Abstract
Molecular analysis encouraged discovery of genetic diversity and relationships of cultivated melon (Cucumis melo L.). We sequenced nine inter- and intra-genic regions of the chloroplast genome, about 5500 bp, using 60 melon accessions and six reference accessions of wild species of Cucumis to show intra-specific variation of the chloroplast genome. Sequence polymorphisms were detected among melon accessions and other Cucumis species, indicating intra-specific diversification of the chloroplast genome. Melon accessions were classified into three subclusters by cytoplasm type and then into 12 subgroups. Geographical origin and seed size also differed between the three subclusters. Subcluster Ia contained small-seed melon from Southern Africa and South and East Asia and subcluster Ib mainly consisted of large-seed melon from northern Africa, Europe and USA. Melon accessions of subcluster Ic were only found in West, Central and Southern Africa. Our results indicated that European melon groups and Asian melon groups diversified independently and shared the same maternal lineage with northern African large-seed melon and Southern African small-seed melon, respectively. Cultivated melon of subcluster Ic may have been domesticated independently in Africa. The presence of 11 cytoplasm types in Africa strongly supported African origin of cultivated melon and indicated the importance of germplasm from Africa.
Keywords: chloroplast genome, Cucumis, genetic differentiation, genetic diversity, maternal line, melon, polyphyletic origin
Introduction
Classification for the Cucurbitaceae has been focused by several studies (e.g., Jeffrey 1980). The Cucurbitaceae comprises 15 tribes, and includes 942–978 species of 95 genera (Schaefer and Renner 2011). Genus Cucumis belongs to the tribe Benincaseae and comprises 65 species, most of which grow wild in Africa and includes two important horticultural crops, melon (Cucumis melo L.) and cucumber (Cucumis sativus L.). Melon is divided into weedy melon (Group Agrestis) and cultivated melon. The latter is further classified into Groups Cantalupensis, Inodorus, Dudaim, Conomon, Flexuosus and Momordica (Robinson and Decker-Walters 1997). Groups Cantalupensis and Inodorus are cultivated mainly in Western countries, such as USA, European countries and Russia and have sweet flesh and some fruits are netted. The other groups are distributed in Asia, except Group Agrestis, which is distributed worldwide, and are characterized by low sugar content and smooth skin. These Asian melons are clearly separated from US and European melon by analysis of 35 plant and fruit traits (Liu et al. 2004).
Seed length (Akashi et al. 2002, Fujishita 1983) and complimentary genes causing bitterness of the young fruit placenta of inter-varietal F1 hybrids (Fujishita et al. 1993) also reflect genetic and geographical differentiation among diversified melon groups. Based on the seed length, melon is classified into large-seed type (≥9.0 mm) and small-seed type (<9.0 mm). Melon accessions of Groups Cantalupensis and Inodorus are mostly classified as large-seed type and those of Groups Agrestis and Conomon as small-seed type. In contrast, both seed types are found in Groups Flexuosus, Dudaim and Momordica collected from South to Southeast Asia (Akashi et al. 2002). Genetic differentiation between melon groups of different geographical origins detected by analysis of the above-mentioned complimentary genes separated Asian melon into a South-Southeast Asian melon group and an East Asian melon group (Fujishita et al. 1993). DNA markers were used to classify melon, and genetic differentiation was shown between Groups Cantalupensis and Inodorus and Group Conomon (Monforte et al. 2003, Stepansky et al. 1999). Phylogenetic relationships between melon groups from USA, Europe, Africa and Asia were analyzed using random amplified polymorphic DNA (RAPD) markers (Garcia et al. 1998, López-Sesé et al. 2003, Mliki et al. 2001, Nakata et al. 2005). Although molecular markers of the nuclear genome are highly polymorphic and are suitable to detect polymorphism among melon accessions, a DNA marker specific to each Group has not been established. Therefore, molecular markers whose sequences are relatively conserved compared with nuclear genome markers should be developed to explain the phylogenetic relationship of melon groups of different geographical origins.
The chloroplast genome generally shows maternal inheritance in higher plants (Reboud and Zeyl 1994) and has a similar gene arrangement among plant species and a low rate of base substitution (Clegg et al. 1994, Hiratsuka et al. 1989, Palmer 1985, Perl-Treves and Galun 1985, Provan et al. 1999). Nakamura et al. (1997) detected sequence polymorphism in the linker sequence between Rpl16 and Rpl14 genes and designated it as a plastid subtype ID (PS-ID) sequence. Polymorphism in the PS-ID sequence was detected between subspecies japonica and indica of rice (Nakamura et al. 1997) and the maternal lineage of Japanese rice cultivars was investigated by PS-ID analysis (Ishikawa et al. 2002a, 2002b). Chung and Staub (2003) developed 23 consensus chloroplast simple sequence repeat (ccSSR) markers for Cucurbitaceae crops based on the complete chloroplast genome sequence of Nicotiana tabacum (NC_001879). Analysis of ccSSR markers using 18 accessions of Cucumis species showed that cucumber and melon are distinct from African wild species of Cucumis (Chung et al. 2006). Renner et al. (2007) and Sebastian et al. (2010) compared the DNA sequence of five regions of the chloroplast genome as well as the nuclear internal transcribed spacer (ITS) region and showed that cucumber and melon are related closely to Asian and Australian wild species Cucumis hystrix Chakr. and Cucumis picrocarpus F. Muell., and rather closely to African wild species Cucumis sagittatus Peyr. and Cucumis metuliferus E. Mey. These results clearly showed that sequence polymorphism of the chloroplast genome can explain phylogenetic relationships within Cucumis and suggest its applicability to phylogenetic analysis of melon.
In this study, we determined the nucleotide sequences of nine regions of the chloroplast genome to determine the plastid type of 66 accessions of Cucumis species. Genetic diversity among 60 accessions of melon and six accessions of Cucumis wild species is shown by sequence comparison and their phylogenetic relationships are discussed based on their plastid types.
Materials and Methods
Plant materials
Sixty-six accessions of Cucumis, which included six accessions of four Cucumis wild species and 60 accessions of melon (C. melo L.), were analyzed (Table 1). The six accessions of wild species consisted of one accession each of Cucumis anguria L. from Zimbabwe, C. metuliferus from South Africa and C. sagittatus from Namibia and three accessions of C. hystrix from China. Of the 60 accessions, 18 accessions were selected from six horticultural groups: three accessions of Group Cantalupensis (European cantaloupe: 1, netted melon: 2 [England glasshouse type: 1, American field type: 1]), three accessions of Group Inodorus (Chinese Honeydew: 1, Spanish winter melon: 1, Chinese Hami melon: 1), one accession each of Group Flexuosus (India), Group Momordica (India) and Group Conomon var. makuwa (Japan) and nine accessions of Group Agrestis (Sudan: 2, Cameroon: 1, Senegal: 4, Ghana: 1, Korea: 1). The remaining 42 accessions, consisting of unclassified landraces, were: 21 accessions from Africa (Egypt: 2, Morocco: 1, Ethiopia: 1, Sudan: 1, Chad: 1, Mali: 1, Senegal: 1, Sierra Leone: 1, Cameroon: 1, Zambia: 4, Zimbabwe: 7), 13 accessions from West and Central Asia (Turkey: 5, Syria: 2, Iraq: 1, Iran: 1, Afghanistan: 4) and eight accessions from South Asia (Maldives: 1, India: 7). Because sequence polymorphism was detected between two plants in each of the following three accessions, the two plants of each accession were treated as different accessions (Zambia: PI 505599-1 and -2; PI 505602-1 and -2 ; Turkey: PI 169379-1 and -2) (Table 1). The complete chloroplast genome sequence of cucumber (DQ865976) registered in the GenBank, was also included for data analysis.
Table 1.
Details of 66 accessions of Cucumis species analyzed in this study
| Name of cultivar/Accession No. | Group/Species nameb | Country | Market class/Area | Seed sourcec | Seedd | Fruit size (cm) | Brix (°) | Sample studied bye | Cluster Nof | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Type | Length (mm) | Height | Diameter | Akashi | Tanaka | |||||||
| Rio Gold | Cantalupensis | America | 1 | L | 9.5 | 11.6 | 10.9 | 14.4 | − | + | Ib-2 | |
| Earl’s Favourite | Cantalupensis | England | 1 | L | 9.6 | 16.0 | 15.5 | 14.0 | − | + | Ib-2 | |
| Ogen (780045) | Cantalupensis | France | 1 | L | 10.1 | 13.0 | 14.0 | – | − | + | Ib-1 | |
| Hami-gua 6 | Inodorus | America | Chinese Honeydew | 1 | L | 14.2 | 18.5 | 16.5 | 12.8 | − | + | Ib-3 |
| Tendrala | Inodorus | Spain | 1 | L | 12.5 | 18.0 | 15.0 | 8.6 | − | + | Ib-3 | |
| Hami-gua | Inodorus | China | 1 | L | 11.7 | 25.5 | 12.0 | – | − | + | Ib-2 | |
| PI 614576 | Flexuosus | India (Center) | Madhya Pradesh | 2 | L | 9.0 | 65.0 | 7.5 | 2.0 | + | + | Ia-1 |
| PI 182952 | Momordica | India (West) | Gujarat | 2 | L | 10.0 | 12.5 | 10.0 | 2.8 | + | + | Ib-1 |
| Kinpyo | Conomon | Japan | 1 | S | 6.8 | 9.5 | 7.0 | 13.8 | + | + | Ia-1 | |
| 940111 | Agrestis | Sudan | 1 | S | 6.0 | 7.0 | 4.5 | 5.0 | + | + | Ic-5 | |
| sud-4 | Agrestis | Sudan | 1 | S | 4.9 | – | – | – | − | − | Ic-5 | |
| 940065 | Agrestis | Cameroon | 1 | S | 6.0 | – | – | – | + | + | Ic-5 | |
| 940108 | Agrestis | Senegal | 1 | S | 5.5 | 4.0 | 2.0 | 9.6 | + | + | Ic-3 | |
| PI 436532 | Agrestis | Senegal | 2 | S | 6.5 | 10.3 | 5.7 | 6.6 | + | + | Ic-3 | |
| PI 436534 | Agrestis | Senegal | 2 | S | 4.8 | – | – | – | + | + | Ic-3 | |
| 940112 | Agrestis | Senegal | 1 | S | 5.0 | 4.0 | 3.0 | 5.0 | + | + | Ic-4 | |
| PI 185111 | Agrestis | Ghana | 2 | S | 6.4 | – | – | – | + | + | Ic-1 | |
| Weedy melon | Agrestis | Korea | 3 | S | 6.0 | 4.0 | 3.0 | 5.0 | + | + | Ia-3 | |
|
| ||||||||||||
| PI 525111 | – | Egypt | 2 | L | 13.0 | 14.0 | 7.5 | 5.0 | + | + | Ib-2 | |
| PI 525105 | – | Egypt | 2 | L | 14.0 | 16.0 | 11.5 | 4.0 | + | + | Ib-1 | |
| PI 207661 | – | Morocco | 2 | L | 12.0 | 11.3 | 9.2 | 5.6 | + | + | Ib-3 | |
| PI 193495 | – | Ethiopia | 2 | S | 7.0 | – | – | – | − | − | Ib-1 | |
| 119 | – | Sudan | 1 | L | 11.5 | 7.9 | 5.6 | 4.8 | − | − | Ib-1 | |
| 940281 | – | Chad | 1 | S | 6.0 | 9.0 | 6.5 | 4.0 | + | + | Ic-5 | |
| PI 490388 | – | Mali | 2 | L | 13.0 | – | – | – | + | + | Ic-3 | |
| PI 436533 | – | Senegal | 2 | S | 5.8 | 8.6 | 6.5 | 8.0 | + | + | Ic-2 | |
| PI 320993 | – | Sierra Leone | 2 | L | 11.5 | 14.0 | 11.3 | 6.2 | + | + | Ia-1 | |
| Cam-84-3 | – | Cameroon | 1 | S | 7.0 | 12.0 | 7.0 | 5.4 | + | + | Ic-5 | |
| PI 505599-1 | – | Zambia | 2 | S | 5.7 | 14.5 | 9.5 | 4.0 | + | + | Ia-1 | |
| PI 505599-2 | – | Zambia | 2 | S | 7.6 | 14.5 | 9.5 | 4.0 | + | + | Ic-6 | |
| PI 505602-1 | – | Zambia | 2 | S | 7.9 | 13.5 | 7.5 | 3.0 | + | + | Ia-1 | |
| PI 505602-2 | – | Zambia | 2 | S | 7.9 | 13.5 | 7.5 | 3.0 | + | + | Ic-6 | |
| PI 482411 | – | Zimbabwe | 2 | S | 7.0 | 12.0 | 7.0 | 4.0 | + | + | Ia-1 | |
| PI 482424 | – | Zimbabwe | 2 | S | 7.0 | 11.0 | 8.0 | 4.0 | + | + | Ia-3 | |
| PI 482429 | – | Zimbabwe | 2 | S | 8.0 | 15.0 | 9.0 | 4.0 | + | + | Ia-3 | |
| PI 482398 | – | Zimbabwe | 2 | S | 6.0 | 8.0 | 5.0 | 5.0 | + | + | Ic-6 | |
| PI 482413 | – | Zimbabwe | 2 | S | 6.0 | 6.0 | 5.5 | 5.0 | + | + | Ic-6 | |
| PI 482422 | – | Zimbabwe | 2 | S | 8.6 | 7.8 | 5.6 | 5.5 | − | − | Ic-6 | |
| PI 482396 | – | Zimbabwe | 2 | S | 7.0 | 9.5 | 5.2 | 6.2 | − | − | Ic-6 | |
| PI 169379-1 | – | Turkey | 2 | L | 10.0 | 31.0 | 11.5 | 5.0 | + | + | Ib-1 | |
| PI 169379-2 | – | Turkey | 2 | L | 10.0 | 31.0 | 11.5 | 5.0 | + | + | Ia-1 | |
| PI 172818 | – | Turkey | 2 | L | 11.0 | 17.0 | 12.0 | 10.0 | + | + | Ia-3 | |
| Karasu melon | – | Turkey | 1 | L | 9.0 | 9.0 | 9.5 | 8.0 | + | + | Ib-2 | |
| PI 176928 | – | Turkey | 2 | L | 10.0 | 15.0 | 10.0 | 9.4 | + | + | Ib-2 | |
| PI 534605 | – | Syria | 2 | L | 12.0 | 12.7 | 10.4 | 3.0 | + | + | Ib-1 | |
| PI 181872 | – | Syria | 2 | L | 11.0 | 14.8 | 14.5 | 4.4 | + | + | Ib-2 | |
| PI 435290 | – | Iraq | 2 | L | 13.5 | 6.5 | 4.7 | 4.0 | + | + | Ib-2 | |
| PI 143231 | – | Iran | 2 | L | 14.0 | 22.0 | 14.0 | 7.0 | + | + | Ib-1 | |
| PI 125931 | – | Afghanistan | 2 | L | 12.0 | 13.5 | 11.5 | 12.6 | + | + | Ib-1 | |
| PI 125961 | – | Afghanistan | 2 | L | 14.0 | – | – | – | + | + | Ib-1 | |
| PI 126047 | – | Afghanistan | 2 | L | 15.0 | – | – | – | + | + | Ib-2 | |
| PI 126054 | – | Afghanistan | 2 | L | 12.0 | 10.5 | 10.0 | 9.5 | + | + | Ia-3 | |
| PI 536480 | – | Maldives | 2 | S | 8.0 | 22.0 | 9.7 | 4.6 | + | + | Ia-1 | |
| PI 116666 | – | India (West) | Punjab | 2 | L | 9.0 | 18.5 | 10.0 | 3.2 | + | + | Ia-1 |
| PI 116738 | – | India (West) | Punjab | 2 | S | 7.0 | 11.0 | 7.0 | 4.5 | + | + | Ib-2 |
| PI 614588 | – | India (Center) | Madhya Pradesh | 2 | L | 8.5 | 7.0 | 5.0 | 3.4 | + | + | Ia-2 |
| PI 124435 | – | India (Center) | Madhya Pradesh | 2 | L | 11.0 | 45.0 | 25.0 | 5.5 | + | + | Ib-2 |
| PI 614542 | – | India (Center) | Madhya Pradesh | 2 | S | 7.0 | 14.0 | 9.5 | 6.8 | + | + | Ia-1 |
| PI 164585 | – | India (South) | Tamil Nadu | 2 | L | 10.0 | 12.3 | 9.0 | 5.2 | + | + | Ib-2 |
| PI 124096 | – | India (South) | Andra Pradesh | 2 | L | 10.0 | 16.0 | 9.5 | 6.3 | + | + | Ib-1 |
|
| ||||||||||||
| PI 292190 | C. metuliferus | South Africa | 2 | – | 6.0 | – | – | – | − | − | III | |
| PI 482383 | C. anguria | Zimbabwe | 2 | – | 5.0 | – | – | – | − | − | III | |
| PI 282441 | C. sagittatus | Namibia | 2 | – | 5.0 | – | – | – | − | − | III | |
| CYS68-1 | C. hystrix | China | 4 | – | 3.6 | – | – | – | − | − | II | |
| CYS68-2 | C. hystrix | China | 4 | – | 3.6 | – | – | – | − | − | II | |
| Sm021-3 | C. hystrix | China | 4 | – | 3.4 | – | – | – | − | − | II | |
Tendral o Invernale a Buccia Verde.
–: Local landraces whose horticultural group or variety was not specified as shown in the text.
1: National Institute of Vegetable and Tea Science (NIVTS), Japan. 2: North Central Regional Plant Introduction Station, Iowa State University (USDA-ARS), USA. 3: Okayama University, Japan. 4: Kunming Institute of Botany, PRC.
S: small-seed melon, L: large-seed melon.
Plants also analyzed by Akashi et al. (2002) and Tanaka et al. (2006) are indicated by “+”.
Cluster number refers to Fig. 1.
Seeds of these accessions were mostly provided by the National Institute of Vegetable and Tea Science (NIVTS), Japan, the North Central Regional Plant Introduction Station, Iowa State University (USDA-ARS), USA (Table 1) and they were cultivated in the field or in a glasshouse of Okayama University, Japan.
Fruit characteristics, such as height and diameter of fruit and soluble solid content (Brix grade) were measured for two fruits of 52 melon accessions. Length and width of the representative seeds were measured for all accessions, which were then classified into a large-seed type (≥9.0 mm) and a small-seed type (<9.0 mm), according to Akashi et al. (2002) (Table 1). As shown in Table 1, of the 60 melon accessions examined here, 49 were used in our previous RAPD analysis (Akashi et al. 2006), and 55 were used for dCAPS analysis of the linker sequence between Rpl16 and Rpl14 (Tanaka et al. 2006).
DNA extraction
Seeds were sown on a filter paper and were grown at 26°C in a 16 h light-8 h dark cycle at light intensity 46.5 μMs−1 m−2. Ten-day-old seedlings were individually ground in liquid nitrogen, and total DNA was extracted by using the procedure of Murray and Thompson (1980) with minor modifications.
Primer design
The seven target regions of the chloroplast genome were: inter-genic regions TrnK-MatK (Cmcp1), PsbK-PsbI (Cmcp3), AtpH-AtpI (Cmcp5), PsaI-Ycf4 (Cmcp15) and NdhF-Rpl32 (Cmcp6), and intron 1 of Rps16 (Cmcp2) and NdhA (Cmcp11), among which Cmcp1 included 127 bp of maturase K sequence (Table 2). The primer pair was designed to amplify these regions based on the chloroplast sequences of tobacco (N. tabacum: NC_001879) and cucumber (C. sativus: DQ119058, DQ865975, DQ865976, AJ970307) registered in the GenBank. For Cmcp5 and Cmcp11, additional primers, F2 and R2, were designed for complete sequencing. Another two target regions of the chloroplast genome regions were inter-genic regions of Rpl16-Rpl14 (PS-ID) and PsbC-TrnS (ccSSR7), for which the specific primer pairs were developed by Nakamura et al. (1997) and Chung and Staub (2003), respectively. Among these regions, Cmcp6 and Cmcp11 are in the small single-copy region of the melon chloroplast genome (JF412791) and the remaining seven regions are in the large single-copy region.
Table 2.
Primer sequence and number of polymorphic site of nine chloroplast genome regions of 67 accessions of Cucumis species
| Regiona | Gene name | Marker name | Forward (F), Reverse (R) primer (5′ to 3′)b | Marker position (bp) | Aligned length (bp)c | Gap (bp)c | Total no. of site (bp)c | SNPc | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Position | Length | No. | % | ||||||||
| LSC | TrnK-MatK | Cmcp1 | F | AACCCGGAACTAGTCGGATGG | 1,638–1,658 | 388 | 390 | 3 | 387 | 21 | 5.4 |
| R | TCGGAATTATTGGAAGAATTCTT | 2,069–2,047 | 388 | – | 388 | 3 | 0.8 | ||||
| Rps16 intron1 | Cmcp2 | F | GAAGTTTTCTCCTCGTACGGC | 5,231–5,251 | 609 | 617 | 14 | 603 | 30 | 5.0 | |
| R | GATGGAGCTCGAGTAGAAAGTAT | 5,883–5,861 | 612 | 4 | 608 | 3 | 0.5 | ||||
| PsbK-PsbI | Cmcp3 | F | TGTTTGGCAAGCTGCTGTAAG | 7,996–8,016 | 377 | 387 | 24 | 363 | 32 | 8.8 | |
| R | GAGAGTAAGCATTACACAATCTCCAAG | 8,420–8,394 | 373 | 5 | 368 | 2 | 0.5 | ||||
| AtpH-AtpI | Cmcp5 | F1 | AATAACGGAAGCGGCAGAAAT | 14,556–14,576 | 976 | 946 | 53 | 893 | 49 | 5.5 | |
| R1 | AGCTCTTATTTTTGCAACTTTAGC | 15,576–15,553 | 920 | – | 920 | 6 | 0.7 | ||||
| F2 | GGTTGTCTTATAGCAATTCGATTC | 15,086–15,109 | |||||||||
| R2 | CATGAACTTAGATTAATAAAATATTG | 15,288–15,263 | |||||||||
| PsbC-TrnS | ccSSR 7 | F | CGGGAAGGGCTCGKGCAG | 37,185–37,202 | 297 | 276 | 12 | 264 | 14 | 5.3 | |
| R | GTTCGAATCCCTCTCTCTCCTTTT | 37,523–37,500 | 276 | 5 | 271 | 1 | 0.4 | ||||
| PsaI-Ycf4 | Cmcp15 | F | TCGAGGTATCCATTCTATGAC | 61,339–61,359 | 534 | 569 | 44 | 525 | 37 | 7.0 | |
| R | ATCCATATACGTTCTGATCGC | 61,914–61,894 | 557 | 25 | 532 | 1 | 0.2 | ||||
| Rpl16-Rpl14 | PS-ID | A | AAAGATCTAGATTTCGTAAACAACATAGAGGAAGAA | 83,600–83,565 | 504 | 474 | 14 | 460 | 13 | 2.8 | |
| B | ATCTGCAGCATTTAAAAGGGTCTGAGGTTGAATCAT | 83,025–83,060 | 474 | 4 | 470 | 2 | 0.4 | ||||
|
| |||||||||||
| SSC | NdhF-Rpl32 | Cmcp6 | F | GAGATGAACGGTATGATCCATGA | 114,328–114,350 | 679 | 720 | 258 | 462 | 29 | 6.3 |
| R | TCCGTTTTTTTGATATAGAAGTGCG | 115,049–115,025 | 676 | 3 | 673 | 6 | 0.9 | ||||
| NdhA intron1 | Cmcp11 | F1 | TATAGGTTGACGCCACAAATTC | 122,070–122,091 | 1,213 | 1,254 | 88 | 1,166 | 59 | 5.1 | |
| R1 | ATCAATATCTCTACGTGTGATTCG | 123,328–123,305 | 1,215 | 33 | 1,182 | 6 | 0.5 | ||||
| F2 | TTGTAATTGGATTCTTTCCATT | 122,650–122,671 | |||||||||
| R2 | GATATTTTTTATTGTTTCTTATCTCC | 122,634–122,609 | |||||||||
|
| |||||||||||
| Total | 5,577 | 5,633 | 510 | 5,123 | 284 | 5.5 | |||||
| 5,491 | 79 | 5,412 | 30 | 0.6 | |||||||
LSC and SSC indicate large- and small-single copy region in the chloroplast genome, respectively. Gene name, maker position and marker length refer to the melon chloroplast genome (accession No. JF412791).
K = T or G
The upper side and the lower side value of each region were calculated for 67 accessions of Cucumis and 60 melon accessions, respectively.
Total number of site = (Aligned length) − (Gap).
PCR amplification and sequencing
PCR amplification was done in a 40 μl mixture (100 ng total DNA, 1 × ExTaqTM buffer [10 mM Tris-HCl at pH 8.3, 50 mM KCl, 20 mM MgCl2], 0.25 U ExTaqTM polymerase [TaKaRa, Japan], 0.1 mM dNTPs and 0.25 μM of each primer) by using an i-Cycler (Bio-Rad, USA). The PCR cycle was: initial denaturing at 95°C for 3 mins with hot start, 35 PCR cycles at 95°C for 30 secs, 57°C for 30 secs and 72°C for 30 secs. The final extension step was at 72°C for 3 mins. For Cmcp11, the annealing reaction was modified to 62 °C.
For direct sequencing, the PCR products were purified by using a WizardR SV Gel and a PCR Clean-UP System (Promega, USA). The sequencing reaction was run by using BigDyeTMCycle Sequence Ready Reaction DNA (Applied Biosystems, USA) and a specific primer for each region. The nucleotide sequence was determined for both strands by using an ABI PRISMR 3730 DNA Analyzer (Applied Biosystems, USA), except for the PS-ID region whose sequence for a reverse strand could not be determined because of two SSRs near the annealing site of the reverse primer.
Classification of large- and small-seed types based on seed weight
To determine the worldwide distribution patterns of large- and small-seed melon, seed size type of 7,396 melon accessions were estimated based on 100 seed weight data of the GRIN database (USDA-ARS, http://www.ars-grin.gov/npgs/). Since seed weight apparently depends on the condition and degree of ripening, it is difficult to accurately classify into large- and small-seed melon just based on 100 seed weight. However, a preliminary analysis of seed length and 100 seed weight, using 99 melon accessions, indicated that melon accessions with 100 seed weight below or over 2.5 g could be classified as small- or large-seed melon, respectively, with an accuracy of 87.9%. Therefore, in this study, 7,396 melon accessions were roughly classified as large- and small-seed melon by these criteria.
Data analysis
Sequences of nine chloroplast regions were aligned using CLUSTAL W (Thompson et al. 1994). For phylogenetic analyses, all gaps and inversions were removed from the sequences, because the mutational process of chloroplast SSRs is not clear (Provan et al. 2001). Phylogenetic relationships were determined by using the neighbor joining (NJ) method with MEGA 5 (Tamura et al. 2011). Genetic distance (GD) was calculated by using the maximum composite likelihood method (Tamura et al. 2004) and a phylogenetic tree was constructed by using the NJ method with a bootstrapping of 1000 replications. The phylogenetic relationships were also analyzed by using a maximum parsimony (MP) method with PAUP* version 4.0b10 (Swofford 2002). The MP method was run as described by Yamane and Kawahara (2005).
Results
Sequence analysis
The total length of determined nucleotide sequence differed between accessions, and 5633 nucleotides were aligned with nucleotide gaps (510 bp total), comprising nine regions of the chloroplast genome: TrnK-MatK (Cmcp1), Rps16 intron 1 (Cmcp2), PsbK-PsbI (Cmcp3), AtpH-AtpI (CmCp5), PsbC-TrnS (ccSSR7), PsaI-Ycf4 (Cmcp15), Rpl16-Rpl14 (PS-ID), NdhF-Rpl32 (Cmcp6) and NdhA intron 1 (Cmcp11) (Table 2 and Supplemental Table 1). Alignment of sequences between C. melo cv. ‘Earl’s Favourite’, Arabidopsis thaliana (NC_000932), N. tabacum (NC_001879) and C. sativus (DQ865976) showed 68% to 98% identities across nine regions (Table 3), confirming amplification of the chloroplast genome in Cucumis accessions. Within the genus Cucumis, 67 accessions, including C. sativus (DQ865976), shared an identity of over 93% (Supplemental Table 2). The sequence homology between ‘Earl’s Favourite’ and C. sativus was over 96% (Table 3), and single nucleotide polymorphisms (SNP) were detected at 284 sites (5.5%) among 68 accessions of Cucumis (Table 2). The most polymorphic region was Cmcp3 where the number of SNPs was observed at 32 sites (8.8%).
Table 3.
Sequence homology (%) of of nine chloroplast genome regions between four plant species
| No. | Name of species | Name of cultivar/Accession No.a | No. | |||
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | 2 | 3 | 4 | |||
| 1 | Cucumis melo | Earl’s Favourite | – | |||
| 2 | Cucumis sativus | DQ865976 | 96–98 | – | ||
| 3 | Nicotiana tabacum | NC_000932 | 72–86 | 71–87 | – | |
| 4 | Arabidopsis thaliana | NC_001879 | 68–87 | 68–87 | 64–84 | – |
Accession numbers are of DDBJ and NCBI.
Among the 60 melon accessions, the total length of sequence determined in nine regions was from 5433 to 5479 bp (Table 4) and their homology was over 99% (Supplemental Table 2). Intra-specific SNPs were detected at 30 sites (0.6%) (Table 2), among which six sites were detected in Cmcp6, which was the most polymorphic region at frequency 0.9%. The frequency of SNPs did not differ greatly between the large single-copy region and the small single-copy region, at one polymorphism every 0.5% and 0.6%, respectively. And five insertions and/or deletions (InDel1-5) and 12 simple sequence repeats (SSR1-12) were also detected in addition to 30 SNPs (SNP1-30) (Table 4). SNP2 (C or A) and SNP3 (G or C) were detected in the coding sequence at 55 bp and 114 bp downstream from the transcription start site of maturase K, respectively. The former was a nonsynonymous substitution, and the latter was a synonymous substitution.
Table 4.
Sequence polymorphism at 47 sites of nine chloroplast genome regions polymorphic among 60 accessions of melon
| Speciesa | Cluster No.b | No. of accessions | Sequence length (bp)c | Cmcp1 | Cmcp2 | Cmcp3 | Cmcp5 | ccSSR7 | Cmcp15 | PS-ID | Cmcp6 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| SNP1 | SNP2 | SNP3 | SNP4 | SNP5 | SNP6 | SNP7 | SNP8 | SNP9 | SNP10 | SNP11 | SNP12 | SNP13 | SNP14 | SNP15 | SNP16 | SNP17 | SNP18 | SNP19 | SNP20 | SNP21 | SNP22 | SNP23 | SNP24 | |||||
| C. melo | I | a-1 | 10 | 5457 | C | C | G | C | T | C | C | G | C | T | G | T | T | G | T | A | A | A | A | C | C | C | A | G |
| C. melo | I | a-2 | 1 | 5457 | C | C | G | C | T | C | C | G | C | T | G | T | T | G | T | A | A | A | A | C | C | C | A | G |
| C. melo | I | a-3 | 5 | 5456 | C | C | G | C | T | C | C | G | C | T | G | T | T | G | T | A | A | A | A | C | C | C | A | G |
| C. melo | I | b-1 | 11 | 5479 | C | A | G | C | T | C | C | A | A | T | A | T | T | G | T | A | A | T | G | C | C | C | A | G |
| C. melo | I | b-2 | 12 | 5478 | C | A | G | C | T | C | C | A | A | T | A | T | T | G | T | A | A | T | G | C | C | C | A | G |
| C. melo | I | b-3 | 3 | 5474 | C | A | G | C | T | C | C | A | A | T | A | T | T | G | T | A | A | T | G | C | C | C | A | G |
| C. melo | I | c-1 | 1 | 5433 | T | C | C | A | G | A | C | G | C | C | G | A | C | T | T | C | C | T | G | A | C | C | A | G |
| C. melo | I | c-2 | 1 | 5472 | T | C | C | C | G | A | A | G | C | C | G | A | C | T | T | C | C | T | G | A | C | C | A | G |
| C. melo | I | c-3 | 4 | 5471 | T | C | C | C | G | A | A | G | C | C | G | A | C | T | T | C | C | T | G | A | C | C | A | A |
| C. melo | I | c-4 | 1 | 5472 | T | C | C | C | G | A | A | G | C | C | G | A | C | T | T | C | C | T | G | A | A | C | A | A |
| C. melo | I | c-5 | 5 | 5448 | C | C | C | C | G | A | C | G | C | C | G | A | C | T | C | C | C | T | G | A | C | G | G | G |
| C. melo | I | c-6 | 6 | 5455 | C | C | C | C | G | A | C | G | C | C | G | A | C | T | C | C | C | T | G | A | C | G | G | G |
| C. hystrix | II | 2 | 5468 | C | C | G | A | G | A | A | T | C | T | G | C | C | G | T | A | C | T | G | C | A | C | A | G | |
| C. hystrix | II | 1 | 5468 | C | C | G | A | G | A | A | T | C | T | G | C | A | G | T | A | C | T | G | C | A | C | A | G | |
| C. sativus | II | 1 | 5460 | C | C | G | – | G | A | C | T | C | T | G | C | C | G | T | A | C | T | G | C | A | C | A | G | |
| C. metuliferus | III | 1 | 5460 | C | C | G | C | G | A | A | G | C | T | G | C | C | G | T | A | C | T | G | C | A | C | A | A | |
| C. sagittatus | III | 1 | 5239 | C | C | G | C | G | A | C | G | C | T | G | C | C | G | G | A | C | T | – | – | A | C | A | G | |
| C. anguria | III | 1 | 5436 | C | C | G | – | G | A | C | G | C | T | G | C | C | G | G | A | A | T | G | C | A | – | A | G | |
| Speciesa | Cluster No.b | No. of ac cessions | Sequence length (bp)c | Cmcp11d | ccSSR7 | Cmcp15 | Cmcp11 | Cmcp2 | Cmcp3 | Cmcp15 | PS-ID | Cmcp6 | Cmcp11 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
| SNP25 | SNP26 | SNP27 | SNP28 | SNP29 | SNP30 | InDel1 | InDel2 | InDel3 | InDel4 | InDel5 | SSR1 | SSR2 | SSR3 | SSR4 | SSR5 | SSR6 | SSR7 | SSR8 | SSR9 | SSR10 | SSR11 | SSR12 | |||||
| C. melo | I | a-1 | 10 | 5457 | G | T | G | T | G | T | 0 | 0 | +18 | +4 | −5 | (A)5 | (T)7 | (A)10 | (A)17 | (T)11 | (T)11 | (T)13 | (A)2 | (T)2 | (A)9 | (T)8 | (T)8 |
| C. melo | I | a-2 | 1 | 5457 | C | T | G | T | T | T | 0 | 0 | +18 | +4 | −5 | (A)5 | (T)7 | (A)10 | (A)17 | (T)11 | (T)11 | (T)13 | (A)2 | (T)2 | (A)9 | (T)8 | (T)8 |
| C. melo | I | a-3 | 5 | 5456 | C | T | G | T | T | T | 0 | 0 | +18 | +4 | −5 | (A)5 | (T)7 | (A)10 | (A)16 | (T)11 | (T)11 | (T)13 | (A)2 | (T)2 | (A)9 | (T)8 | (T)8 |
| C. melo | I | b-1 | 11 | 5479 | C | T | G | T | T | T | 0 | +23 | +18 | +4 | −5 | (A)5 | (T)7 | (A)10 | (A)15 | (T)11 | (T)11 | (T)14 | (A)2 | (T)2 | (A)9 | (T)8 | (T)8 |
| C. melo | I | b-2 | 12 | 5478 | C | T | G | T | T | T | 0 | +23 | +18 | +4 | −5 | (A)5 | (T)7 | (A)10 | (A)15 | (T)11 | (T)10 | (T)14 | (A)2 | (T)2 | (A)9 | (T)8 | (T)8 |
| C. melo | I | b-3 | 3 | 5474 | C | T | G | T | T | T | −5 | +23 | +18 | +4 | −5 | (A)5 | (T)7 | (A)10 | (A)15 | (T)11 | (T)11 | (T)14 | (A)2 | (T)2 | (A)9 | (T)8 | (T)8 |
| C. melo | I | c-1 | 1 | 5433 | – | C | A | G | T | C | 0 | 0 | 0 | +4 | −6 | (A)4 | (T)8 | (A)13 | (A)13 | (T)9 | (T)11 | (T)13 | (A)3 | (T)3 | (A)10 | (T)2 | (T)9 |
| C. melo | I | c-2 | 1 | 5472 | C | C | A | G | T | C | 0 | +23 | +18 | +4 | −6 | (A)4 | (T)7 | (A)12 | (A)15 | (T)9 | (T)11 | (T)13 | (A)2 | (T)2 | (A)10 | (T)2 | (T)9 |
| C. melo | I | c-3 | 4 | 5471 | C | C | A | G | T | C | 0 | +23 | +18 | +4 | −6 | (A)4 | (T)7 | (A)12 | (A)14 | (T)9 | (T)11 | (T)13 | (A)1 | (T)3 | (A)10 | (T)2 | (T)9 |
| C. melo | I | c-4 | 1 | 5472 | C | C | A | G | T | C | 0 | +23 | +18 | +4 | −6 | (A)4 | (T)7 | (A)12 | (A)14 | (T)9 | (T)11 | (T)13 | (A)1 | (T)4 | (A)10 | (T)2 | (T)9 |
| C. melo | I | c-5 | 5 | 5448 | C | C | A | G | T | C | 0 | 0 | +18 | −1 | −6 | (A)4 | (T)8 | (A)13 | (A)12 | (T)9 | (T)12 | (T)15 | (A)2 | (T)2 | (A)10 | (T)3 | (T)10 |
| C. melo | I | c-6 | 6 | 5455 | C | C | A | G | T | C | 0 | +7 | +18 | −1 | −6 | (A)4 | (T)8 | (A)13 | (A)12 | (T)9 | (T)12 | (T)15 | (A)2 | (T)2 | (A)10 | (T)3 | (T)10 |
| C. hystrix | II | 2 | 5468 | – | C | G | T | T | C | 0 | 0 | 0 | 0 | −5 | (A)3 | (T)5 | (A)9 | (A)13 | (T)9 | (T)11 | (T)12 | (A)2 | (T)5 | (A)13 | (T)2 | (T)9 | |
| C. hystrix | II | 1 | 5468 | – | C | G | T | T | C | 0 | 0 | 0 | 0 | −5 | (A)3 | (T)5 | (A)9 | (A)13 | (T)9 | (T)11 | (T)12 | (A)2 | (T)5 | (A)13 | (T)2 | (T)9 | |
| C. sativus | II | 1 | 5460 | – | C | G | T | T | C | 0 | 0 | 0 | 0 | −5 | (A)3 | (T)6 | (A)9 | (A)14 | (T)9 | (T)11 | (T)11 | (A)2 | (T)5 | (A)15 | (T)2 | (T)8 | |
| C. metuliferus | III | 1 | 5460 | – | C | G | T | T | C | 0 | 0 | 0 | −1 | 0 | (A)3 | (T)6 | (A)18 | (A)14 | (T)12 | (T)5 | (T)9 | (A)2 | (T)4 | (A)11 | (T)2 | (T)10 | |
| C. sagittatus | III | 1 | 5239 | – | C | G | T | T | C | 0 | 0 | 0 | 0 | 0 | (A)3 | (T)6 | (A)17 | (A)11 | (T)15 | (T)8 | (T)9 | – | – | (A)12 | (T)2 | (T)12 | |
| C. anguria | III | 1 | 5436 | – | C | G | T | T | C | 0 | −7 | 0 | 0 | 0 | (A)3 | (T)6 | (A)14 | (A)14 | (T)13 | (T)11 | (T)8 | (A)2 | (T)4 | (A)10 | (T)2 | (T)11 | |
Single nucleotide polymorphism, insertion or deletion and simple sequence repeat are represented by “SNP”, “In/Del” and “SSR”, respectively. SNPs data of wild species was also indicated. Absence of sequence at SNP and SSR sites was indicated by “−”.
Cluster number refers to Fig. 1.
Sequence length indicating total number of nucleotides of nine chloroplast genome regions.
SNP25 was located in the motif sequence of In/Del3.
Phylogenetic relationships between Cucumis species
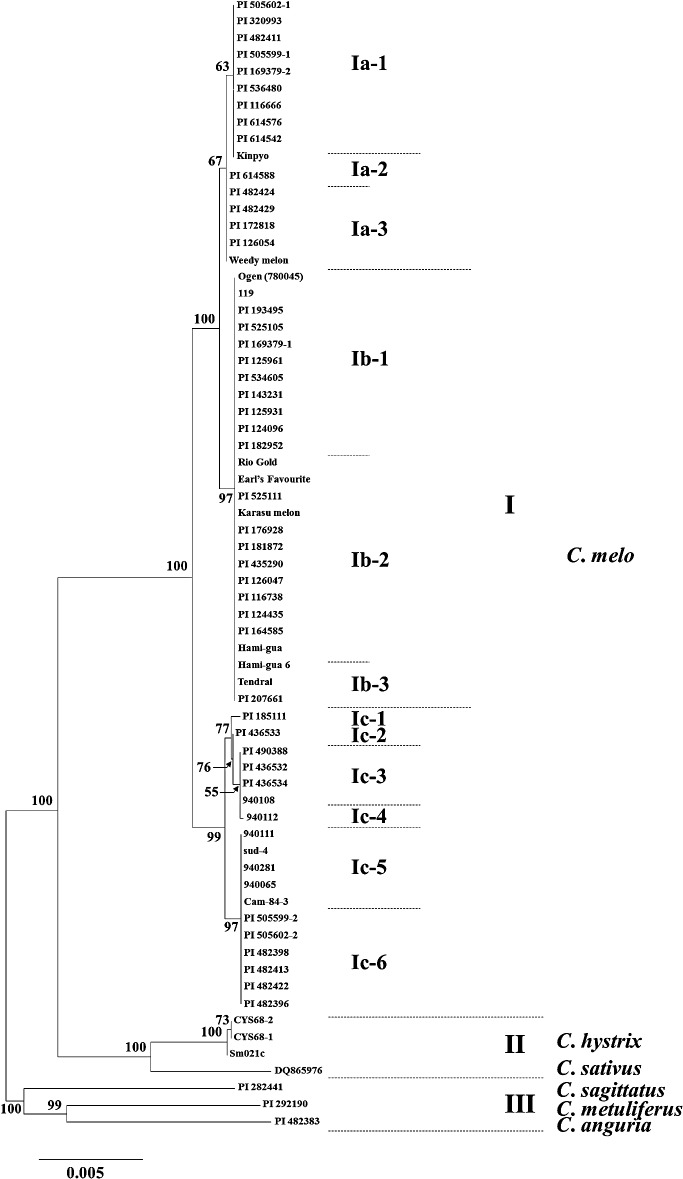
Sixty-seven accessions of Cucumis, including the reference sequence of C. sativus (DQ865976), were divided into three clusters in the NJ tree constructed with the GD based on 284 SNPs (Fig. 1). Cluster I comprised all melon accessions. Cluster II comprised C. hystrix and C. sativus, even though these two species have different chromosome numbers, 2n = 14 and 2n = 24, respectively. Cluster III comprised African wild species C. anguria, C. metuliferus and C. sagittatus. Cluster I was slightly closer to cluster II at GD 0.01692 to 0.01950 than to cluster III at GD 0.02205 to 0.02440 (Fig. 1 and Table 5). The GD between C. hystrix and C. sativus was 0.00974 and was significantly shorter than the average distance between clusters II and III (GD = 0.02400). The GD between African wild species of cluster III was 0.01935 to 0.02213 (Table 5). Among them, C. sagittatus related most closely to C. melo accessions of cluster I (GD = 0.02205–0.02253) and then to C. metuliferus (GD = 0.02311–0.02387).
Fig. 1.
Neighbor-joining tree showing relationships of 67 accessions of Cucumis species from SNPs in nine chloroplast genome regions. Bootstrap values over 50% for 1000 replicates are shown beside the branch. Tendral: Tendral o Invernale a Buccia Verde.
Table 5.
Genetic distance (GD) between eight groups of Cucumis accessions, calculated from the frequency of nucleotide in 284 SNPs
| No. | Name of species | Cluster No. | No. of accession | No. | GDwithin groupa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||||
| 1 | C. melo | I | a | 16 | – | 0.00019 | |||||||
| 2 | b | 26 | 0.00132 | – | 0 | ||||||||
| 3 | c | 18 | 0.00426 | 0.00446 | – | 0.00084 | |||||||
| 4 | C. hystrix | II | 3 | 0.01692 | 0.01701 | 0.01721 | – | 0.00012 | |||||
| 5 | C. sativus | 1 | 0.01888 | 0.01896 | 0.01950 | 0.00974 | – | nc | |||||
| 6 | C. sagittatus | III | 1 | 0.02205 | 0.02253 | 0.02233 | 0.02145 | 0.02350 | – | nc | |||
| 7 | C. metuliferus | 1 | 0.02311 | 0.02358 | 0.02387 | 0.02330 | 0.02566 | 0.02200 | – | nc | |||
| 8 | C. anguria | 1 | 0.02380 | 0.02407 | 0.02440 | 0.02534 | 0.02636 | 0.02213 | 0.01935 | – | nc | ||
GD not calculated within a group is indicated by “nc”.
Melon accessions of cluster I were divided into three subclusters (Fig. 1 and Table 4). Subclusters Ia, Ib and Ic comprised 16, 26 and 18 accessions, respectively. The GD between subclusters Ia and Ic and between subclusters Ib and Ic were 0.00426 and 0.00446, respectively (Table 5). In contrast, the GD between subclusters Ia and Ib was 0.00132, indicating that subcluster Ic was rather distinct from subclusters Ia and Ib. The alignment of their sequences showed that melon accessions of subcluster Ic were uniquely characterized by eight SNPs (SNP3, 10, 12, 14, 16, 20, 27, 28) and subclusters Ia and Ib were unique by seven SNPs (SNP5, 6, 12, 13, 17, 26, 30) (Table 4). Differentiation between subcluster Ic and subclusters Ia and Ib was also detected by seven additional polymorphisms (InDel5; SSR1, 3, 5, 10, 11, 12). The uniqueness of subclusters Ia and Ib were represented by three sites (SNP18, 19; SSR4) and five sites (SNP2, 8, 9, 11; SSR7), respectively.
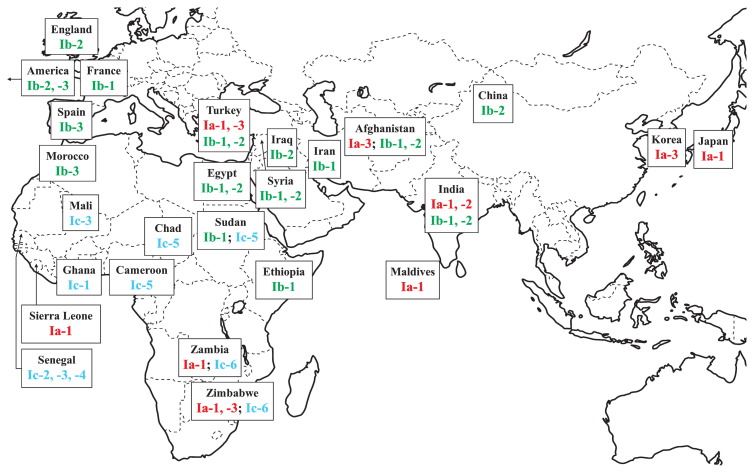
Genetic composition of melon groups
The eighteen melon accessions of subcluster Ic were all landraces of African origin (Fig. 2) and were divided into six subgroups (Ic-1, -2, -3, -4, -5, -6) based on the polymorphism at 20 sites (Table 4). Subgroups Ic-5 and Ic-6, which differed at InDel2, were separated from the other subgroups by four SNPs (SNP1, 15, 22, 23), one indel (InDel4) and five SSRs (SSR4, 6, 7, 11, 12). Subgroups Ic-1, Ic-2, Ic-3 and Ic-4 were classified by four SNPs (SNP4, 7, 21, 24), two indels (InDel2, 3) and five SSRs (SSR2, 3, 4, 8, 9). The GD within subcluster Ic, corresponding to nucleotide diversity (π) according to Nei and Li (1979), was 0.00084 (Table 5).
Fig. 2.
Geographical distribution of cytoplasm types in melon landraces. Cluster number refers to Fig. 1.
Twenty-six melon accessions of subcluster Ib comprised accessions from USA and Europe (UK, France, Spain), northern Africa (Sudan, Ethiopia, Morocco, Egypt), West and Central Asia (Turkey, Syria, Iraq, Iran, Afghanistan), South Asia (India) and East Asia (China, Fig. 2). The chloroplast genome sequence of these melon accessions was identical for over 5000 bp, except polymorphic sites SSR6 and Indel1, by which subcluster Ib was divided into subgroups Ib-1, -2 and -3 (Fig. 1 and Table 4). Twelve accessions of subgroup Ib-2 were unique in the repeat number of SSR motif (T) at SSR6, and three accessions of subgroup Ib-3 had a deletion of 5 bp (ATATT) at Indel1.
Subcluster Ia comprised 16 melon landraces from West and Southern Africa (Sierra Leone, Zambia, Zimbabwe), West and Central Asia (Turkey, Afghanistan), South Asia (Maldives, India) and East Asia (Korea, Japan) (Fig. 2). These melon accessions were divided into subgroup Ia-1 and subgroups Ia-2 and Ia-3 by SNP25 and 29 in Cmcp11 and the latter two subgroups differed by SSR4 in Cmcp3 (Table 4). The GD within subcluster Ia was 0.00019 (Table 5).
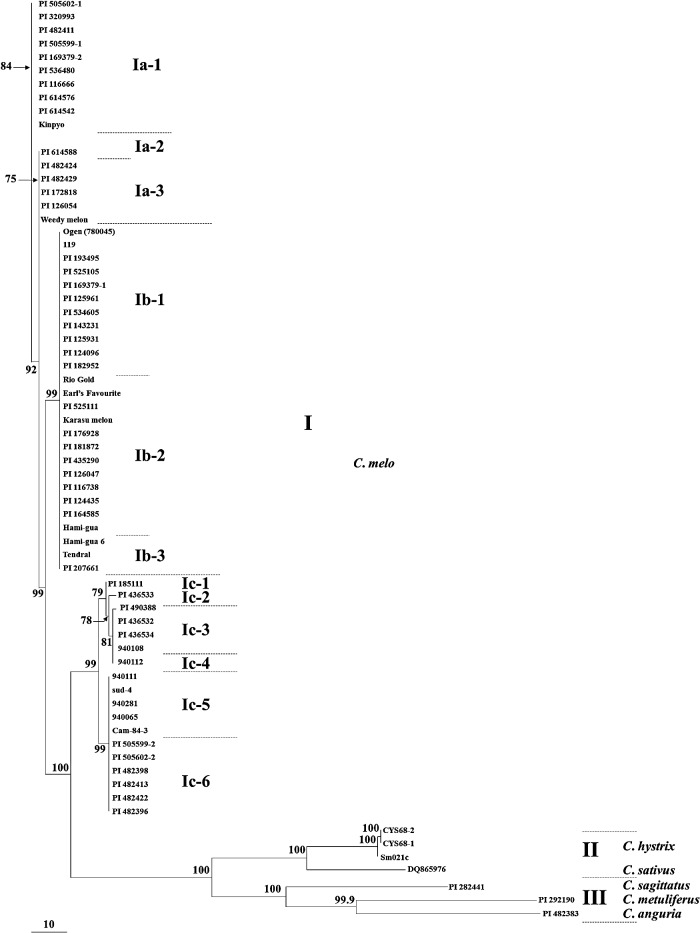
By combining the sequences of the nine regions, four parsimony trees were retained based on 133 parsimony informative sites; consistency index and retention index showed higher values which was 0.9290 and 0.9712, respectively. These four trees were the same as the NJ tree on the diagram between melon and other Cucumis species and among subcluster Ia, Ib and Ic (Figs. 1, 3).
Fig. 3.
Parsimony tree showing relationships of 67 accessions of Cucumis species, from SNPs of nine chloroplast genome regions. Length = 330 steps; consistency index = 0.9290; retention index = 0.9712. Bootstrap values for 1000 replicates are shown beside the branch; values less than 50% are not shown. Tendral: Tendral o Invernale a Buccia Verde.
Worldwide distribution patterns of large- and small-seed types
Melon accessions (7,396) in the GRIN database were divided into large- and small-seed melon based on the 100 seed weight (Table 6). The frequency of large-seed melon was 93.7% (1,782/1,901 accessions) in Europe, USA and Russia and 96.9% (2,538/2,619 accessions) in West and Central Asia. The frequency of small-seed melon increased from West Asia towards the east from 51.4% (1,092/2,123 accessions) in South Asia to 62.2% (194/312 accessions) in East Asia. In Africa, the frequency of large-seed melon differed among areas at 98.6% in northern Africa (275/279 accessions) and 26.4% in southern Africa (32/121 accessions), respectively. These findings demonstrate geographical differences in seed size: large-seed melon is mainly cultivated in USA, Europe, West and Central Asia and northern Africa and small-seed melon is frequent in southern Africa, South and East Asia. Indian melon was diversified, and both large- and small-seed melon showed similar frequencies.
Table 6.
Varietal and geographical variation of 100 seeds weight among 7,396 accessions of melon
| Area | Country | No. of accessions | 100 seeds weight (g)a | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||||||||||
| 0– | 0.5– | 1.0– | 1.5– | 2.0– | 2.5– | 3.0– | 3.5– | 4.0– | 4.5– | 5.0– | 5.5– | 6.0– | 6.5– | 7.0– | 7.5– | 8.0– | 8.5– | 9.0– | 9.5– | |||
|
|
|
|||||||||||||||||||||
| Small-seed type | Large-seed type | |||||||||||||||||||||
| North America | 224 | – | 40 | 8 | 7 | 14 | 35 | 59 | 43 | 10 | 5 | 1 | – | – | – | 1 | – | – | – | 1 | – | |
| Central America | 40 | – | 2 | 7 | 4 | 2 | 5 | 5 | 1 | 6 | 5 | 1 | 1 | – | – | – | 1 | – | – | – | – | |
| South America | 39 | – | 2 | 1 | – | – | – | 6 | 9 | 7 | 12 | 1 | 1 | – | – | – | – | – | – | – | – | |
|
| ||||||||||||||||||||||
| Europe | 1,520 | 1 | – | 1 | 7 | 19 | 51 | 125 | 213 | 294 | 271 | 235 | 144 | 88 | 37 | 21 | 8 | 3 | 1 | 1 | – | |
| Russia/Former Soviet Union | 78 | – | – | 1 | – | 3 | 9 | 12 | 14 | 19 | 14 | 3 | – | 2 | 1 | – | – | – | – | – | – | |
| West and Central Asia | Turkey | 660 | – | – | 1 | 5 | 15 | 37 | 78 | 133 | 157 | 110 | 72 | 32 | 11 | 4 | 4 | – | 1 | – | – | – |
| Afghanistan | 962 | – | 2 | 2 | 1 | 9 | 30 | 59 | 98 | 157 | 205 | 170 | 135 | 61 | 19 | 5 | 5 | 2 | – | 1 | 1 | |
| Other | 997 | – | 7 | 1 | 10 | 28 | 44 | 83 | 130 | 157 | 205 | 134 | 107 | 49 | 22 | 12 | 6 | 1 | 1 | – | – | |
|
| ||||||||||||||||||||||
| Africa | North | 279 | – | – | 1 | – | 3 | 4 | 9 | 34 | 49 | 71 | 52 | 33 | 21 | 1 | 1 | – | – | – | – | – |
| West and Center | 23 | – | – | 5 | 3 | – | – | 1 | 5 | 4 | 3 | 2 | – | – | – | – | – | – | – | – | – | |
| South | 121 | – | 1 | 14 | 39 | 35 | 23 | 5 | 2 | 2 | – | – | – | – | – | – | – | – | – | – | – | |
|
| ||||||||||||||||||||||
| South Asia | Pakistan | 73 | – | – | 2 | – | 7 | 15 | 21 | 8 | 10 | 4 | 1 | 2 | 2 | – | 1 | – | – | – | – | – |
| India | 1,999 | 1 | 57 | 202 | 291 | 501 | 378 | 269 | 142 | 101 | 44 | 10 | 3 | – | – | – | – | – | – | – | – | |
| Maldives | 33 | – | 19 | 2 | 1 | 1 | 5 | 4 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | |
| Myanmar | 18 | – | – | 6 | 2 | – | 1 | 5 | 3 | 1 | – | – | – | – | – | – | – | – | – | – | – | |
| Southeast Asia | 18 | – | 2 | 2 | 1 | 3 | 2 | 2 | 2 | 4 | – | – | – | – | – | – | – | – | – | – | – | |
|
| ||||||||||||||||||||||
| East Asia | China | 202 | – | – | 23 | 55 | 19 | 7 | 19 | 18 | 21 | 17 | 9 | 10 | 2 | 2 | – | – | – | – | – | – |
| Korea | 28 | – | – | 12 | 12 | 2 | 1 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | |
| Japan | 82 | – | 2 | 20 | 42 | 7 | 7 | 1 | 3 | – | – | – | – | – | – | – | – | – | – | – | – | |
|
| ||||||||||||||||||||||
| 7,396 | 2 | 134 | 311 | 480 | 668 | 654 | 764 | 859 | 999 | 966 | 691 | 468 | 236 | 86 | 45 | 20 | 7 | 2 | 3 | 1 | ||
The data of 100 seeds weight was obtained from the GRIN database of USDA-ARS (http://www.ars-grin.gov/npgs/).
Discussion
The nucleotide sequence of nine inter- and intra-genic regions of the chloroplast genome, in total 5239 bp to 5479 bp, was compared for 66 Cucumis accessions, including wild species of C. sagittatus, C. anguria, C. metuliferus and C. hystrix with one reference accession of cucumber (C. sativus). Sequence polymorphism was successfully detected among the melon accessions (C. melo) (Table 4). Sixty melon accessions, including weedy melon, were separated into three subclusters of cytoplasm (types Ia, Ib and Ic) based on polymorphism at 47 sites (SNPs: 30, InDel: 5, SSR: 12). This is the first report to show intra-specific diversification of cytoplasm type within C. melo, while interspecific variation among 113 accessions including 17 melon accessions, was successfully shown by Sebastian et al. (2010). The NJ and parsimony trees showed the three cytoplasm types differentiated in the direction of (Ic, (Ib, Ia)) (Figs. 1, 3). These cytoplasm types were classified further into 12 cytoplasm types Ia (-1, -2, -3), Ib (-1, -2, -3) and Ic (-1, -2, -3, -4, -5, -6), by 3, 2 and 20 polymorphic sites, respectively (Table 4). The assumption is reasonable that these polymorphisms accumulated in the chloroplast genome after the differentiation of the three types in melon.
Groups Cantalupensis and Inodorus from Greece (Staub et al. 2004), Spain (López-Sesé et al. 2003) and Hungary (Szabó et al. 2005) have genetic affinities among themselves. Six accessions of Groups Cantalupensis and Inodorus, used in this study, were also classified together as the subcluster Ib which consists mainly of large-seed melon (Table 1). However, subcluster Ib was further divided into three subgroups, Ib-1, Ib-2 and Ib-3. Subgroups Ib-2 and Ib-3 were differentiated from subgroup Ib-1, independently, because of a sequence slippage ([T]11 → [T]10) at SSR6 (PS-ID region, Rpl16-Rpl14) in Ib-2 and a deletion of the motif sequence (ATATT) at InDel1 (PsbC-TrnS, ccSSR7) in Ib-3, respectively (Table 4). Melon accessions of subgroups Ib-1 and Ib-2 were distributed in diverse areas, such as northern Africa, West Asian countries and India (Fig. 2), suggesting that the cytoplasmic diversification in large-seed melon should have occurred before the spread of melon from Africa. Subgroup Ib-2 contained Groups Cantalupensis and Inodorus accessions; ‘Rio Gold’ (netted melon, USA) and ‘Earl’s Favourite’ (netted melon, UK) of Group Cantalupensis and ‘Hami-gua’ (China) of Group Inodorus. In contrast, Group Inodorus was not in subgroup Ib-1 and Group Cantalupensis was not in subgroup Ib-3. Although the number of melon accessions analyzed was limited, these results indicate that Groups Cantalupensis and Inodorus were established in independent maternal lines.
Group Conomon, cultivated in East and South Asia, was classified into subcluster Ia together with Group Agrestis from Asia (Table 1). Although only one accession of each group was tested in this study, Nhi et al. (2010) confirmed that 24 accessions of Groups Conomon and Agrestis from Vietnam and 14 accessions of Group Conomon from China uniformly had “A” at SNP18, which was a diagnostic sequence of subcluster Ia (Table 4). In contrast, all accessions of Group Agrestis from Africa were classified as subcluster Ic together with 10 accessions of African cultivated melon (Fig. 1 and Table 1). These results indicated that Group Agrestis, a companion weed in a crop field, is diversified in its maternal line and shares the same maternal line with cultivated melon in each area. Several studies on diversity in the nuclear genome have provided evidence that Group Agrestis shares a common gene pool with small-seed melon or landraces in each area (Akashi et al. 2002, McCreight et al. 2004, Nakata et al. 2005). Therefore, these results suggested that Group Agrestis was not an ancestral type of cultivated melon, but was established independently as an escape from cultivated melon in each area.
According to Pitrat (2008), Groups Conomon and Agrestis from Asia and Groups Cantalupensis and Inodorus from Europe and USA were classified as different subspecies, that is, subsp. agrestis and subsp. melo, respectively. Genetic differentiation between Groups Conomon and Agrestis from Asia and Groups Cantalupensis and Inodorus from Europe and USA has been clearly shown by analysis of nuclear genome markers (Aierken et al. 2011, Monforte et al. 2003, Stepansky et al. 1999). In this study, Asian melon with small seeds was classified as subcluster Ia by six SNPs (SNP 2, 8, 9, 11, 18, 19) and was genetically differentiated from European and US melon with large seeds in subcluster Ib (Fig. 1 and Table 4). Such a differentiation clearly indicated that Asian melon with small seed and US and European melon with large seed were established independently in different maternal lines. However, melon accessions of subcluster Ia also included large-seed melon from Turkey (PI 169379-2, PI 172818), Afghanistan (PI 126054) and India (PI 116666, PI 614588). Based on the results summarized in Table 6, North American, European, North African and West and Central Asian melon mainly consisted of large-seed type (≥2.5 g) and East Asian melon included small-seed type (<2.5 g). However in India, both large- and small-seed melons were at similar frequencies, as also reported by Tanaka et al. (2007). In our previous study, using dCAPS marker of SNP18, 71 melon accessions from South Asia were found to be a mixture comprising not only large-seed melon of subgroup Ib and small-seed melon of subgroup Ia but also large-seed melon of subgroup Ia and small-seed melon of subgroup Ib (Tanaka et al. 2006). In the present study, among the Indian melon accessions, large-seed accessions PI 116666 and PI 614588 were classified as subcluster Ia and small-seed accession PI 116738 was classified as sub-cluster Ib (Fig. 1). These results suggested that spontaneous hybridization between large- and small-seed types occurred in India and resulted in establishing large-seed melon of sub-cluster Ia and small-seed melon of subcluster Ib.
Africa is considered the center of evolution of Cucumis species, and archaeological remains and historical records hypothetically infer the origin of cultivated melon in Africa (Bates and Robinson 1995, Robinson and Decker-Walters 1997). Twenty-nine accessions of African melon were diversified and were assigned to 11 cytoplasm types, among which five cytoplasm types (subclusters Ia and Ib) were commonly found also in other continents and the other six cytoplasm types (subcluster Ic) were unique to Africa (Fig. 2). Although the number and area coverage of African melon landraces examined is limited, the geographical distribution pattern clearly differed between the cytoplasm types. Melon accessions of subcluster Ib, which included large-seed melon from Europe and USA, were only in northern Africa, such as Egypt, Morocco, Ethiopia and Sudan. In contrast, melon accessions of subcluster Ia, which included Asian small-seed melon, were mainly in Southern Africa, such as Zambia and Zimbabwe. Noteworthy is that the African accessions included five subgroups of subclusters Ia and Ib. Melon accessions of subcluster Ic were endemic to Western, Central and Southern Africa, and those of subgroup Ic-6 were from Southern Africa (Zambia and Zimbabwe). Similar geographical variation was observed in seed size. Three of four melon accessions (75%) were large-seed melon from northern Africa, and 78.6% and 100% of melon accessions were small-seed melon from West and Central Africa and Southern Africa, respectively (Table 1). These results may suggest that large-seed melon Groups Cantalupensis and Inodorus were established from northern African melon landraces in maternal line Ib.
Groups Conomon and Agrestis, small-seed melon commonly found in Asia, share the same maternal line with Southern African small-seed melon, even though these two regions are geographically distant. RAPD polymorphism indicated genetic differentiation between melon landraces from northern and Southern Africa, which are related closely to European and US melon and East Asian melon, respectively (Mliki et al. 2001). Nakata et al. (2005) also indicated a genetic relationship between South African melon and East Asian melon. The third cytoplasm type, Ic, differed distinctly from cytoplasm types Ia (GD = 0.00426) and Ib (GD = 0.00445) (Table 5), which suggests that the cultivated melon accessions of subcluster Ic were established independently from those of subclusters Ia and Ib. Further studies using more accessions of African melon are required to validate these hypotheses on the multiple origin of cultivated melon.
In this study, we analyzed one reference accession of C. sativus and six accessions of wild species C. hystrix, C. anguria, C. sagittatus and C. metuliferus (Table 1). These species are genetically close to C. melo from analysis of chromosome pairing (Dane et al. 1980), pollen-pistil interaction (Kho et al. 1980), self-incompatibility (Deakin et al. 1971, Den Nijis and Visser 1985) and seed fertility of F1 hybrids (Norton and Granberry 1980, Singh and Yadava 1984). Isozyme analysis (Perl-Treves et al. 1985), RFLP analysis (Perl-Treves and Galun 1985), southern blotting analysis of satellite DNA (Helm and Hemleben 1997) and sequence analysis of the ITS region (Garcia-Mas et al. 2004) indicate that C. melo is genetically related to C. sagittatus and C. metuliferus, rather than C. anguria. However, analysis of the chloroplast genome indicated that C. melo is more closely related to Asian species of Cucumis, such as C. sativus and C. hystrix, compared with African wild species, even though the chromosome number differs between C. melo and C. sativus (Chung et al. 2006, Renner et al. 2007). In this study, cluster I of only melon accessions was related more closely to cluster II of C. sativus and C. hystrix and then to cluster III of C. sagittatus, C. metuliferus and C. anguria (Figs. 1, 3 and Table 5). These phylogenetic relationships supported the previous results of Chung et al. (2006) and Renner et al. (2007) and provided an approximate relationship between Cucumis species. However, the genetic distance was not small, even between C. melo and C. hystrix (Table 5) and thus identifying the direct wild ancestor of cultivated melon was difficult. C. picrocarpus from Australia is related more closely to melon than to C. hystrix (Sebastian et al. 2010). Intra-specific variation of the chloroplast genome sequence, detected for C. melo, C. hystrix (Table 4) and C. metuliferus (Rennar et al. 2007), may provide important clues, and thus intra-specific variation of the chloroplast genome should be surveyed for each Cucumis species.
Acknowledgments
The authors thanks Dr. K.R. Reitsma, Iowa State University, USA, Dr. N. Fujishita, former Osaka Prefecture University, Japan, and Dr. Y. Sakata, National Agricultural Research Center for Kyushu Okinawa Region, for kindly supplying the seeds. This study was partly supported by the Sasakawa Scientific Research Grant from the Japan Science Society (No. 17-218), the Japan Society for the Promotion of Science (JSPS) Asian CORE Program entitled “Cooperative Research and Educational Center for Important Plant Genetic Resources in East Asia”, the Special Research of Hirosaki University entitled “Research Project for Promoting the Utilization of Archeological Remains”, and the JSPS Grant-in-Aid for Scientific Research (A) (No. 23255007) and Grant-in-Aid for Young Scientists (B) (No. 23701015). This is contribution number XXI from the Sato Project of the Research Institute for Humanity and Nature (RIHN), Japan.
Literature Cited
- Aierken, Y., Akashi, Y., Nhi, P.T.P., Halidan, Y., Tanaka, K., Long, B., Nishida, H., Long, C., Wu, M.Z., and Kato, K. (2011) Molecular analysis of the genetic diversity of Chinese Hami melon and its relationship to the melon germplasm from Central and South Asia. J. Jpn. Soc. Hort. Sci. 80: 52–65 [Google Scholar]
- Akashi, Y., Fukuda, N., Wako, T., Masuda, M., and Kato, K. (2002) Genetic variation and phylogenetic relationships in East and South Asian melons, Cucumis melo L., based on the analysis of five isozymes. Euphytica 125: 385–396 [Google Scholar]
- Akashi, Y., Tanaka, K., Nishida, H., Kato, K., Khaing, M.T., Yi, S.S., and Chou, T.T. (2006) Genetic diversity and phylogenetic relationship among melon accessions from Africa and Asia revealed by RAPD analysis. In: Holmes, G.J. (ed.) Proceedings of Cucurbitaceae 2006 Asheville, North Carolina, USA, September 17–21, pp. 317–325 [Google Scholar]
- Bates, D.M., and Robinson, R.W. (1995) Cucumbers, melons and water-melons. In: Smartt, J., and Simmonds, N.W. (eds.) Evolution of crop plants, 2nd ed., Longman Scientific, Harlow, pp. 89–96 [Google Scholar]
- Chung, S.M., and Staub, J.E. (2003) The development and evaluation of consensus chloroplast primer pairs that possess highly variable sequence regions in a diverse array of plant taxa. Theor. Appl. Genet. 107: 757–767 [DOI] [PubMed] [Google Scholar]
- Chung, S.M., Staub, J.E., and Chen, J.F. (2006) Molecular phylogeny of Cucumis species as revealed by consensus chloroplast SSR marker length and sequence variation. Genome 49: 219–229 [DOI] [PubMed] [Google Scholar]
- Clegg, M.T., Gaut, B.S., Learn, G.H., and Morton, B.R. (1994) Rates and patterns of chloroplast DNA evolution. Proc. Nat. Acad. Sci. USA 91: 6795–6801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dane, F., Denna, D.W., and Tsuchiya, T. (1980) Evolutionary studies of wild species in the genus Cucumis. Z PFLANZENZUECHT 85: 89–109 [Google Scholar]
- Deakin, J.R., Bohn, G.W., and Whitaker, T.W. (1971) Interspecific hybridization in Cucumis. Econ. Bot. 25: 195–211 [Google Scholar]
- Den Nijis, A.P.M., and Visser, D.L. (1985) Relationships between African species of the genus Cucumis L. estimated by the production, vigour and fertility of F1 hybrids. Euphytica 34: 279–290 [Google Scholar]
- Fujishita, N. (1983) Genetic diversity and phylogenetic differentiation in melon. Curr. Top. Plant Breed. 24: 3–21 [Google Scholar]
- Fujishita, N., Furukawa, H., and Morii, S. (1993) Distribution of three genotypes for bitterness of F1 immature fruit in Cucumis melo. Jpn.J. Breed. 43 (Suppl. 2): 206 [Google Scholar]
- Garcia, E., Jamilena, M., Alvarez, J.I., Arnedo, T., Oliver, J.L., and Lozano, R. (1998) Genetic relationships among melon breeding lines revealed by RAPD markers and agronomic traits. Theor. Appl. Genet. 96: 878–885 [Google Scholar]
- Garcia-Mas, J., Monforte, A.J., and Arús, P. (2004) Phylogenetic relationships among Cucumis species based on the ribosomal internal transcribed spacer sequence and microsatellite markers. Plant Syst. Evol. 248: 191–203 [Google Scholar]
- Helm, M.A., and Hemleben, V. (1997) Characterization of a new prominent satellite DNA of Cucumis metuliferus and differential distribution of satellite DNA in cultivated and wild species of Cucumis and in related genera of Cucurbitaceae. Euphytica 94: 219–226 [Google Scholar]
- Hiratsuka, J., Shimada, H., Whittier, R., Ishibashi, T., Sakamoto, M., Mori, M., Kondo, C., Honji, Y., Sun, C.R., and Meng, B.Y. (1989) The complete sequence of the rice (Oryza sativa) chloroplast genome: Intermolecular recombination between distinct tRNA(G)-genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol. Gen. Genet. 217: 185–194 [DOI] [PubMed] [Google Scholar]
- Ishikawa, R., Nakamura, I., Nishihara, T., Kikuchi, M., Senda, M., Akada, S., Harada, T., and Niizeki, M. (2002a) Origin of cytoplasm substituted rice cultivars found in Japan. Theor. Appl. Genet. 105: 608–613 [DOI] [PubMed] [Google Scholar]
- Ishikawa, R., Sato, Y.I., Tang, T., and Nakamura, I. (2002b) Different maternal origins of Japanese lowland and upland rice populations. Theor. Appl. Genet. 104: 976–980 [DOI] [PubMed] [Google Scholar]
- Jeffrey, C. (1980) A review of the Cucurbitaceae. Bot. J. Linn. Soc. 81: 233–247 [Google Scholar]
- Kho, Y.O., Den Nijs, A.P.M., and Franken, J. (1980) Interspecific hybridization in Cucumis L. II. The crossability of species, an investigation of in vivo pollen tube growth and seed set. Euphytica 29: 661–672 [Google Scholar]
- Liu, L., Kakihara, F., and Kato, M. (2004) Characterization of six varieties of Cucumis melo L. based on morphological and physiological characters, including shelf-life of fruit. Euphytica 135: 305–313 [Google Scholar]
- López-Sesé, A.I., Staub, J.E., and Gómez-Guillamón, M.L. (2003) Genetic analysis of Spanish melon (Cucumis melo L.) germplasm using a standardized molecular-marker array and geographically diverse reference accessions. Theor. Appl. Genet. 108: 41–52 [DOI] [PubMed] [Google Scholar]
- McCreight, J.D., Staub, J.E., López-Sesé, A.I., and Chung, S.M. (2004) Isozyme variation in Indian and Chinese melon (Cucumis melo L.) germplasm collections. J. Am. Soc. Hort. Sci. 129: 811–818 [Google Scholar]
- Mliki, A., Staub, J.E., Zhangyong, S., and Ghorbel, A. (2001) Genetic diversity in melon (Cucumis melo L.): An evaluation of African germplasm. Genet. Resour. Crop Evol. 48: 587–597 [Google Scholar]
- Monforte, A.J., Garcia-Mas, J., and Arús, P. (2003) Genetic variability in melon based on microsatellite variation. Plant Breed. 122: 153–157 [Google Scholar]
- Murray, G.C., and Thompson, W.F. (1980) Rapid isolation of high molecular weight DNA. Nucleic Acids Res. 8: 4321–4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, I., Kameya, N., Kato, Y., Yamanaka, S.I., Jomori, H., and Sato, Y. (1997) A proposal for identifying the short ID sequence which addresses the plastid subtype of higher plants. Breed. Sci. 47: 385–388 [Google Scholar]
- Nakata, E., Staub, J.E., López-Sesé, A.I., and Katzir, N. (2005) Genetic diversity in Japanese melon (Cucumis melo L.) as assessed by random amplified polymorphic DNA and simple sequence repeat markers. Genet. Resour. Crop Evol. 52: 405–419 [Google Scholar]
- Nei, M., and Li, W.H. (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 76: 5269–5273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nhi, P.T.P., Akashi, Y., Hang, T.T.M., Tanaka, K., Aierken, Y., Yamamoto, T., Nishida, H., Long, C., and Kato, K. (2010) Genetic diversity in Vietnamese melon landraces revealed by the analyses of morphological traits and nuclear and cytoplasmic molecular markers. Breed. Sci. 60: 255–266 [Google Scholar]
- Norton, J.D., and Granberry, D.M. (1980) Characteristics of progeny from an interspecific cross of Cucumis melo with C. metuliferus. J. Am. Soc. Hort. Sci. 105: 174–180 [Google Scholar]
- Palmer, J.D. (1985) Comparative organization of chloroplast genomes. Annu. Rev. Genet. 19: 325–354 [DOI] [PubMed] [Google Scholar]
- Perl-Treves, R., and Galun, E. (1985) The Cucumis plastome: physical map, intrageneric variation and phylogenetic relationships. Theor. Appl. Genet. 71: 417–429 [DOI] [PubMed] [Google Scholar]
- Perl-Treves, R., Zamir, D., Navot, N., and Galun, E. (1985) Phylogeny of Cucumis based on isozyme variability and its comparison with plastome phylogeny. Theor. Appl. Genet. 71: 430–436 [DOI] [PubMed] [Google Scholar]
- Pitrat, M. (2008) Melon. In: Prohens, J., and Nuez, F. (eds.) Handbook of plant breeding. Vegetables I. Asteraceae, Brassicaceae, Chenopoidicaceae, and Cucurbitaceae, Springer, USA, pp. 283–315 [Google Scholar]
- Provan, J., Soranzo, N., Wilson, N.J., Goldstein, D.B., and Powell, W. (1999) A low mutation rate for chloroplast microsatellites. Genetics 153: 943–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provan, J., Powell, W., and Hollingsworth, P.M. (2001) Chloroplast microsatellites: new tools for studies in plant ecology and evolution. Trends Ecol. Evol. 16: 142–147 [DOI] [PubMed] [Google Scholar]
- Reboud, X., and Zeyl, C. (1994) Organelle inheritance in plants. Heredity 72: 132–140 [Google Scholar]
- Renner, S.S., Schaefer, H., and Kocyan, A. (2007) Phylogenetics of Cucumis (Cucurbitaceae): Cucumber (C. sativus) belongs in an Asian/Australian clade far from melon (C. melo). BMC Evol. Biol. 7: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, R.W., and Decker-Walters, D.S. (1997) Major and minor crops. In: Robinson, R.W., and Decker-Walters, D.S. (eds.) Cucurbits. Crop production science in horticulture series 6, Cab International, New York, pp. 58–112 [Google Scholar]
- Schaefer, H., and Renner, S.S. (2011) Cucurbitaceae. In: Kubitzki,, K. (ed.) The families and genera of vascular plants vol. 10, Sapindales, Cucurbitales, Myrtaceae, Springer, Berlin, pp. 112–174 [Google Scholar]
- Sebastian, P., Schaefer, H., Telford, I.R.H., and Renner, S.S. (2010) Cucumber (Cucumis sativus) and melon (C. melo) have numerous wild relatives in Asia and Australia, and the sister species of melon is from Australia. Proc. Natl. Acad. Sci. USA 107: 14269–14273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, A.K., and Yadava, K.S. (1984) An analysis of interspecific hybrids and phylogenetic implications in Cucumis (Cucurbitaceae). Plant Syst. Evol. 147: 237–252 [Google Scholar]
- Staub, J.E., López-Sesé, A.I., and Fanourakis, N. (2004) Diversity among melon landraces (Cucumis melo L.) from Greece and their genetic relationships with other melon germplasm of diverse origins. Euphytica 136: 151–166 [Google Scholar]
- Stepansky, A., Kovalski, I., and Perl-Treves, R. (1999) Intraspecific classification of melons (Cucumis melo L.) in view of their phenotypic and molecular variation. Plant Syst. Evol. 217: 313–332 [Google Scholar]
- Swofford, D.L. (2002) PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4.0b10. Sinauer Associates, Sunderland, MA, USA [Google Scholar]
- Szabó, Z., Gyulai, G., Humphreys, M., Horváth, L., Bittsánszky, A., Lágler, R., and Heszky, L. (2005) Genetic variation of melon (C. melo) compared to an extinct landrace from the Middle Ages (Hungary) I. rDNA, SSR and SNP analysis of 47 cultivars. Euphytica 146: 87–94 [Google Scholar]
- Tamura, K., Nei, M., and Kumar, S. (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 101: 11030–11035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., and Kumar, S. (2011) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, K., Fukunaga, K., Akashi, Y., Nishida, H., Kato, K., and Khaing, M.T. (2006) Polyphyletic origin of cultivated melon inferred from analysis of its chloroplast genome. In: Holmes,, G.J. (ed.) Proceedings of Cucurbitaceae 2006, Asheville, North Carolina, USA, September 17–21, pp. 372–379 [Google Scholar]
- Tanaka, K., Nishitani, A., Akashi, Y., Sakata, Y., Nishida, H., Yoshino, H., and Kato, K. (2007) Molecular characterization of South and East Asian melon, Cucumis melo L., and the origin of Group Conomon var. makuwa and var. conomon revealed by RAPD analysis. Euphytica 153: 233–247 [Google Scholar]
- Thompson, J.D., Higgins, D.G., and Gibson, T.J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22: 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane, K., and Kawahara, T. (2005) Intra- and interspecific phylogenetic relationships among diploid Triticum-Aegilops species (Poaceae) based on base-pair substitutions, indels, and microsatellites in chloroplast noncoding sequences. Am. J. Bot. 92: 1887–1898 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.