. Stem cell depletion through epidermal deletion of rac1. Science. 2005;309:933–935. doi: 10.1126/science.1113579. [DOI] [PubMed] [Google Scholar]
- 16.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 17.Rundhaug JE, Gimenez-Conti I, Stern MC, Budunova IV, Kiguchi K, Bol DK, et al. Changes in protein expression during multistage mouse skin carcinogenesis. Mol Carcinog. 1997;20:125–136. [PubMed] [Google Scholar]
- 18.Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, et al. Hif overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of Hif stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Astuti D, Latif F, Dallol A, Dahia PL, Douglas F, George E, et al. Gene mutations in the succinate dehydrogenase subunit sdhb cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69:49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niemann S, Muller U. Mutations in sdhc cause autosomal dominant paraganglioma, type 3. Nat Genet. 2000;26:268–270. doi: 10.1038/81551. [DOI] [PubMed] [Google Scholar]
- 21.Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, et al. Mutations in sdhd, a mitochondrial complex ii gene, in hereditary paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 22.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, et al. P53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in vhl-deficient renal cell carcinoma by repression of c-myc activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Sayeed A, Meng Z, Luciani G, Chen LC, Bennington JL, Dairkee SH. Negative regulation of ucp2 by TGF-beta signaling characterizes low and intermediate-grade primary breast cancer. Cell Death Dis. 2010;1:e53. doi: 10.1038/cddis.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derdak Z, Mark NM, Beldi G, Robson SC, Wands JR, Baffy G. The mitochondrial uncoupling protein-2 promotes chemoresistance in cancer cells. Cancer Res. 2008;68:2813–2819. doi: 10.1158/0008-5472.CAN-08-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derdak Z, Fulop P, Sabo E, Tavares R, Berthiaume EP, Resnick MB, et al. Enhanced colon tumor induction in uncoupling protein-2 deficient mice is associated with nf-kappab activation and oxidative stress. Carcinogenesis. 2006;27:956–961. doi: 10.1093/carcin/bgi335. [DOI] [PubMed] [Google Scholar]
- 27.Mills EM, Xu D, Fergusson MM, Combs CA, Xu Y, Finkel T. Regulation of cellular oncosis by uncoupling protein 2. J Biol Chem. 2002;277:27385–27392. doi: 10.1074/jbc.M111860200. [DOI] [PubMed] [Google Scholar]
- 28.Gates AC, Bernal-Mizrachi C, Chinault SL, Feng C, Schneider JG, Coleman T, et al. Respiratory uncoupling in skeletal muscle delays death and diminishes age-related disease. Cell Metab. 2007;6:497–505. doi: 10.1016/j.cmet.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Korshunov SS, Korkina OV, Ruuge EK, Skulachev VP, Starkov AA. Fatty acids as natural uncouplers preventing generation of O2.- and H2O2 by mitochondria in the resting state. FEBS Lett. 1998;435:215–218. doi: 10.1016/s0014-5793(98)01073-4. [DOI] [PubMed] [Google Scholar]
- 30.Wallace DC. Mitochondria and cancer: Warburg addressed. Cold Spring Harb Symp Quant Biol. 2005;70:363–374. doi: 10.1101/sqb.2005.70.035. [DOI] [PubMed] [Google Scholar]
- 31.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting Hif-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 32.Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guigal N, Rodriguez M, Cooper RN, Dromaint S, Di Santo JP, Mouly V, et al. Uncoupling protein-3 (ucp3) mrna expression in reconstituted human muscle after myoblast transplantation in rag2−/−/gamma c/c5(−) immunodeficient mice. J Biol Chem. 2002;277:47407–47411. doi: 10.1074/jbc.M208048200. [DOI] [PubMed] [Google Scholar]
- 35.Rice RH, Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979;18:681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- 36.Oakes SA. Mitochondria control calcium entry at the immunological synapse. Proc Natl Acad Sci USA. 2007;104:15171–15172. doi: 10.1073/pnas.0707798104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacAskill AF, Atkin TA, Kittler JT. Mitochondrial trafficking and the provision of energy and calcium buffering at excitatory synapses. Eur J Neurosci. 2010;32:231–240. doi: 10.1111/j.1460-9568.2010.07345.x. [DOI] [PubMed] [Google Scholar]
- 38.Biswas G, Adebanjo OA, Freedman BD, Anandatheerthavarada HK, Vijayasarathy C, Zaidi M, et al. Retrograde ca2+ signaling in c2c12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: A novel mode of inter-organelle crosstalk. Embo J. 1999;18:522–533. doi: 10.1093/emboj/18.3.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholls DG, Snelling R, Rial E. Proton and calcium circuits across the mitochondrial inner membrane. Biochem Soc Trans. 1984;12:388–390. doi: 10.1042/bst0120388. [DOI] [PubMed] [Google Scholar]
- 40.Tamiji S, Beauvillain JC, Mortier L, Jouy N, Tual M, Delaporte E, et al. Induction of apoptosis-like mitochondrial impairment triggers antioxidant and bcl-2-dependent keratinocyte differentiation. J Invest Dermatol. 2005;125:647–658. doi: 10.1111/j.0022-202X.2005.23885.x. [DOI] [PubMed] [Google Scholar]
- 41.Lapouge G, Youssef KK, Vokaer B, Achouri Y, Michaux C, Sotiropoulou PA, et al. Identifying the cellular origin of squamous skin tumors. Proc Natl Acad Sci USA. 2011;108:7431–7436. doi: 10.1073/pnas.1012720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 UCP3 increases expression of differentiaion markers in vivo and is up-regulated in response to calcium induced differentiation in vitro. (a) Immunohistochemistry for basal keratinocyte marker keratin 5 (K5) and early and late keratinocyte differentiation markers keratin 1 (K1). loricrin, and involucrin in adult (7 week old) mouse epidermis. Scale bars = 50 microns. (b) RT-PCR for UCP3 and (32 microglobulin (β2M) mRNA expression in wild type FVB/N primary keratinocytes in response to calcium induced differentiation for 48 hours at 0.05 mM, 0.1 mM, and 1.2 mM calcium, (c) Immunoblot for hUCP3 and cytochrome C (Cyt. C) expression in isolated mitochondria from HaCat and HeLa cell lines grown in 1.2 mM calcium for 48 hours.
Supplemental Figure 2 UCP3 increases expression of keratinocyte bulge stem cell and differentiation markers across developmental stages. (a) Immunohistochemistry for bulge stem cell marker keratin 15 (K15), basal keratinocyte marker keratin 5 (K5) and early and late keratinocyte differentiation markers keratin 1 (K1), loricrin, and involucrin across developmental stages in newborn and 3 week old mouse epidermis. Scale bars = 50 microns.
Supplemental Figure 3 UCP3 increases expression of keratinocyte bulge stem cell marker CD34 across developmental stages, (a) Immunohistochemistry for stem cell marker CD34 in skin sections from newborn, 3-week, and 7-week aged mice. Arrows indicate positive immunoreactivity in the follicular bulge regions of wild type epidermis. Scale bars = 50 microns.
Supplemental Figure 4 K5-UCP3 epidermis shows no difference in bSC apoptosis following TPA treatment. TUNEL staining for apoptotic cells in skin sections from wild type FVB/N and K5-UCP3 epidermis 36 hours after application of TPA (2.5μg/200μl acetone - vehicle). Wild type FVB/N uterus was used as a positive assay control. Scale bars = 50 microns.
Supplemental Figure 5 K5-UCP3 epidermis shows no difference in bSC functionality. (a) H & E staining showing sebaceous gland morphology in wild type FVB/N (WT) and K5-UCP3 epidermis. Scale bars = 50 microns. (b) Rates of wound healing after epidermal abrasion (days). Error bars are means +/− SEM.