Abstract
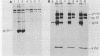
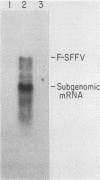
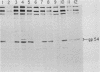
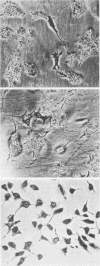
Friend spleen focus-forming virus shuts down its gene expression frequently (ca. 10(-3) per generation) in a cis-dominant hereditable fashion in various murine cells but much less frequently in rat cells (less than 10(-6) per generation). Thus, nonexpresser variants were isolated at high frequency from murine cell lines by immunoselection directed against virus-encoded cell surface glycoproteins and also simply by subcloning cells from lines which had been cultured for many generations. Studies of independently infected cell clones indicate that shutdown is a property of the cell line rather than of the specific proviral site. Nucleic acid blot analyses suggest that shutdown correlates with decreased transcription. Moreover, preliminary evidence indicates that other murine retroviruses also shut down frequently in murine but not in rat cells and that shutdown of replication-competent murine leukemia viruses with accompanying loss in interference to superinfection may be the rate-limiting reaction enabling cells to acquire multiple proviruses in their chromosomes. High-frequency shutdown in vivo would have important pathological consequences.
Full text
PDF



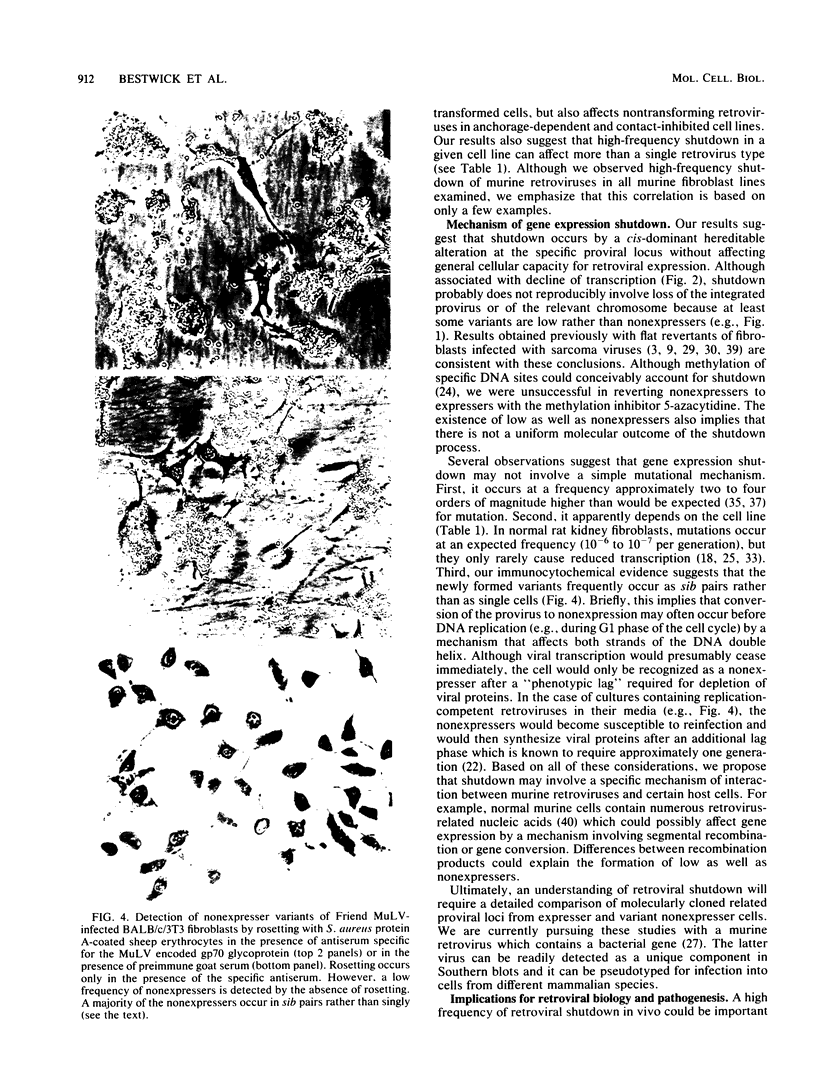


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baliga V., Ferrer J. F. Expression of the bovine leukemia virus and its internal antigen in blood lymphocytes. Proc Soc Exp Biol Med. 1977 Nov;156(2):388–391. doi: 10.3181/00379727-156-39942. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Bensinger W. I., Robbins K. C., Greenberger J. S., Aaronson S. A. Different mechanisms for morphologic reversion of a clonal population of murine sarcoma virus-transformed nonproducer cells. Virology. 1977 Apr;77(2):750–761. doi: 10.1016/0042-6822(77)90496-2. [DOI] [PubMed] [Google Scholar]
- Bestwick R., Ruta M., Kiessling A., Faust C., Linemeyer D., Scolnick E., Kabat D. Genetic structure of Rauscher spleen focus-forming virus. J Virol. 1983 Mar;45(3):1217–1222. doi: 10.1128/jvi.45.3.1217-1222.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M., Stowring L., Ventura P., Haase A. T. Gene expression in visna virus infection in sheep. Nature. 1981 Jul 16;292(5820):240–242. doi: 10.1038/292240a0. [DOI] [PubMed] [Google Scholar]
- Breindl M., Bacheler L., Fan H., Jaenisch R. Chromatin conformation of integrated Moloney leukemia virus DNA sequences in tissues of BALB/Mo mice and in virus-infected cell lines. J Virol. 1980 May;34(2):373–382. doi: 10.1128/jvi.34.2.373-382.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Cloyd M. W., Linemeyer D. L., Lander M. R., Rands E., Lowy D. R. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature. 1982 Jan 7;295(5844):25–31. doi: 10.1038/295025a0. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Doig D., Nishio J. Antibody-induced modulation of Friend virus cell surface antigens decreases virus production by persistent erythroleukemia cells: influence of the Rfv-3 gene. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5784–5788. doi: 10.1073/pnas.76.11.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C. T., Stehelin D., Bishop J. M., Varmus H. E. Characteristics of virus-specific RNA in avian sarcoma virus-transformed BHK-21 cells and revertants. Virology. 1977 Jan;76(1):313–330. doi: 10.1016/0042-6822(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Doig D., Chesebro B. Antibody-induced loss of Friend virus leukemia cell surface antigens occurs during progression of erythroleukemia in F1 mice. J Exp Med. 1978 Nov 1;148(5):1109–1121. doi: 10.1084/jem.148.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. A., Fan H. Immunoselection and characterization of Moloney murine leukemia virus-infected cell lines deficient in surface gag antigen expression. Virology. 1981 Aug;113(1):95–108. doi: 10.1016/0042-6822(81)90139-2. [DOI] [PubMed] [Google Scholar]
- Evans L. H., Dresler S., Kabat D. Synthesis and glycosylation of polyprotein precursors to the internal core proteins of Friend murine leukemia virus. J Virol. 1977 Dec;24(3):865–874. doi: 10.1128/jvi.24.3.865-874.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Chute H., Chao E., Feuerman M. Construction and characterization of Moloney murine leukemia virus mutants unable to synthesize glycosylated gag polyprotein. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5965–5969. doi: 10.1073/pnas.80.19.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Jaenisch R., MacIsaac P. Low-multiplicity infection of Moloney murine leukemia virus in mouse cells: effect on number of viral DNA copies and virus production in producer cells. J Virol. 1978 Dec;28(3):802–809. doi: 10.1128/jvi.28.3.802-809.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenyö E. M., Klein E., Klein G., Swiech K. Selection of an immunoresistant Moloney lymphoma subline with decreased concentration of tumor-specific surface antigens. J Natl Cancer Inst. 1968 Jan;40(1):69–89. [PubMed] [Google Scholar]
- Fenyö E. M., Nordenskjöld M. C., Klein E. Membrane immunofluorescence on cultued indicator cells as a measure of virus production by mouse L cells and by two Moloney lymphoma sublines differing in immunosensitivity. Ann N Y Acad Sci. 1971 Jun 21;177:121–129. doi: 10.1111/j.1749-6632.1971.tb35038.x. [DOI] [PubMed] [Google Scholar]
- Firestone G. L., Payvar F., Yamamoto K. R. Glucocorticoid regulation of protein processing and compartmentalization. Nature. 1982 Nov 18;300(5889):221–225. doi: 10.1038/300221a0. [DOI] [PubMed] [Google Scholar]
- Fitting T., Ruta M., Kabat D. Mutant cells that abnormally process plasma membrane glycoproteins encoded by murine leukemia virus. Cell. 1981 Jun;24(3):847–858. doi: 10.1016/0092-8674(81)90110-0. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Narayan P., Griffin D., Price D. Slow persistent infection caused by visna virus: role of host restriction. Science. 1977 Jan 14;195(4274):175–177. doi: 10.1126/science.188133. [DOI] [PubMed] [Google Scholar]
- Hardy W. D., Jr, McClelland A. J., Zuckerman E. E., Snyder H. W., Jr, MacEwen E. G., Francis D., Essex M. Development of virus non-producer lymphosarcomas in pet cats exposed to FeLv. Nature. 1980 Nov 6;288(5786):90–92. doi: 10.1038/288090a0. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Humphries E. H., Glover C., Reichmann M. E. Rous sarcoma virus infection of synchronized cells establishes provirus integration during S-phase DNA synthesis prior to cellular division. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2601–2605. doi: 10.1073/pnas.78.4.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioachim H. L., Sabbath M. Redistribution and modulation of Gross murine leukemia virus antigens induced by specific antibodies. J Natl Cancer Inst. 1979 Jan;62(1):169–180. [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Kabat D., Ruta M., Murray M. J., Polonoff E. Immunoselection of mutants deficient in cell surface glycoproteins encoded by murine erythroleukemia viruses. Proc Natl Acad Sci U S A. 1980 Jan;77(1):57–61. doi: 10.1073/pnas.77.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Montandon P. E., Montandon F., Fan H. Methylation state and DNase I sensitivity of chromatin containing Moloney murine leukemia virus DNA in exogenously infected mouse cells. J Virol. 1982 Nov;44(2):475–486. doi: 10.1128/jvi.44.2.475-486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S. Revertants of mouse cells transformed by murine sarcoma virus. V. Loss of MSV-specific nucleotide sequences from cellular RNA. Virology. 1978 Dec;91(2):444–452. doi: 10.1016/0042-6822(78)90390-2. [DOI] [PubMed] [Google Scholar]
- Porzig K. J., Robbins K. C., Aaronson S. A. Cellular regulation of mammalian sarcoma virus expression: a gene regulation model for oncogenesis. Cell. 1979 Apr;16(4):875–884. doi: 10.1016/0092-8674(79)90102-8. [DOI] [PubMed] [Google Scholar]
- Rojko J. L., Hoover E. A., Quackenbush S. L., Olsen R. G. Reactivation of latent feline leukaemia virus infection. Nature. 1982 Jul 22;298(5872):385–388. doi: 10.1038/298385a0. [DOI] [PubMed] [Google Scholar]
- Ruta M., Bestwick R., Machida C., Kabat D. Loss of leukemogenicity caused by mutations in the membrane glycoprotein structural gene of Friend spleen focus-forming virus. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4704–4708. doi: 10.1073/pnas.80.15.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta M., Clarke S., Boswell B., Kabat D. Heterogeneous metabolism and subcellular localization of a potentially leukemogenic membrane glycoprotein encoded by Friend erythroleukemia virus. Isolation of viral and cellular processing mutants. J Biol Chem. 1982 Jan 10;257(1):126–134. [PubMed] [Google Scholar]
- Ruta M., Kabat D. Plasma membrane glycoproteins encoded by cloned Rauscher and Friend spleen focus-forming viruses. J Virol. 1980 Sep;35(3):844–853. doi: 10.1128/jvi.35.3.844-853.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Troxler D. H., Boyars J. K., Parks W. P., Scolnick E. M. Friend strain of spleen focus-forming virus: a recombinant between mouse type C ecotropic viral sequences and sequences related to xenotropic virus. J Virol. 1977 May;22(2):361–372. doi: 10.1128/jvi.22.2.361-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Wyke J. Revertants of an ASV-transformed rat cell line have lost the complete provius or sustained mutations in src. Virology. 1981 Jan 15;108(1):28–46. doi: 10.1016/0042-6822(81)90525-0. [DOI] [PubMed] [Google Scholar]