Abstract
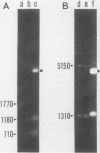
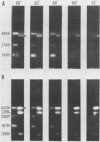
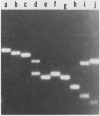
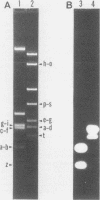
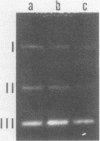
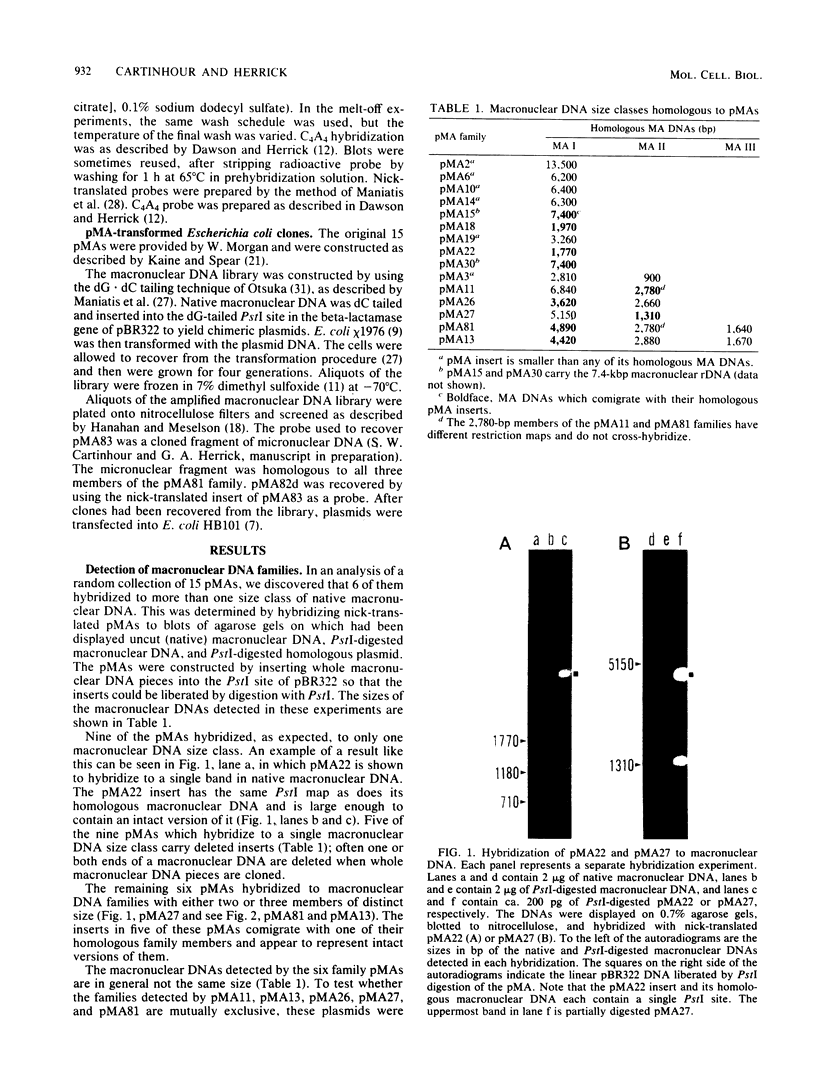
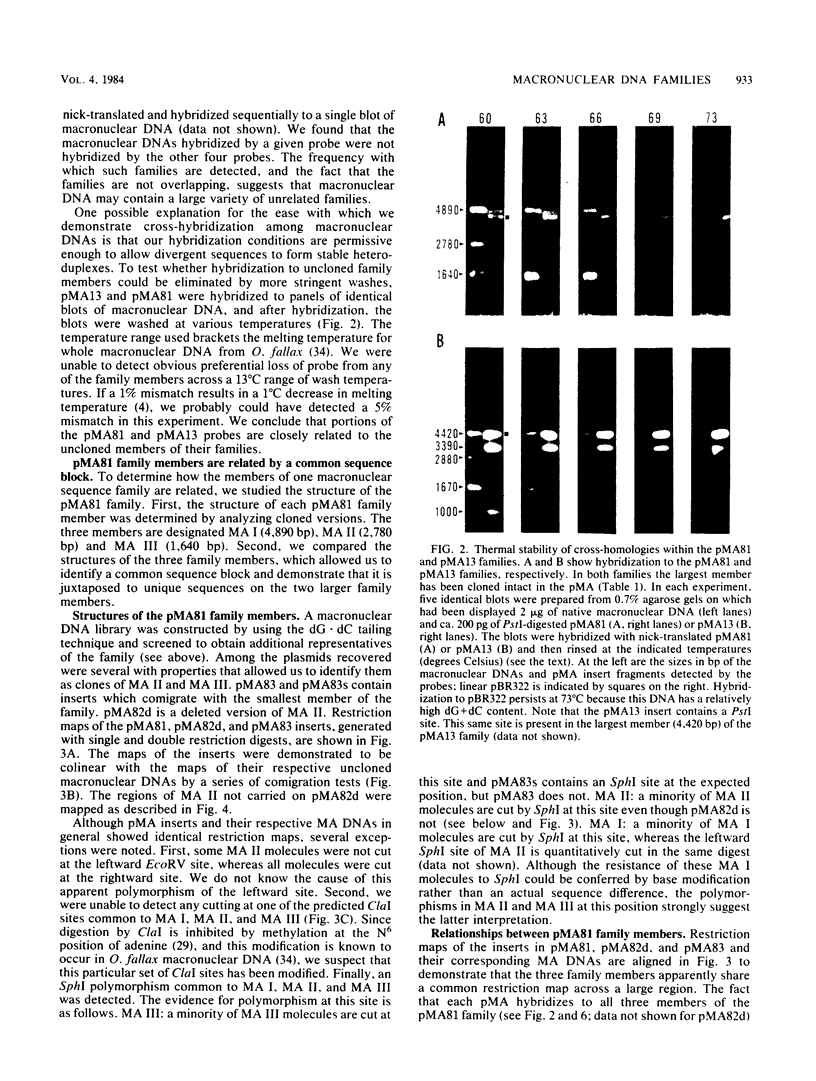
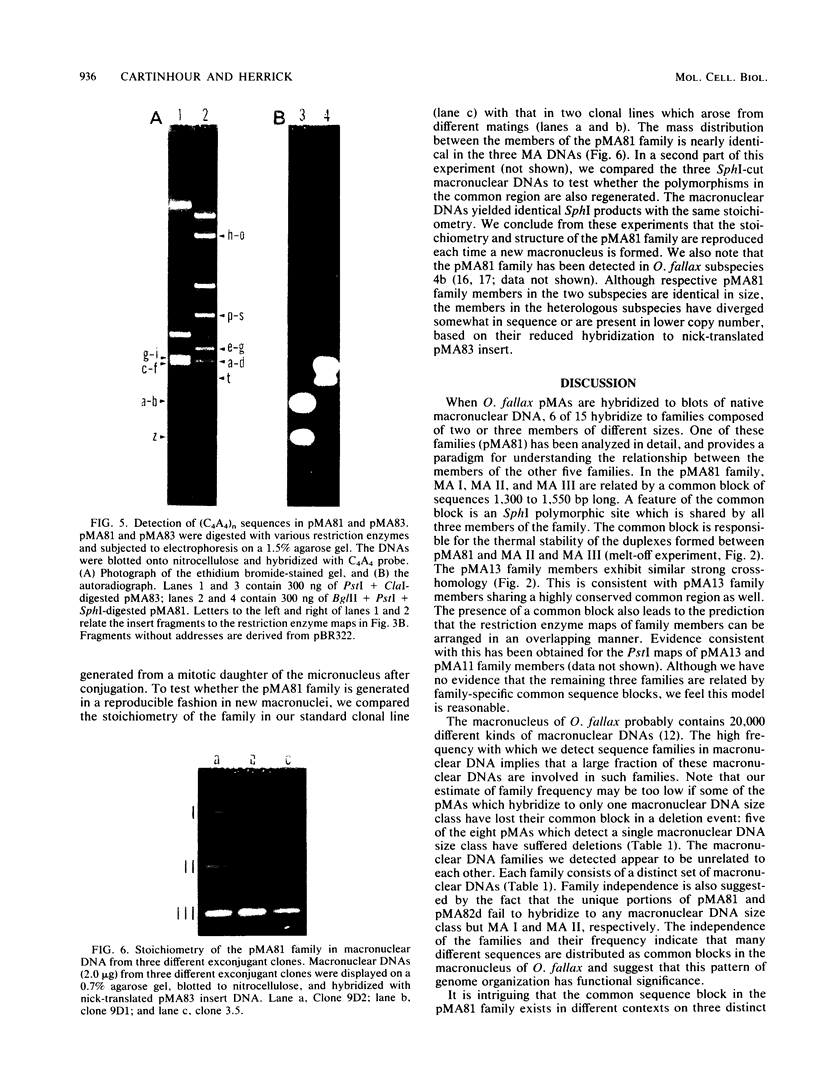
The three members of a cross-hybridizing family of macronuclear DNAs (4,890, 2,780, and 1,640 base pairs) from the protozoan Oxytricha fallax have in common a conserved sequence block 1,300 to 1,550 base pairs long. Adjacent to the common block in the two larger DNAs are sequences which are unique to them, whereas the smallest DNA contains few if any additional sequences. The family reappears when the macronucleus is replaced after conjugation and can be detected in another O. fallax subspecies. In a random collection of cloned macronuclear DNAs, 6 of 15 hybridize to macronuclear DNA families. This high frequency suggests that families sharing common sequence blocks have an important role in macronuclear function.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammermann D., Steinbrück G., von Berger L., Hennig W. The development of the macronucleus in the ciliated protozoan Stylonychia mytilus. Chromosoma. 1974 May 10;45(4):401–429. doi: 10.1007/BF00283386. [DOI] [PubMed] [Google Scholar]
- Ammermann D. The micronucleus of the ciliate Stylonychia mytilus; its nucleic acid synthesis and its function. Exp Cell Res. 1970 Jul;61(1):6–12. doi: 10.1016/0014-4827(70)90251-x. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Brenner D. J., Neufeld B. R., Britten R. J. Reduction in the rate of DNA reassociation by sequence divergence. J Mol Biol. 1973 Dec 5;81(2):123–135. doi: 10.1016/0022-2836(73)90184-8. [DOI] [PubMed] [Google Scholar]
- Borst P., Cross G. A. Molecular basis for trypanosome antigenic variation. Cell. 1982 Jun;29(2):291–303. doi: 10.1016/0092-8674(82)90146-5. [DOI] [PubMed] [Google Scholar]
- Boswell R. E., Jahn C. L., Greslin A. F., Prescott D. M. Organization of gene and non-gene sequences in micronuclear DNA of Oxytricha nova. Nucleic Acids Res. 1983 Jun 11;11(11):3651–3663. doi: 10.1093/nar/11.11.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R., Kant J. A. Organization of the rat gamma-fibrinogen gene: alternative mRNA splice patterns produce the gamma A and gamma B (gamma ') chains of fibrinogen. Cell. 1982 Nov;31(1):159–166. doi: 10.1016/0092-8674(82)90415-9. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Dawson D., Herrick G. Micronuclear DNA sequences of Oxytricha fallax homologous to the macronuclear inverted terminal repeat. Nucleic Acids Res. 1982 May 11;10(9):2911–2924. doi: 10.1093/nar/10.9.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsevier S. M., Lipps H. J., Steinbrück G. Histone genes in macronuclear DNA of the ciliate Stylonychia mytilus. Chromosoma. 1978 Dec 6;69(3):291–306. doi: 10.1007/BF00332133. [DOI] [PubMed] [Google Scholar]
- Grimes G. W., Hammersmith R. L. Analysis of the effects of encystment and excystment on incomplete doublets of Oxytricha fallax. J Embryol Exp Morphol. 1980 Oct;59:19–26. [PubMed] [Google Scholar]
- Hammersmith R. L., Grimes G. W. Effects of cystment on cells of Oxytricha fallax possessing supernumerary dorsal bristle rows. J Embryol Exp Morphol. 1981 Jun;63:17–27. [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Sloan J. S., Kelly J. D. A Drosophila metabolic gene transcript is alternatively processed. Cell. 1983 Sep;34(2):405–414. doi: 10.1016/0092-8674(83)90374-4. [DOI] [PubMed] [Google Scholar]
- Honjo T. Immunoglobulin genes. Annu Rev Immunol. 1983;1:499–528. doi: 10.1146/annurev.iy.01.040183.002435. [DOI] [PubMed] [Google Scholar]
- Kaine B. P., Spear B. B. Nucleotide sequence of a macronuclear gene for actin in Oxytricha fallax. Nature. 1982 Feb 4;295(5848):430–432. doi: 10.1038/295430a0. [DOI] [PubMed] [Google Scholar]
- Kaine B. P., Spear B. B. Putative actin genes in the macronucleus of Oxytricha fallax. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5336–5340. doi: 10.1073/pnas.77.9.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B. O., Yao M. C. Tandemly repeated hexanucleotide at Tetrahymena rDNA free end is generated from a single copy during development. Cell. 1982 Nov;31(1):177–182. doi: 10.1016/0092-8674(82)90417-2. [DOI] [PubMed] [Google Scholar]
- King C. R., Piatigorsky J. Alternative RNA splicing of the murine alpha A-crystallin gene: protein-coding information within an intron. Cell. 1983 Mar;32(3):707–712. doi: 10.1016/0092-8674(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Lauth M. R., Spear B. B., Heumann J., Prescott D. M. DNA of ciliated protozoa: DNA sequence diminution during macronuclear development of Oxytricha. Cell. 1976 Jan;7(1):67–74. doi: 10.1016/0092-8674(76)90256-7. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Heumann J. M., Herrick G., Prescott D. M. The gene-size DNA molecules in Oxytricha. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):483–492. doi: 10.1101/sqb.1978.042.01.051. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M. Purification and characterization of two new modification methylases: MClaI from Caryophanon latum L and MTaqI from Thermus aquaticus YTI. Nucleic Acids Res. 1981 Dec 21;9(24):6795–6804. doi: 10.1093/nar/9.24.6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock A. RNA and macronuclear transcription in the ciliate Stylonychia mytilus. Chromosoma. 1981;83(2):209–220. doi: 10.1007/BF00286790. [DOI] [PubMed] [Google Scholar]
- Otsuka A. Recovery of DNA fragments inserted by the "tailing" method: regeneration of PstI restriction sites. Gene. 1981 May;13(4):339–346. doi: 10.1016/0378-1119(81)90013-5. [DOI] [PubMed] [Google Scholar]
- Pluta A. F., Kaine B. P., Spear B. B. The terminal organization of macronuclear DNA in Oxytricha fallax. Nucleic Acids Res. 1982 Dec 20;10(24):8145–8154. doi: 10.1093/nar/10.24.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae P. M., Spear B. B. Macronuclear DNA of the hypotrichous ciliate Oxytricha fallax. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4992–4996. doi: 10.1073/pnas.75.10.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spear B. B., Lauth M. R. Polytene chromosomes of Oxytricha: biochemical and morphological changes during macronuclear development in a ciliated protozoan. Chromosoma. 1976 Jan 27;54(1):1–13. doi: 10.1007/BF00331828. [DOI] [PubMed] [Google Scholar]
- Swanton M. T., Greslin A. F., Prescott D. M. Arrangement of coding and non-coding sequences in the DNA molecules coding for rRNAs in Oxytricha sp. DNA of ciliated protozoa. VII. Chromosoma. 1980;77(2):203–215. doi: 10.1007/BF00329545. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]