Abstract
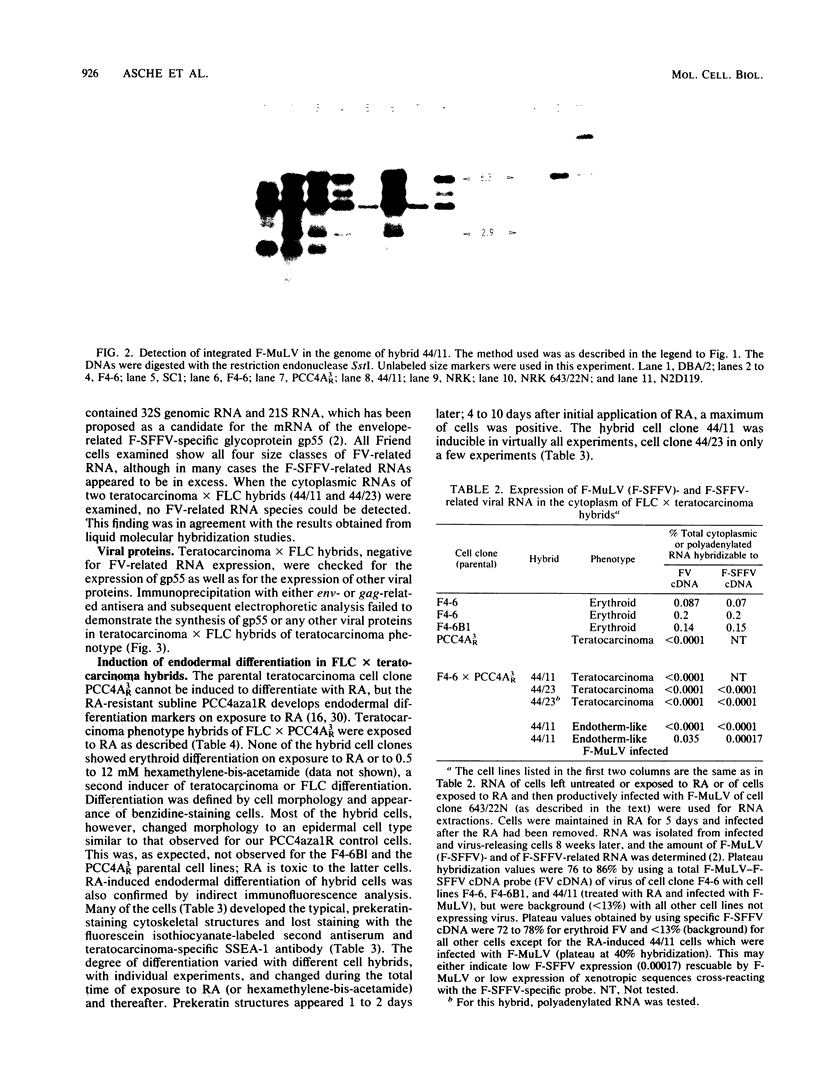
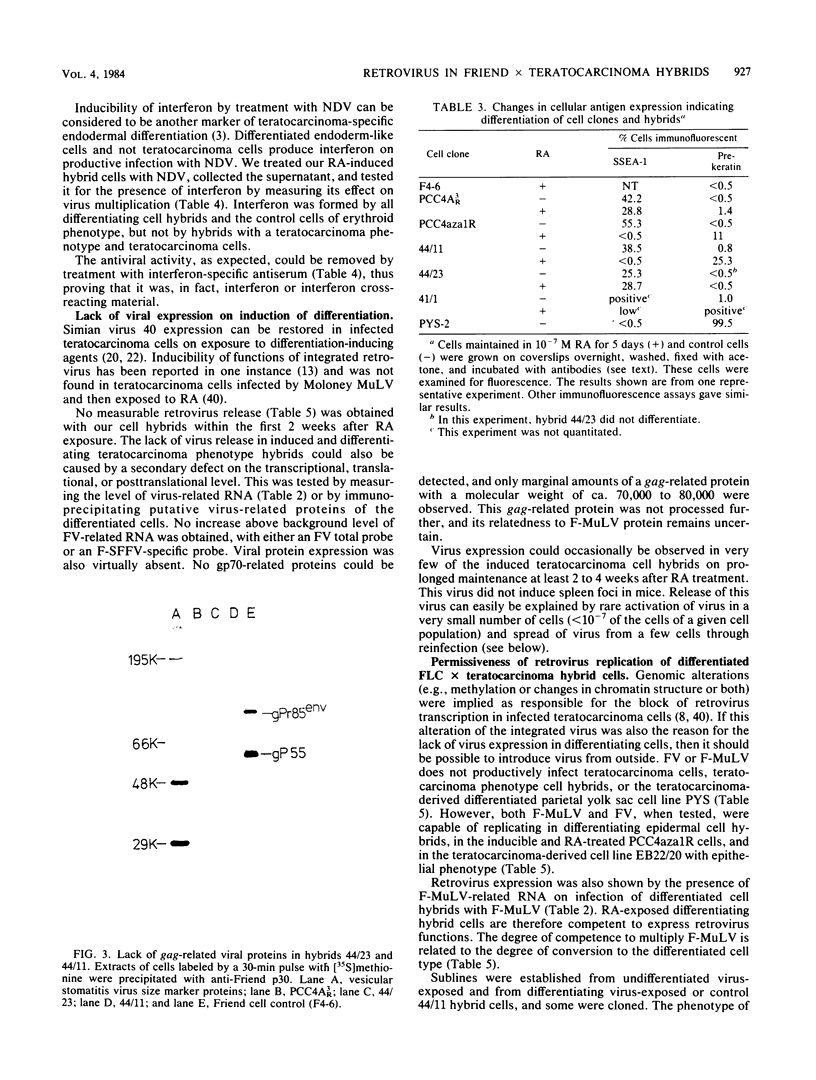
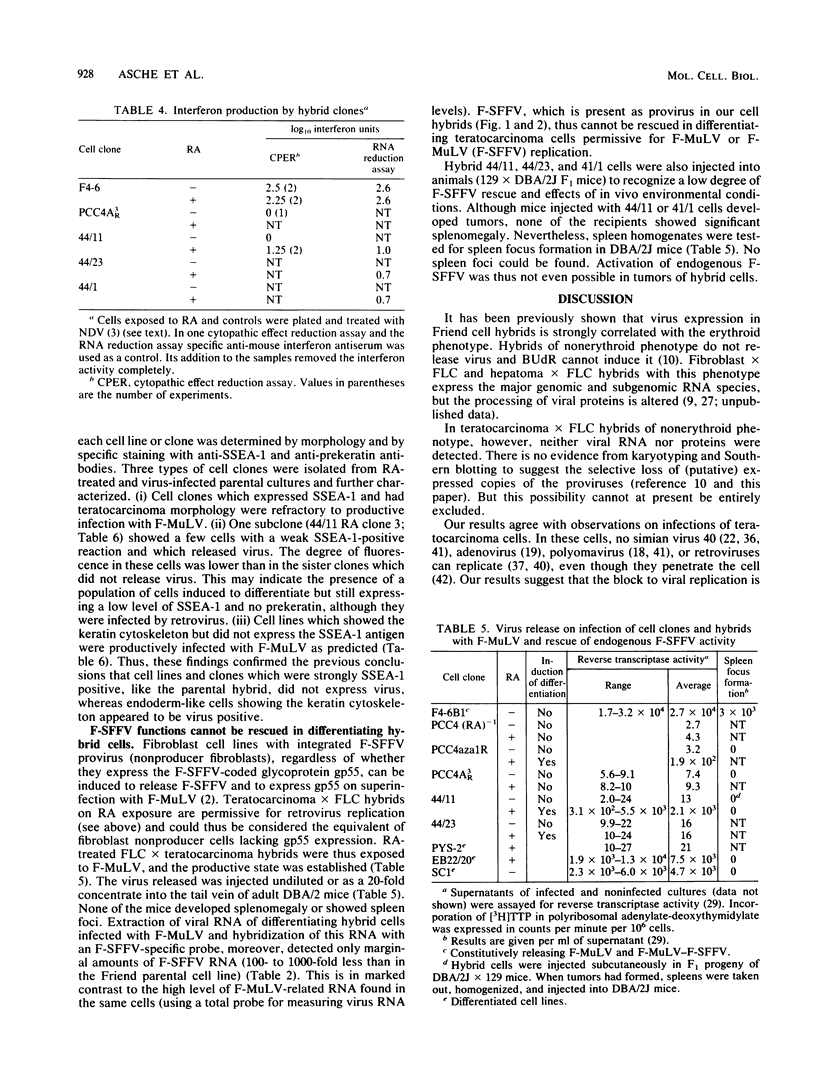
Two types of hybrids between cells with erythroid phenotype (Friend cells) and teratocarcinoma cells can be distinguished: cell hybrids with an erythroid phenotype, which release or can be induced to release large amounts of Friend spleen focus-forming virus (F-SFFV) on exposure to bromodeoxyuridine and cell hybrids with a teratocarcinoma phenotype, which do not release Friend virus and are not inducible for F-SFFV release. In this paper, we attempted to relate these differences to the expression of F-SFFV and Friend murine leukemia virus (F-MuLV) functions. Teratocarcinoma phenotype hybrids retained F-SFFV-and F-MuLV-related provirus sequences. They did not express F-SFFV- or F-MuLV-related RNA or proteins. The hybrids differentiated to endoderm-like cells on exposure to retinoic acid or hexamethylene-bis -acetamide. These cells, in contrast to the teratocarcinoma phenotype (uninduced) cells expressing SSEA-1-like antigens, did not express SSEA-1-like antigens; they formed typical, prekeratin-staining cytoskeletal structures and could be induced to release mouse interferon. The differentiating cells, but not the uninduced teratocarcinoma hybrids, were infected productively with F-MuLV or the F-MuLV--F-SFFV complex. They, however, did not express endogenous F-SFFV. Endogenous F-SFFV functions could not be rescued by infection with F-MuLV. Induction of teratocarcinoma hybrids with retinoic acid did not activate endogenous F-MuLV or F-SFFV transcription or protein synthesis. These data demonstrated two control mechanisms of Friend virus repression: one which acted trans during formation of the cell hybrids and was maintained only in teratocarcinoma phenotype cells and the other which acted cis and was still operative during induction of endodermal differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilello J. A., Colletta G., Warnecke G., Koch G., Frisby D., Pragnell I. B., Ostertag W. Analysis of the expression of spleen focus-forming virus (SFFV)-related RNA and gp55, a Friend and Rauscher virus-specific protein. Virology. 1980 Dec;107(2):331–344. doi: 10.1016/0042-6822(80)90301-3. [DOI] [PubMed] [Google Scholar]
- Burke D. C., Graham C. F., Lehman J. M. Appearance of interferon inducibility and sensitivity during differentiation of murine teratocarcinoma cells in vitro. Cell. 1978 Feb;13(2):243–248. doi: 10.1016/0092-8674(78)90193-9. [DOI] [PubMed] [Google Scholar]
- Deisseroth A., Bode U., Fontana J., Hendrick D. Expression of human alpha-globin genes in hybrid mouse erythroleukaemia cells depends on differentiated state of human donor cell. Nature. 1980 May 1;285(5759):36–38. doi: 10.1038/285036a0. [DOI] [PubMed] [Google Scholar]
- Fagg B., Ostertag W. Friend erythroleukemia virus complex: role of viral components in modifying erythroid differentiation in mice. J Natl Cancer Inst. 1982 Mar;68(3):457–460. [PubMed] [Google Scholar]
- Frisby D. P., Weiss R. A., Roussel M., Stehelin D. The distribution of endogenous chicken retrovirus sequences in the DNA of galliform birds does not coincide with avian phylogenetic relationships. Cell. 1979 Jul;17(3):623–634. doi: 10.1016/0092-8674(79)90270-8. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Wilson M. C. Delayed de novo methylation in teratocarcinoma suggests additional tissue-specific mechanisms for controlling gene expression. Nature. 1983 Jan 6;301(5895):32–37. doi: 10.1038/301032a0. [DOI] [PubMed] [Google Scholar]
- Greiser-Wilke I., Ostertag W., Goldfarb P., Lang A., Furusawa M., Conscience J. F. Inducibility of spleen focus-forming virus by BrdUrd is controlled by the differentiated state of the cell. Proc Natl Acad Sci U S A. 1981 May;78(5):2995–2999. doi: 10.1073/pnas.78.5.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbers K., Schnieke A., Stuhlmann H., Jähner D., Jaenisch R. DNA methylation and gene expression: endogenous retroviral genome becomes infectious after molecular cloning. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7609–7613. doi: 10.1073/pnas.78.12.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Huebner K., Tsuchida N., Green C., Croce C. M. A murine teratocarcinoma stem cell line carries suppressed oncogenic virus genomes. J Exp Med. 1979 Aug 1;150(2):392–405. doi: 10.1084/jem.150.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob H., Boon T., Gaillard J., Nicolas J., Jacob F. Tératocarcinome de la spuris: isolement, culture et propriétés de cellules a potentialités multiples. Ann Microbiol (Paris) 1973 Oct;124(3):269–282. [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E., Sherman M. I. Stimulation of differentiation of several murine embryonal carcinoma cell lines by retinoic acid. Exp Cell Res. 1979 Dec;124(2):381–391. doi: 10.1016/0014-4827(79)90213-1. [DOI] [PubMed] [Google Scholar]
- Jähner D., Stuhlmann H., Stewart C. L., Harbers K., Löhler J., Simon I., Jaenisch R. De novo methylation and expression of retroviral genomes during mouse embryogenesis. Nature. 1982 Aug 12;298(5875):623–628. doi: 10.1038/298623a0. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Bertolotti R., Ninio M., Weiss M. C. Short-lived cytoplasmic regulators of gene expression in cell cybrids. Nature. 1981 Apr 23;290(5808):717–720. doi: 10.1038/290717a0. [DOI] [PubMed] [Google Scholar]
- Katinka M., Vasseur M., Montreau N., Yaniv M., Blangy D. Polyoma DNA sequences involved in control of viral gene expression in murine embryonal carcinoma cells. Nature. 1981 Apr 23;290(5808):720–722. doi: 10.1038/290720a0. [DOI] [PubMed] [Google Scholar]
- Kelly F., Boccara M. Susceptibility of teratocarcinoma cells to adenovirus type 2. Nature. 1976 Jul 29;262(5567):409–411. doi: 10.1038/262409a0. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Pan S., Solter D., Linnenbach A., Croce C., Huebner K. Expression of H-2, laminin and SV40 T and TASA on differentiation of transformed murine teratocarcinoma cells. Nature. 1980 Dec 11;288(5791):615–618. doi: 10.1038/288615a0. [DOI] [PubMed] [Google Scholar]
- Lehman J. M., Speers W. C., Swartzendruber D. E., Pierce G. B. Neoplastic differentiation: characteristics of cell lines derived from a murine teratocarcinoma. J Cell Physiol. 1974 Aug;84(1):13–27. doi: 10.1002/jcp.1040840103. [DOI] [PubMed] [Google Scholar]
- Linnenbach A., Huebner K., Croce C. M. DNA-transformed murine teratocarcinoma cells: regulation of expression of simian virus 40 tumor antigen in stem versus differentiated cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4875–4879. doi: 10.1073/pnas.77.8.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney M. W. Hemoglobin synthesis in cell hybrids formed between teratocarcinoma and Friend erythroleukemia cells. Cell. 1977 Nov;12(3):653–662. doi: 10.1016/0092-8674(77)90265-3. [DOI] [PubMed] [Google Scholar]
- Morgan R. H., Henry J. A., Hooper M. L. Isolation of cell lines from differentiating embryonal carcinoma cultures. Exp Cell Res. 1983 Oct 15;148(2):461–473. doi: 10.1016/0014-4827(83)90167-2. [DOI] [PubMed] [Google Scholar]
- Mével-Ninio M., Weiss M. C. Immunofluorescence analysis of the time-course of extinction, reexpression, and activation of albumin production in rat hepatoma-mouse fibroblast heterokaryons and hybrids. J Cell Biol. 1981 Aug;90(2):339–350. doi: 10.1083/jcb.90.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa O., Yokota Y., Ishida H., Sugahara T. Independent mechanisms involved in suppression of the Moloney leukemia virus genome during differentiation of murine teratocarcinoma cells. Cell. 1983 Apr;32(4):1105–1113. doi: 10.1016/0092-8674(83)90294-5. [DOI] [PubMed] [Google Scholar]
- Ostertag W., Pragnell I. B. Differentiation and viral involvement in differentiation of transformed mouse and rat erythroid cells. Curr Top Microbiol Immunol. 1981;94-95:143–208. doi: 10.1007/978-3-642-68120-2_4. [DOI] [PubMed] [Google Scholar]
- Ostertag W., Roesler G., Krieg C. J., Kind J., Cole T., Crozier T., Gaedicke G., Steinheider G., Kluge N., Dube S. Induction of endogenous virus and of thymidine kinase by bromodeoxyuridine in cell cultures transformed by Friend virus. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4980–4985. doi: 10.1073/pnas.71.12.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostertag W., Vehmeyer K., Fagg B., Pragnell I. B., Paetz W., Le Bousse M. C., Smadja-Joffe F., Klein B., Jasmin C., Eisen H. Myeloproliferative virus, a cloned murine sarcoma virus with spleen focus-forming properties in adult mice. J Virol. 1980 Feb;33(2):573–582. doi: 10.1128/jvi.33.2.573-582.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulin D., Jakob H., Jacob F., Weber K., Osborn M. In vitro differentiation of mouse teratocarcinoma cells monitored by intermediate filament expression. Differentiation. 1982;22(2):90–99. doi: 10.1111/j.1432-0436.1982.tb01231.x. [DOI] [PubMed] [Google Scholar]
- Pragnell I. B., Fusco A., Arbuthnott C., Smadja-Joffe F., Klein B., Jasmin C., Ostertag W. Analysis of the myeloproliferative sarcoma virus genome: limited changes in the prototype lead to altered target cell specificity. J Virol. 1981 Jun;38(3):952–957. doi: 10.1128/jvi.38.3.952-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racevskis J., Koch G. Viral protein synthesis in Friend erythroleukemia cell lines. J Virol. 1977 Jan;21(1):328–337. doi: 10.1128/jvi.21.1.328-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S. K., Linemeyer D., Feild J., Troxler D., Scolnick E. M. Characterization of a protein found in cells infected with the spleen focus-forming virus that shares immunological cross-reactivity with the gp70 found in mink cell focus-inducing virus particles. J Virol. 1979 Jun;30(3):787–798. doi: 10.1128/jvi.30.3.787-798.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler J., Matthaei K. I., Sherman M. I. Isolation and characterization of mouse mutant embryonal carcinoma cells which fail to differentiate in response to retinoic acid. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1077–1080. doi: 10.1073/pnas.78.2.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal S., Khoury G. Differentiation as a requirement for simian virus 40 gene expression in F-9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5611–5615. doi: 10.1073/pnas.76.11.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekikawa K., Levine A. J. Isolation and characterization of polyoma host range mutants that replicate in nullipotential embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1100–1104. doi: 10.1073/pnas.78.2.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A. 1978 Nov;75(11):5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speers W. C., Birdwell C. R., Dixon F. J. Chemically induced bidirectional differentiation of embryonal carcinoma cells in vitro. Am J Pathol. 1979 Dec;97(3):563–584. [PMC free article] [PubMed] [Google Scholar]
- Stewart C. L., Stuhlmann H., Jähner D., Jaenisch R. De novo methylation, expression, and infectivity of retroviral genomes introduced into embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4098–4102. doi: 10.1073/pnas.79.13.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzendruber D. E., Lehman J. M. Neoplastic differentiation: interaction of simian virus 40 and polyoma virus with murine teratocarcinoma cells in vitro. J Cell Physiol. 1975 Apr;85(2 Pt 1):179–187. doi: 10.1002/jcp.1040850204. [DOI] [PubMed] [Google Scholar]
- Teich N. M., Weiss R. A., Martin G. R., Lowy D. R. Virus infection of murine teratocarcinoma stem cell lines. Cell. 1977 Dec;12(4):973–982. doi: 10.1016/0092-8674(77)90162-3. [DOI] [PubMed] [Google Scholar]
- Vasseur M., Katinka M., Herbomel P., Yaniv M., Blangy D. Physical and biological features of polyoma virus mutants able to infect embryonal carcinoma cell lines. J Virol. 1982 Sep;43(3):800–808. doi: 10.1128/jvi.43.3.800-808.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]