Abstract
Samples from a pristine raised peat bog runoff in Austria, the Tannermoor creek, were analysed for their iron linked to natural organic matter (NOM) content. Dissolved organic carbon < 0.45 μm (DOC) was 41–64 mg L−1, iron 4.4–5.5 mg L−1. Samples were analysed applying asymmetric field flow fractionation (AsFlFFF) coupled to UV–vis absorption, fluorescence and inductively coupled plasma mass spectrometry (ICP-MS). The samples showed an iron peak associated with the NOM peak, one sample exhibiting a second peak of iron independent from the NOM peak. As highland peat bogs with similar climatic conditions and vegetation to the Tanner Moor are found throughout the world, including areas adjacent to the sea, we examined the behaviour of NOM and iron in samples brought to euhaline (35‰) conditions with artificial sea salt. The enhanced ionic strength reduced NOM by 53% and iron by 82%. Size exclusion chromatography (SEC) of the samples at sea-like salinity revealed two major fractions of NOM associated with different iron concentrations. The larger one, eluting sharply after the upper exclusion limits of 4000–5000 g mol−1, seems to be most important for iron chelating. The results outline the global importance of sub-mountainous and mountainous raised peat bogs as a source of iron chelators to the marine environment at sites where such peat bogs release their run-offs into the sea.
Keywords: Peat bog, Complexed iron, NOM, DOM, DOC
Introduction
Natural organic matter (NOM) has many important functions in surface-water systems (Battin et al. 2009) and is of mixed origin (Micic et al. 2011). These functions include, among others, the co-transport of metals and the stabilization or destabilization of natural colloids, depending on its concentration (Laglera and van den Berg 2009; Kumpulainen et al. 2008; Yang et al. 2010, 2011). The NOM content of surface waters depends on many different factors. Dissolved organic matter (DOM) is technically defined as the fraction of NOM passing a <0.45 μm filter. Typical DOM concentrations vary between 1 and 50 mg L−1. DOM is part of the carbon cycle in aquatic systems and is connected via various physico-chemical reactions with other carbon compartments, such as organic carbon in biota, inorganic carbon and the particulate organic carbon fraction (Sigg and Stumm 1994). A high percentage of DOM (50–90%) consists of humic substances (HS) (Farjalla et al. 2009; Thurman 1985), a class of compounds which has been of interest to generations of scientists due to its manifold properties. In 1936, Waksman and Hutchings (1936) already described one possible natural synthesis of humic substances from lignin by microorganisms. Since then other kinds of natural HS formation have been described. Stevenson (1994) outlined three major possibilities for natural synthesis. All have one thing in common: the oxidative decomposition of organic material leading to reactive intermediate products, which in turn react with other intermediate products (MacCarthy 2001). Following this theory Ziechmann (1996) described humic substances as being the result of a more or less accidental chain of reactions. This makes it very difficult to present a general formula to describe their chemical structure. Attempts to identify individual formulas of constituents have been made, amongst others, by Stenson et al. (2003). That attempt revealed 5000 different formulas from one river in the USA and demonstrating the immense diversity of chemical structures within this group of compounds.
Moreover, humic particles are assumed to have an individual structure within the so-called humic system, which consists of humics and integrated non-humics interacting via weak chemical bounds. Planar marginal groups with chinoid or phenolic character are thought to stabilize the humic system through weak chemical interactions in between humic particles Ziechmann (1972, 1996). The character of binding sites for non-humics, especially for metal ions, is not fully understood, but there is strong evidence that electrostatic attraction of humic matter as well as abundant sites formed by oxygen-containing ligands, such as carboxylic, phenolic or other weak acid groups, are major factors (Tipping 2002). This ability of humic substances to act as chelators for metals is one of their outstanding properties. Apart from the previously mentioned effects on electrostatic and non-electrostatic stabilization of colloids, this property enhances the metal-carrying capacity of fluvial systems (Stolpe and Hassellov 2007).
During estuarine mixing the particulate and dissolved inorganic and organic matter >10 kD flocculate due to the major change in pH and, most importantly, to the ionic strength of the water (Dai and Martin 1995). Therefore, the global river input of bio-available iron to the ocean has been estimated to be marginal (De Baar and De Jong 2001). Recent evidence from the Mississippi River plume, however, shows that certain organic iron complexes escape the estuarine mixing zone and contribute essentially to the fertility of coastal waters (Rose and Waite 2003; Powell and Wilson-Finelli 2003). To date the form in which colloidal iron is bioavailable remains unclear, but it is apparently accessible to microorganisms relying on siderophore-based transport systems (Batchelli et al. 2010). The net bioavailability and reactivity of iron can also be increased by photo chemical reactions of iron–organic–ligand complexes, such as direct photolysis via light absorption and ligand-to-metal charge transfer, or secondary reactions with photochemically produced radicals (Barbeau 2006).
It has been shown that approximately 22% of the original iron load of humic rich peat bog water remains soluble at sea-like salinities in water from peat bogs in Austria and Scotland (Krachler et al. 2005, 2010) after being mixed with artificial and natural sea water. Therefore, iron fertilization by humic-rich rivers of coastal regions might play a yet underestimated role.
In addition, terrestrially derived humic substances apparently play a major role as chelators for iron and other trace metals (Laglera and van den Berg 2009) in the marine environment. This holds true especially for high nutrient low chlorophyll (HNLC) areas where iron limits primary production (Breitbarth et al. 2010).
The Tanner Moor, a pristine submountainous raised peat bog in Lower Austria, releases water with elevated NOM and iron content (Krachler et al. 2005). Highland peat bogs subject to similar climatic conditions and vegetation are found throughout the world, including regions adjoining the marine environment. This calls for more closely examining the connections between particle size and metal-binding capability of NOM < 0.45 μm in original samples from the Tanner Moor as well as in water samples brought to euhaline conditions. This approach could help to understand the influence of terrestrially derived humic substances for the marine environment everywhere, where such peat bogs release their runoff into coastal waters.
Materials and methods
Sampling site
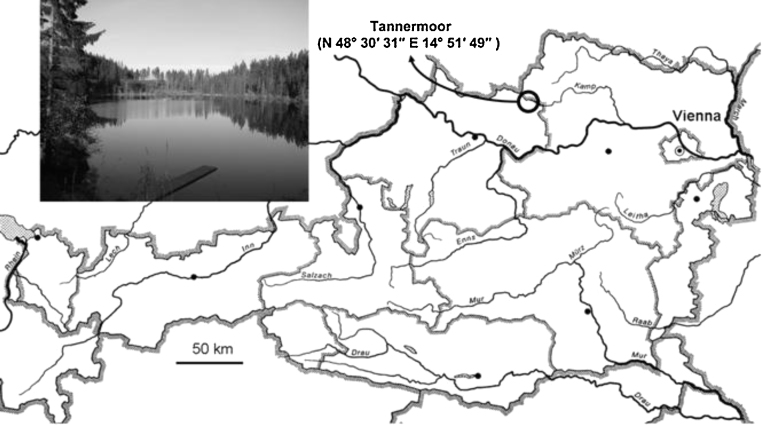
The Tanner Moor (Fig. 1, 48°30′31″N, and 14°51′49″E) is one of the largest raised peat bogs in Austria covering a total area of 1.2 km2. It was formed at the end of the last glacial period, app. 12,000 years ago, at an altitude of 930 m above sea level. This pristine area is now a protected conservation area within the NATURA2000 convention of the European Union. Its higher vegetation is dominated by the mountain pine Pinus mugo, which covers about 1.0 km2. Common spruce Picea abies also surrounds the area. Deciduous trees are represented by the silver birch Betula pendula and the downy birch Betula pubescens (Liebenau 2010). The site can be classified as a typical krummholz-raised-peat-bog in submountainous and mountainous locations, comparable to other peat bogs of Central- and Eastern Europe, as described by Neuhäusl (1972).
Fig. 1.
Geographical situation of the Tannermoor in Austria: photo of the lake “Rubener Teich” fed by the Tannermoor creek.
Sampling and analysis
Water samples were taken from the main brook draining the bog into a pond in March 2009 and 2010. The runoff conditions on both dates were similar, with remnant snow on the ground, but ice-free surface waters. The pH, specific conductance and dissolved oxygen saturation were determined in situ using portable instruments (WTW). Samples for further analysis were taken with a polyethylene (PE) dipper into acid prewashed PE bottles that were rinsed with bog water three times in situ, before being filled, and transported to the laboratory cooled, where they were immediately filtered through 0.45 μm cellulose–acetate filters (Sartorius).
DOC and element analyses
Dissolved organic carbon (DOC) was measured from the filtered samples as NPOC – non purgeable organic carbon – with a TOC-VCPH total organic carbon analyser (Shimadzu), bringing the samples to pH ≈ 2 with HCl (p.a. by Fluka) and sparging them with carrier gas for 5 min prior to combustion. A potassium hydrogen phthalate solution was used for calibration; the accuracy was tested to be ±5%. Concentrations of the 28 most probably occurring elements (metals, silicium and phosphor) were determined using inductively coupled plasma–optical emission spectrometry (ICP-OES) (Optima 5300 DL, Perkin-Elmer) using Rh as internal reference standard, showing a recovery range between 95 and 105%.
AsFlFFF – UV–vis absorption – fluorescence – ICP-MS
Flow field flow fractionation has been widely used to characterize of aquatic colloids and macromolecules (Plathe et al. 2010; von der Kammer et al. 2011, and references therein). The fractionator used was a model Eclipse 3+ Asymmetric Flow Field Flow Fractionation (AsFlFFF) from Wyatt Technology (Dernbach, Germany). A polytetrafluoroethylene spacer of 0.75 mm height determined area, shape and height of the channel in which the fractionation took place. The accumulation wall consisted of a 300 g mol−1 nominal cut-off polyether sulfone membrane (Postnova Analytics, Landsberg, Germany). Flows were controlled with an Agilent Technologies 1200 series isocratic pump equipped with a micro-vacuum degasser. In order to maximize sample recovery a 0.6 mM ammonium carbonate solution was used as carrier liquid. The AsFlFFF was coupled to a multiwavelength ultraviolet/visible (UV–vis) diode array detector (DAD, Agilent Technologies 1200 series, absorption wavelength 250 nm), and a fluorescence detector (Agilent Technologies 1200 series, excitation 250 nm, emission 410 nm). AsFlFFF parameters and run specifications are given in detail in Table 2. A total of 100 μL sample solution was injected for each analysis. To analyse iron in the water samples, the AsFlFFF system was coupled to an ICP-MS (Agilent Technologies 7700×). Prior to sample analysis at the ICP-MS a multi-point calibration was performed. Iron was calibrated with the coupled AsFlFFF. The standards were injected via a switch valve that was installed between the diode array detector and the ICP-MS. As indicated in Table 2 (AsFIFFF and ICP-MS operational parameters) a collision cell with He as cell gas was used. Recovery runs were performed by injecting samples without the application of a cross flow. The plots of signal versus elution volume were integrated using the OriginPro 7.5 software (OriginLab Corporation, Northampton, USA). This yielded the peak area, as described in Neubauer et al. (2011). The recovery percentage (R%) for all measured signals was calculated using
Table 2.
AsFIFFF and ICP-MS operational parameters.
| RF power (W) 1550 tip to tip channel length (cm) 19.5 |
| Focus flow rate (mL min−1) 1.5 |
| Focus time (min) 2 |
| Channel flow rate (mL min−1) 1 |
| Cross flow rate (mL min−1) 1.5 |
| Carrier gas (L min−1) 1.04 (Ar) |
| Collision cell gas (mL min−1) 4 (He) |
| Dwell time (s) 0.1 |
| Isotopes 56Fe, 57Fe, 103Rh |
where A is the peak area of the sample's fractogram, A0 is the corresponding area for the recovery run with no cross flow, and x is the correction factor for the recovery samples which were injected with typically one twelfth of the sample load. In order to test the reproducibility of the analysis, sample Tan 2009 was injected twice, and recovery runs were performed in triplicate. Standard deviation for the measured iron in the replicates was <5% and <3% for the UV–vis and the fluorescence signal, respectively. The concentrations of the Fe standard solutions ranged from 2.5 to 150 μg L−1. Before injection to ICP-MS, the effluent passed through an interface where Rhodium (10 μg L−1) was mixed into the sample stream as internal standard. Table 2 also summarizes the ICP-MS instrument settings and operating parameters. A series of polystyrene sulfonate (PSS Polymer Standards Service GmbH, Mainz, Germany) was used for molecular weight calibration (1100, 3610, 10,600, 63,900, 145,000 g mol−1). The molecular weight of the colloids was calculated after several runs of the samples and the PSS at different ionic strengths of the mobile phase in order to account for different particle–membrane interactions (Neubauer et al. 2011).
Salting-out experiments
Filtered water samples were brought to marine-like saline conditions (35‰) using a mixture of ten different salts, containing the major components of sea salt, as proposed by Kester et al. (1967) (Table 1). Eight litres of sample were put on a shaker in a 10 L PE bottle for 10 days, aerating the content once a day to guarantee oxygen saturation over the whole period of time, in order to simulate conditions in the coastal mixing zone. To determine the minimum reaction time to reach equilibrium of precipitated and soluble components for this mixture, 30 mL of the mixture were sampled, filtered and analysed daily for their iron and DOC content. As reference eight litres of filtered unsalted sample were put on the shaker simultaneously to the salted sample and DOC and iron was measured daily in there as well. The whole experiment was conducted in the laboratory, no sunlight allowed, to avoid photochemical reactions.
Table 1.
Salts used for fall out experiments, composition as proposed by Kester et al. (1967) for natural sea water.
| Salt | Mass (g L−1) |
|---|---|
| NaCl | 23.926 |
| MgCl2·6H2O | 10.831 |
| CaCl2·2H2O | 1.518 |
| Na2SO4 | 4.008 |
| KCl | 0.667 |
| NaHCO3 | 0.196 |
| KBr | 0.098 |
| H3BO3 | 0.026 |
| SrCl2·6H2O | 0.024 |
| NaF | 0.003 |
Solid phase extraction (SPE)
Experiments using AsFIFFF showed only one NOM peak which was not further resolvable. We therefore decided to use size exclusion chromatography (SEC), to determine whether different size fractions of NOM in connection with different iron content could be identified in euhaline samples. To gain sufficient quantities of samples for iron measurement with ICP-OES and to desalt them, samples were concentrated with solid phase extraction (SPE) before further analyses following Louchouarn et al. (2000). After the determined optimal reaction time of 3 days with the sea salt, the still soluble organic matter (DOM) was extracted from the samples after filtering (0.45 μm) and adjusting the pH ≈ 2.0 with HCL (Fluka). To avoid the dissociation of the DOM–iron complexes due to the lower pH, which has been described by van Schaik et al. (2008) as very slow, the sample was pumped through a C18-SPE-cartridge (C-18 Mega Bond Elute/40 g sorbent by Varian) with a velocity of 50 mL min−1 immediately after acidification. After rinsing the cartridge with diluted HCl (pH ≈ 2.0) the cartridge was stored at 4 °C until further processing.
For size exclusion chromatography the DOM was eluted from the column with 100 mL methanol. The thus obtained sample was concentrated to 5 mL on a rotavapor and then diluted with H2O (Millipore) to 100 mL. Recovery rates for DOC using SPE were assumed to app. 40% as shown by Dittmar et al. (2008) for extractions of DOC from natural seawater.
Size exclusion chromatography (SEC)
After preoperational experiments to optimize separation, the following parameters were used: A 50 cm long column by GE Healthcare 1 cm in diameter, loaded with Sephadex-LH 20 by GE Healthcare (exclusion limit 4000–5000 g mol−1) as separation medium, a Pharmacia-6000 pistonpump providing a 0.5 mL m−1 flow rate and 5%Vol methanol in water as eluent. A UV–vis spectrometer (Lambda 35 by Perkin Elmer) with a 0.2 mm flow-through cuvette was used as detector, extinction was measured at a wavelength of 242 nm. Acetone (58.08 g mol−1) and Blue Dextran (2000,000 g mol−1) both from Sigma Aldrich, were used to determine the retention times for the limits of size exclusion. Due to the difficulties in precisely determining the molecular weight of humic substances (Cabaniss et al. 2000) we made no attempt to calibrate the SEC. Iron in the eluted fractions was determined using ICP OES.
Results and discussion
Physico chemical properties and ASIFF characteristics of the filtered samples
Physico-chemical data of two samples from the Tanner Moor are given in Table 3, showing the most abundant of the analysed elements. The relatively low content of dissolved ions and low pH are characteristic for the poorly soluble granite and gneiss geological setting of the Bohemian Massif (Walter and Dorn 1995). This also reflects the activity of peat mosses, which use ion exchange to recover nutrients in exchange for protons from the waterphase of the moor and thus enhance its acidity (Gerken 1983). Due to the location in a peat bog the DOC is relatively high (approximately 40 mg L−1), compared to large world rivers (Meybeck 1982), but is in the range of other peat-draining surface waters (Baum et al. 2007; Gibson et al. 2009).
Table 3.
Physico-chemical parameters of the two samples from the Tannermoor creek.
| Parameter | TAN 2009 | TAN 2010 |
|---|---|---|
| pH | 6.4 | 5.2 |
| Specific conductance (μS cm−1) | 42 | 38 |
| Oxygen saturation (%) | 98 | 95 |
| DOC (mg L−1) | 41.0 | 64.3 |
| Na (mg L−1) | 1.2 | 0.9 |
| K (mg L−1) | 0.5 | 0.5 |
| Mg (mg L−1) | 4.9 | 5.2 |
| Ca (mg L−1) | 6.3 | 6.6 |
| Si (mg L−1) | 7.3 | 5.6 |
| Fe (mg L−1) | 5.5 | 4.4 |
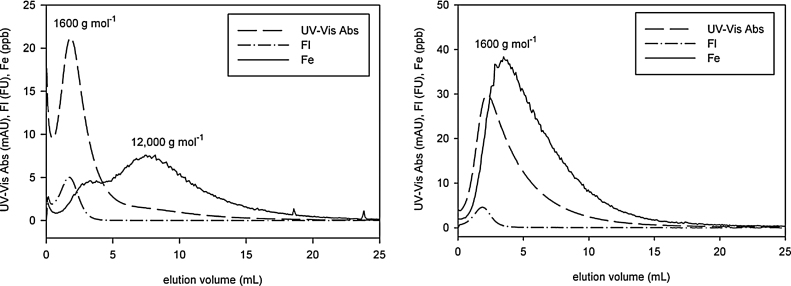
Recoveries for iron, UV–vis and fluorescence in Tan 2009 were 96%, 28% and 33% respectively. The corresponding values in Tan 2010 were 77%, 96% and 82%. Note that neither chromophores nor fluorophores are evenly distributed over the molecular weight range covered in AsFlFFF analysis. Many factors affect the loss of natural organic matter in FlowFFF analysis most importantly membrane adsorption and loss through the membrane (compare Neubauer et al. 2011). Molecules smaller than the cut-off for the membrane (300 g mol−1 in our analysis) may leave the channel through the membrane. Ultrafiltration analysis for sample Tan 2009 showed that 46% of the organic carbon passed through 1000 g mol−1 ultrafiltration membranes, but almost none of the iron (<1%). This result suggests that sample Tan 2009 contains a high fraction of very small-sized organic molecules that do not bind iron. This might explain the formation of the iron-dominated colloids.
We characterized the size distribution of Tan 2009 and Tan 2010 with AsFlFFF-ICM-MS. Fig. 2 shows the elution pattern of the UV–vis absorption, fluorescence and the iron signal. In the observed size region (approximately one nanometer to some 10 nanometers) the true light absorption by NOM and the weak light scattering expected from like-sized particles at the chosen wavelength makes the UV–vis detection nearly selective for NOM (von der Kammer et al. 2005). Tan 2010 shows a single peak. The fluorescence signal depicts only the smaller-sized organic molecules, whereas UV–vis absorption portrays a wider range of molecular sizes in the sample. Iron coincides with the NOM, indicating iron complexed by the organic macromolecules. In sample Tan 2009 iron has two clearly separated peaks. The first, smaller peak at app. 1600 g mol−1 elutes together with the NOM peak, indicating iron complexation by NOM. The second iron peak at a higher molecular weight (approximately 12,000 g mol−1) represents another source of iron in this sample and potentially represents small-sized iron minerals. The occurrence or absence of such particles was also observed by Batchelli et al. (2010) in different samples from a peat draining river in Scotland. The mechanism behind the formation of NOM or a NOM-FeOx system in the Tannermoor remains unknown, but ongoing investigation in the lab should shed light on this issue in the near future. Nonetheless, the coexistence of iron and organic matter influences both metal speciation (Hassellöv and von der Kammer 2008; Lyvén et al. 2003) as well as the fraction of iron that can escape the estuarine mixing zone.
Fig. 2.
Chromatograms of the ASFFF of Tan 2009 (left) + 2010 (right) samples.
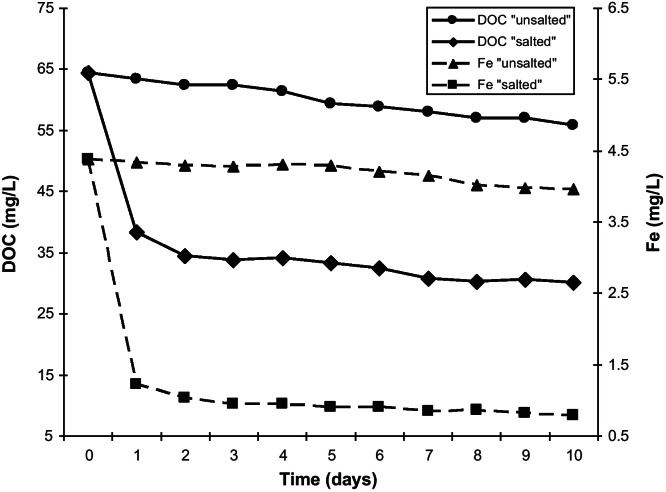
Salting-out experiments
The results of the salting-out experiments (Fig. 3) show the rapid flocculation and precipitation of most part of the DOM measured as DOC (53.3%) and iron (82.1%) in the euhaline samples due to the change in ionic strength. These findings support the results of earlier investigations by, amongst others, Eckert and Sholkovitz (1976), Sholkovitz (1976) and Boyle et al. (1977). The loss in DOC and dissolved iron is significantly lower in the reference versus the salted sample (13.2% and 9.5% respectively). It can be explained by the oxic degradation of humic particles and the oxidation of released Fe(II) and subsequent co-precipitation with DOC. The percentage of soluble iron we found under euhaline conditions here is also comparable to the results of Krachler et al. (2005) and Krachler et al. (2010). Those authors measured iron characteristics with an Fe59 tracer method in river water from the Tanner Moor and in water samples from Scotland during mixing experiments with artificial and natural sea water, in which about 22% of the dissolved iron remained soluble at euhaline conditions.
Fig. 3.
DOC and Fe concentrations during the salting-out experiments with Tannermoor samples and artificial sea salt prepared after Kester et al. (1967).
SEC and iron analyses of euhaline samples
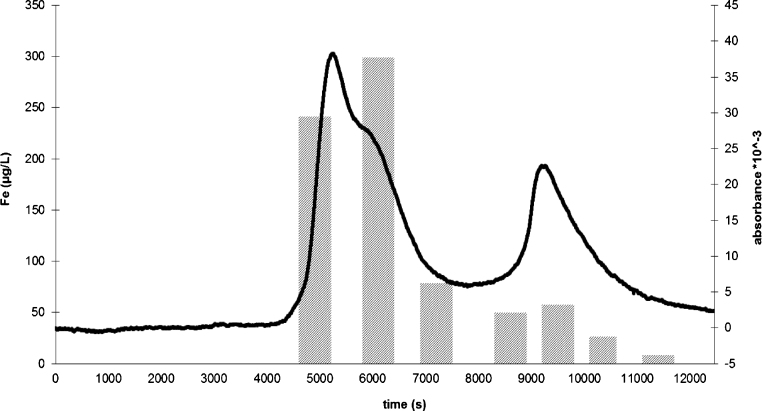
The best achieved size exclusion chromatogram of the “salted out” samples of TAN 2010 overlaid with the iron content of the collected fractions is shown in Fig. 4. TAN 2009 showed an almost identical pattern, the graph is shown as supplementary material at www.pangaea.de. The peak time of the band for Blue Dextran was 4100 s, for acetone 13,530 s. This shows that all the DOM fractions eluted within the exclusion limits of Sephadex LH-20 and had a molecular weight <4000–5000 g mol−1. It also suggests no adsorption between stationary phase and solute. The two major DOM bands are clearly separated indicating the presence of two major size classes of humic particles: the larger one appears to be associated with the largest iron fraction in the sample, whereas the smaller colloids seem to be less important for iron bonding. The shoulder of the first band clearly suggests the presence of more than one size class of colloids, which were not separated with the analytical setup used. These results echo those of Huber et al. (2011), who investigated natural river water and described an identical shoulder at the humic substances peak. Those authors referred to this peak as “building blocks”, humic substance-like material of lower molecular weight, which cannot be removed by flocculation. Piccolo et al. (1992) described the relatively weak resolution of the column separation of humic substances derived from coal in the small size classes of ultrafiltrates (500–10,000 g mol−1) with UV detection, requiring to find another detection device for future investigations.
Fig. 4.
Size exclusion chromatogram of the Tannermoor sample 2010 after saltout experiments (line) combined with the iron content of the fractions (columns).
Although laboratory studies on iron colloid formation in the presence of NOM are available, e.g. Bauer and Blodau (2009), Pullin and Cabaniss (2003), there is a strong need to bridge the gap between lab materials and understanding highly complex natural systems. A large number of environmental parameters such as type of source soil, temperature, changing redox conditions due to drought and rewetting, as well as the dilution and concentration of colloids, etc. might influence colloid formation.
Our samples showed that the Tannermoor is a source of organic ligands for iron that remains stable under euhaline conditions. This points to a high relevance of similarly raised peat bogs as sources for iron–humic complexes, wherever such bogs release their runoff into marine coastal waters. Further investigation is needed to elucidate the chemical composition of these compounds to enhance our understanding of terrestrially derived DOM chelated iron in marine systems.
Acknowledgement
A major part of this work was funded by the Austrian Science Fund (FWF) under project no. P19629, which is gratefully acknowledged.
References
- Barbeau K. Photochemistry of organic iron(III) complexing ligands in oceanic systems. Photochem. Photobiol. 2006;82(6):1505–1516. doi: 10.1562/2006-06-16-IR-935. [DOI] [PubMed] [Google Scholar]
- Batchelli S., Muller F.L., Chang K.C., Lee C.L. Evidence for strong but dynamic iron–humic colloidal associations in humic-rich coastal waters. Environ. Sci. Technol. 2010;44(22):8485–8490. doi: 10.1021/es101081c. [DOI] [PubMed] [Google Scholar]
- Battin T.J., Luyssaert S., Kaplan L.A., Aufdenkampe A.K., Richter A., Tranvik L.J. The boundless carbon cycle. Nat. Geosci. 2009;2(9):598–600. [Google Scholar]
- Bauer M., Blodau C. Arsenic distribution in the dissolved, colloidal and particulate size fraction of experimental solutions rich in dissolved organic matter and ferric iron. Geochim. Cosmochim. Acta. 2009;73(3):529–542. [Google Scholar]
- Baum A., Rixen T., Samiaji J. Relevance of peat draining rivers in central Sumatra for the riverine input of dissolved organic carbon into the ocean. Estuar. Coast. Shelf Sci. 2007;73(3–4):563–570. [Google Scholar]
- Boyle E.A., Edmond J.M., Sholkovitz E.R. Mechanism of iron removal in estuaries. Geochim. Cosmochim. Acta. 1977;41(9):1313–1324. [Google Scholar]
- Breitbarth E., Achterberg E.P., Ardelan M.V., Baker A.R., Bucciarelli E., Chever F., Croot P.L., Duggen S., Gledhill M., Hassellov M., Hassler C., Hoffmann L.J., Hunter K.A., Hutchins D.A., Ingri J., Jickells T., Lohan M.C., Nielsdottir M.C., Sarthou G., Schoemann V., Trapp J.M., Turner D.R., Ye Y. Iron biogeochemistry across marine systems – progress from the past decade. Biogeosciences. 2010;7(3):1075–1097. [Google Scholar]
- Cabaniss S.E., Zhou Q.H., Maurice P.A. Considerations in the use of high-pressure size exclusion chromatography (HPSEC) for determining molecular weights of aquatic humic substances. Water Res. 2000;34(14):3505–3514. [Google Scholar]
- Dai M., Martin J.M. First data on trace metal level and behaviour in two major Arctic river-estuarine systems (Ob and Yenisey) and in the adjacent Kara Sea. Earth Planet. Sci. Lett. 1995;131:127–141. [Google Scholar]
- De Baar H.J., De Jong . Distributions, sources and sinks of iron in seawater. In: Buffle J., Leeuwen H.P., editors. The Biogeochemistry of Iron in Seawater. vol. 7. 2001. (IUPAC Series on Analytical and Physical Chemistry of Environmental Systems). [Google Scholar]
- Dittmar T., Koch B., Hertkorn N., Kattner G. A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater. Limnol. Oceanogr.: Methods. 2008;6:230–235. [Google Scholar]
- Eckert J.M., Sholkovitz E.R. Flocculation of iron, aluminum and humates from river water by electrolytes. Geochim. Cosmochim. Acta. 1976;40(7):847–848. [Google Scholar]
- Farjalla V.F., Amado A.M., Suhett A.L., Meirelles-Pereira F. DOC removal paradigms in highly humic aquatic ecosystems. Environ. Sci. Pollut. Res. Int. 2009;16(5):531–538. doi: 10.1007/s11356-009-0165-x. [DOI] [PubMed] [Google Scholar]
- Gerken B. Verlag Rombach; Freiburg i. Br: 1983. Moore und Sümpfe. Bedrohte Reste der Urlandschaft. [Google Scholar]
- Gibson H.S., Worrall F., Burt T.P., Adamson J.K. DOC budgets of drained peat catchments: implications for DOC production in peat soils. Hydrol. Process. 2009;23(13):1901–1911. [Google Scholar]
- Hassellöv M., von der Kammer F. Iron oxides as geochemical nanovectors for metal transport in soil–river systems. Elements. 2008;4:401–406. [Google Scholar]
- Huber S.A., Balz A., Abert M., Pronk W. Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography – organic carbon detection – organic nitrogen detection (LC-OCD-OND) Water Res. 2011;45(2):879–885. doi: 10.1016/j.watres.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Kester D.R., Duedall I.W., Connors D.N., Pytkowic R.M. Preparation of artificial sea water. Limnol. Oceanogr. 1967;12:176–179. [Google Scholar]
- Krachler R., Jirsa F., Ayromlou S. Factors influencing the dissolved iron input by river water to the open ocean. Biogeosciences. 2005;2(4):311–315. [Google Scholar]
- Krachler R., Krachler R.F., von der Kammer F., Suphandag A., Jirsa F., Ayromlou S., Hofmann T., Keppler B.K. Relevance of peat-draining rivers for the riverine input of dissolved iron into the ocean. Sci. Total Environ. 2010;408(11):2402–2408. doi: 10.1016/j.scitotenv.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Kumpulainen S., von der Kammer F., Hofmann T. Humic acid adsorption and surface charge effects on schwertmannite and goethite in acid, sulphate waters. Water Res. 2008;42(8–9):2051–2060. doi: 10.1016/j.watres.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Laglera L.M., van den Berg C.M.G. Evidence for geochemical control of iron by humic substances in seawater. Limnol. Oceanogr. 2009;54(2):610–619. [Google Scholar]
- Liebenau T. Liebenau; Austria: 2010. Naturschutzgebiet Tannermoor. [Google Scholar]
- Louchouarn P., Opsahl S., Benner R. Isolation and quantification of dissolved lignin from natural waters using solid-phase extraction and GC/MS. Anal. Chem. 2000;72(13):2780–2787. doi: 10.1021/ac9912552. [DOI] [PubMed] [Google Scholar]
- Lyvén B., Hassellöv M., Turner D.R., Haraldsson C., Andersson K. Competition between iron- and carbon-based colloidal carriers for trace metals in a freshwater assessed using flow field-flow fractionation coupled to ICPMS. Geochim. Cosmochim. Acta. 2003;67(20):3791–3802. [Google Scholar]
- MacCarthy P. The principles of humic substances. Soil Sci. 2001;166(11):738–751. [Google Scholar]
- Meybeck M. Carbon, nitrogen, and phosphorus transport by world rivers. Am. J. Sci. 1982;282(4):401–450. [Google Scholar]
- Micic V., Kruge M.A., Koster J., Hofmann T. Natural, anthropogenic and fossil organic matter in river sediments and suspended particulate matter: a multi-molecular marker approach. Sci. Total Environ. 2011;409(5):905–919. doi: 10.1016/j.scitotenv.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Neubauer E.d.v., Kammer F., Hofmann T. Influence of carrier solution ionic strength and injected sample load on retention and recovery of natural nanoparticles using flow field-flow fractionation. J. Chromatogr. A. 2011;1218(38):6763–6773. doi: 10.1016/j.chroma.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Neuhäusl R. Academia; Praha: 1972. Subkontinentale Hochmoore und ihre Vegetation. p. 121. [Google Scholar]
- Piccolo A., Rausa R., Celano G. Characteristics of molecular-size fractions of humic substances derived from oxidized coal. Chemosphere. 1992;24(9):1381–1387. [Google Scholar]
- Plathe K.L., von der Kammer F., Hassellov M., Moore J., Murayama M., Hofmann T., Hochella M.F. Using FlFFF and aTEM to determine trace metal–nanoparticle associations in riverbed sediment. Environ. Chem. 2010;7(1):82–93. [Google Scholar]
- Powell R.T., Wilson-Finelli A. Importance of organic Fe complexing ligands in the Mississippi River plume. Estuar. Coast. Shelf Sci. 2003;58(4):757–763. [Google Scholar]
- Pullin M.J., Cabaniss S.E. The effects of pH, ionic strength, and iron-fulvic acid interactions on the kinetics of non-photochemical iron transformations, I. Iron(II) oxidation and iron(III) colloid formation. Geochim. Cosmochim. Acta. 2003;67(21):4067–4077. [Google Scholar]
- Rose A.L., Waite T.D. Kinetics of iron complexation by dissolved natural organic matter in coastal waters. Mar. Chem. 2003;84(1–2):85–103. [Google Scholar]
- Sholkovitz E.R. Flocculation of dissolved organic and inorganic matter during mixing of river water and seawater. Geochim. Cosmochim. Acta. 1976;40(7):831–845. [Google Scholar]
- Sigg L., Stumm W. Verlag der Fachvereine; Teubner, Zürich, Stuttgart: 1994. Aquatische Chemie: eine Einführung in die Chemie wässriger Lösungen und natürlicher Gewässer. xiii, p. 498. [Google Scholar]
- Stenson A.C., Marshall A.G., Cooper W.T. Exact masses and chemical formulas of individual Suwannee River fulvic acids from ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectra. Anal. Chem. 2003;75(6):1275–1284. doi: 10.1021/ac026106p. [DOI] [PubMed] [Google Scholar]
- Stevenson F.J. Wiley; New York: 1994. Humus Chemistry: Genesis, Composition, Reactions. xiii, p. 496. [Google Scholar]
- Stolpe B., Hassellov M. Changes in size distribution of fresh water nanoscale colloidal matter and associated elements on mixing with seawater. Geochim. Cosmochim. Acta. 2007;71:3292–3301. [Google Scholar]
- Thurman E.M. Organic geochemistry of natural waters. In: Nijhoff M., editor. vol. 2. Kluwer Academic; Dordrecht, Boston, Hingham, MA, USA: 1985. (Developments in Biogeochemistry). xii, p. 497. [Google Scholar]
- Tipping E. Cambridge University Press; Cambridge: 2002. Cation Binding by Humic Substances. [Google Scholar]
- van Schaik J.M., Persson I., Kleja D.B., Gustafsson J.P. EXAFS study on the reactions between iron and fulvic acid in acid aqueous solutions. Environ. Sci. Technol. 2008;42(7):2367–2373. doi: 10.1021/es072092z. [DOI] [PubMed] [Google Scholar]
- von der Kammer F., Baborowski M., Friese K. Application of a high-performance liquid chromatography fluorescence detector as a nephelometric turbidity detector following field-flow fractionation to analyse size distributions of environmental colloids. J. Chromatogr. A. 2005;1100(1):81–89. doi: 10.1016/j.chroma.2005.09.013. [DOI] [PubMed] [Google Scholar]
- von der Kammer F., Legros S., Larsen E.H., Loeschner K., Hofmann T. Separation and characterization of nanoparticles in complex food and environmental samples by field-flow fractionation. Trends Anal. Chem. 2011;30(3):425–436. [Google Scholar]
- Waksman S.A., Hutchings I.J. Decomposition of lignin by microorganisms. Soil Sci. 1936;42(2):119–130. [Google Scholar]
- Walter R., Dorn P. E. Schweizerbart; Stuttgart: 1995. Geologie von Mitteleuropa. ix, p. 566. [Google Scholar]
- Yang X., Flynn R., von der Kammer F., Hofmann T. Quantifying the influence of humic acid adsorption on colloidal microsphere deposition onto iron-oxide-coated sand. Environ. Pollut. 2010;158(12):3498–3506. doi: 10.1016/j.envpol.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Yang X., Flynn R., von der Kammer F., Hofmann T. Influence of ionic strength and pH on the limitation of latex microsphere deposition sites on iron-oxide coated sand by humic acid. Environ. Pollut. 2011;159(7):1896–1904. doi: 10.1016/j.envpol.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Ziechmann W. Über die Elektronen-Donator- und Acceptoreigenschaften von Huminstoffen. Geoderma. 1972;8(2–3):111–131. [Google Scholar]
- Ziechmann W. Spektrum Akademischer Verlag; Heidelberg: 1996. Huminstoffe und ihre Wirkungen. Spektrum Umwelt. p. 239. [Google Scholar]