Abstract
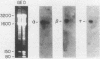
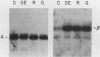
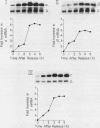
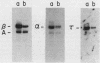
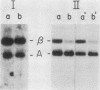
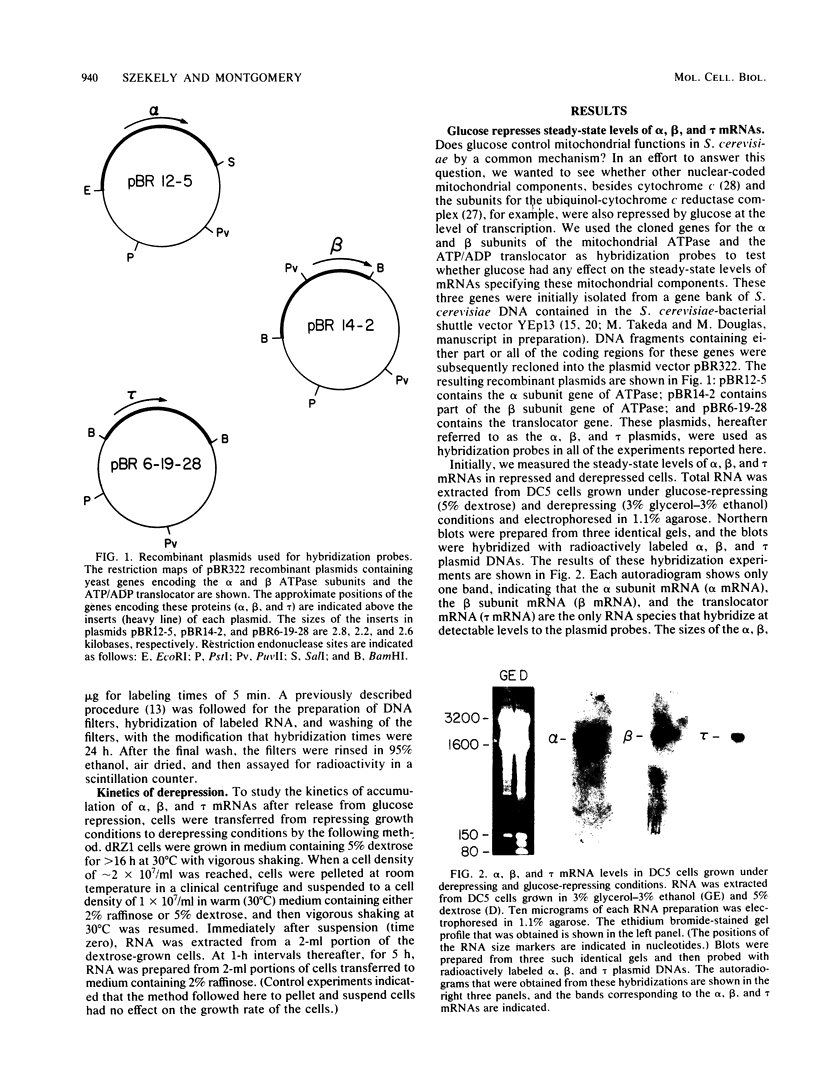
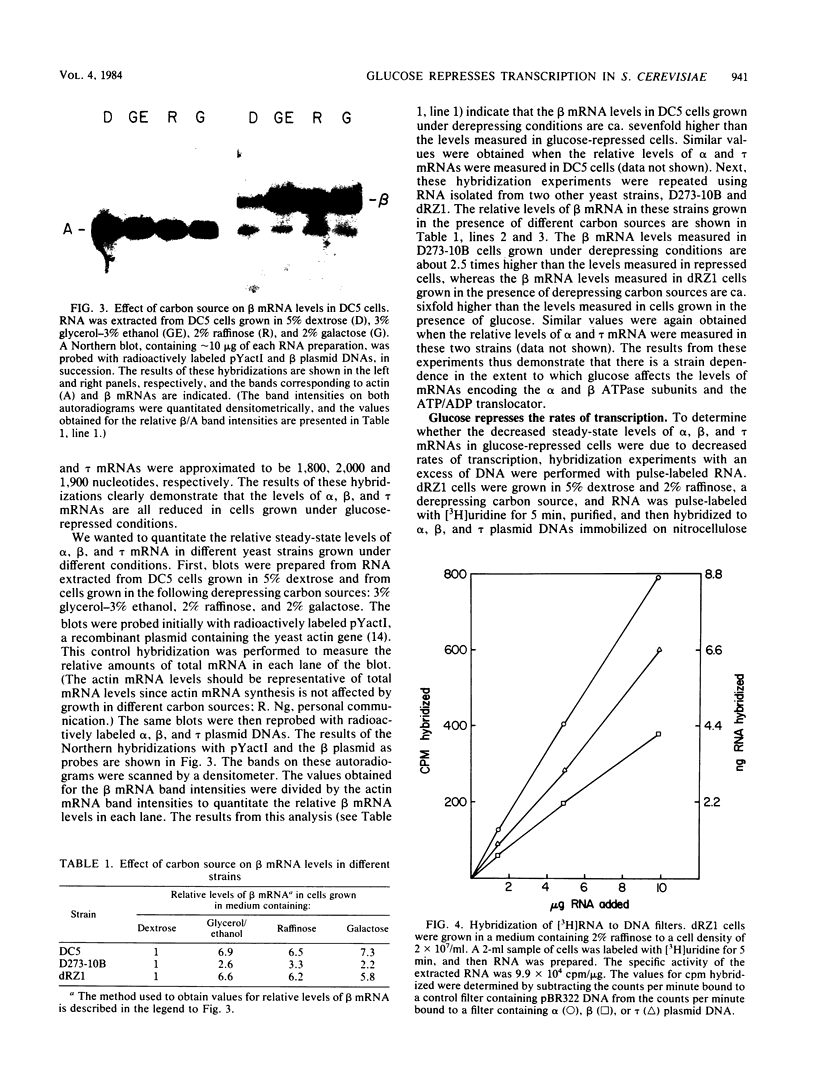
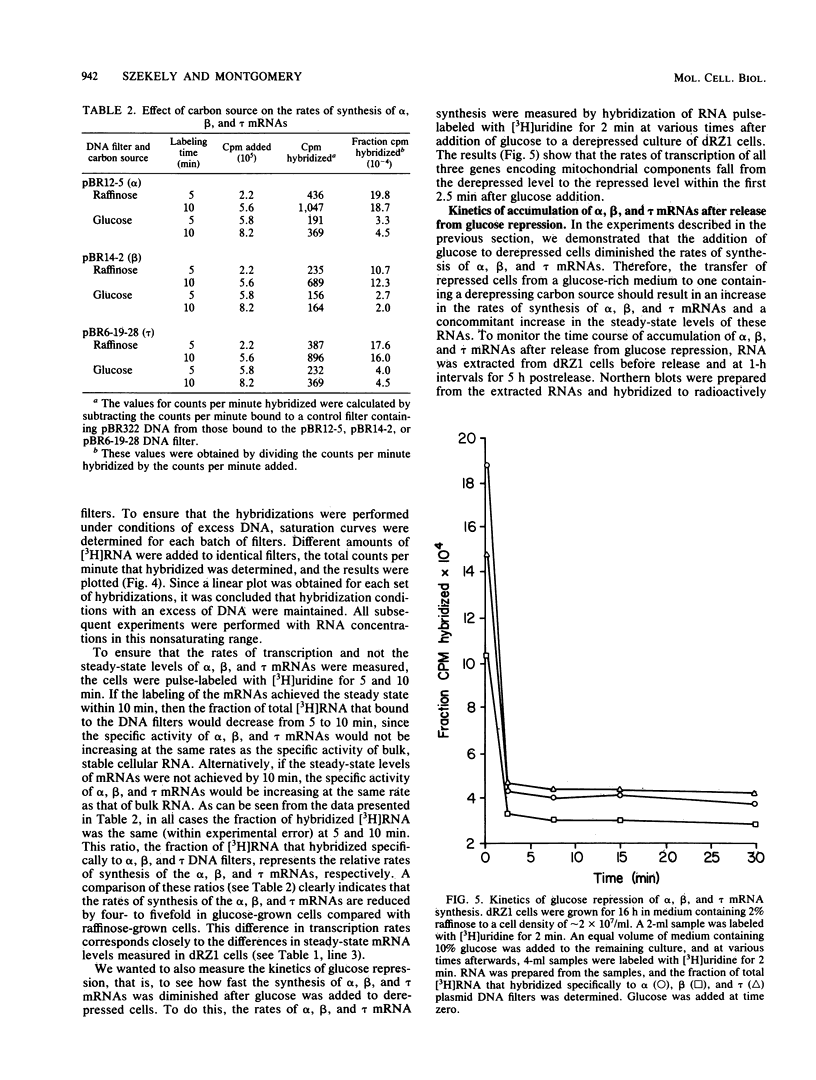
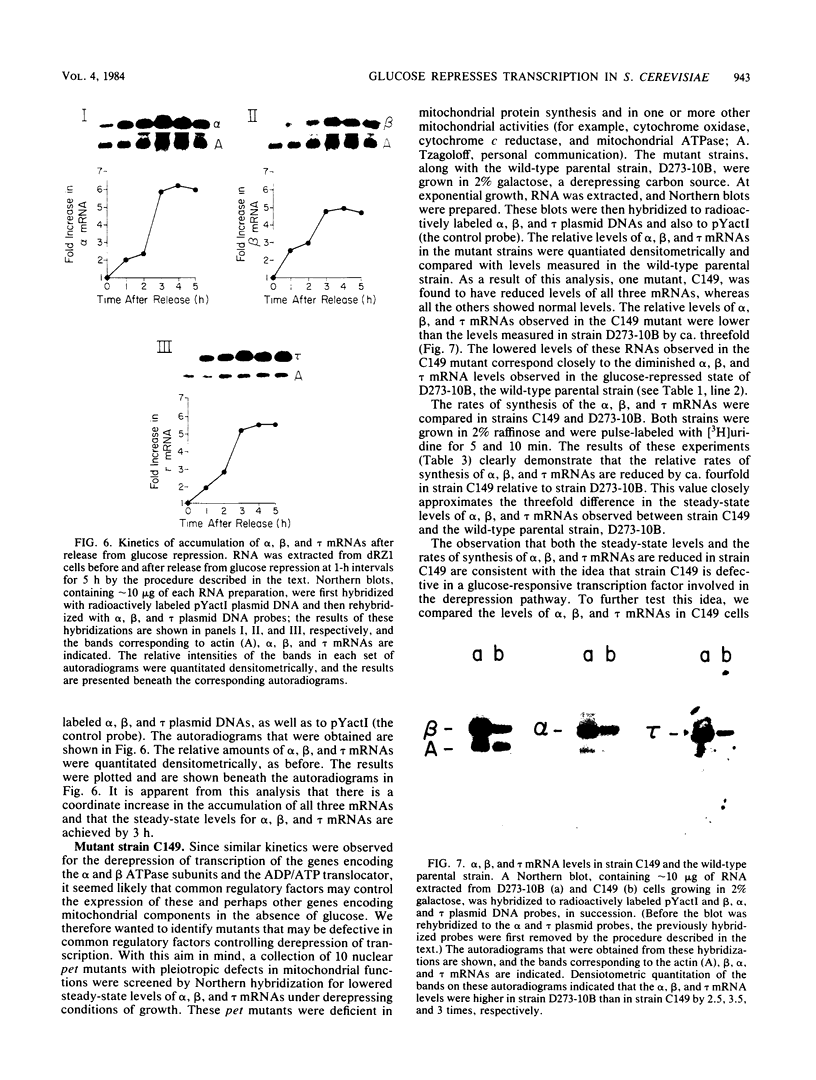
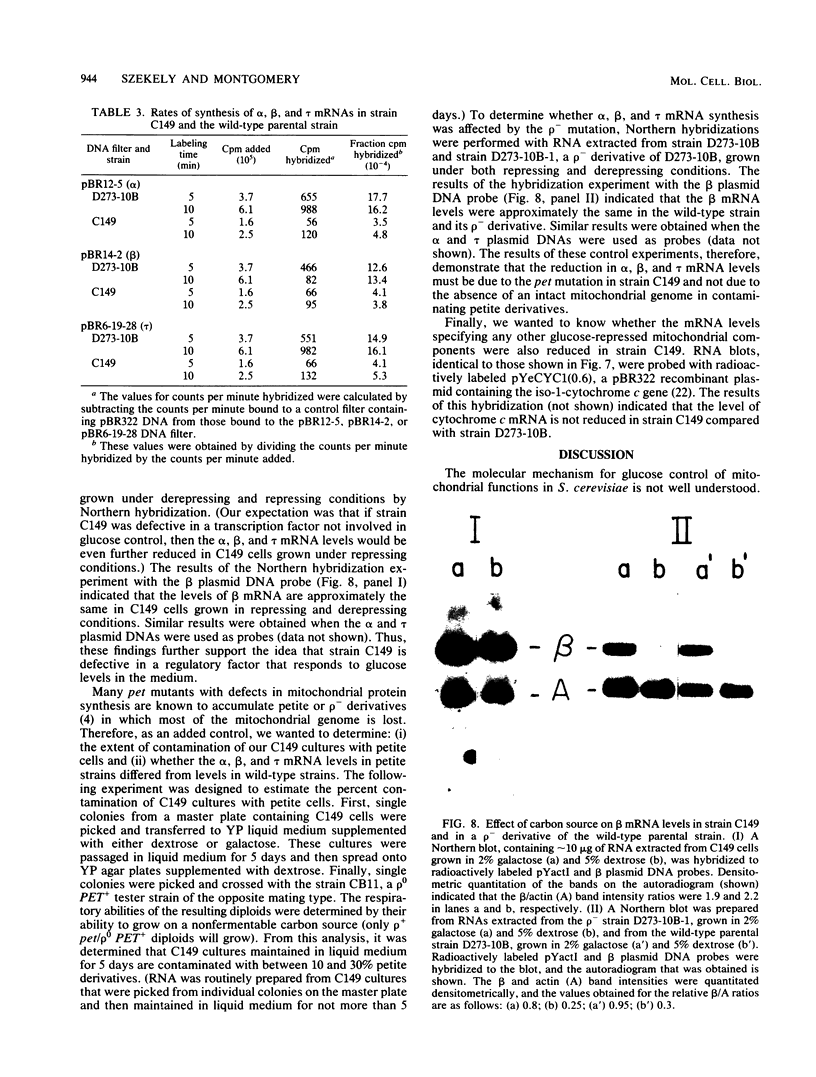
By Northern blot hybridization analysis, we demonstrated that the steady-state levels of mRNAs specifying the alpha subunit of ATPase, the beta subunit of ATPase, and the ATP/ADP translocator are all reduced in cells grown in glucose-rich medium. The extent to which glucose represses the levels of alpha, beta, and translocator mRNAs varies from strain to strain, from 2.5- to 7-fold. Furthermore, by hybridization experiments with an excess of DNA, we showed that glucose represses the rates of synthesis of these mRNAs. The kinetics of repression and depression of transcription were also studied. Finally, a mutant was characterized which appears to be defective in depression of transcription of the genes encoding the alpha and beta ATPase subunits as well as the ATP/ADP translocator.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B. G. Induction of galactokinase in Saccharomyces cerevisiae: kinetics of induction and glucose effects. J Bacteriol. 1972 Aug;111(2):308–315. doi: 10.1128/jb.111.2.308-315.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Böker-Schmitt E., Francisci S., Schweyen R. J. Mutations releasing mitochondrial biogenesis from glucose repression in Saccharomyces cerevisiae. J Bacteriol. 1982 Jul;151(1):303–310. doi: 10.1128/jb.151.1.303-310.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entian K. D., Zimmermann F. K. New genes involved in carbon catabolite repression and derepression in the yeast Saccharomyces cerevisiae. J Bacteriol. 1982 Sep;151(3):1123–1128. doi: 10.1128/jb.151.3.1123-1128.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federoff H. J., Eccleshall T. R., Marmur J. Carbon catabolite repression of maltase synthesis in Saccharomyces carlsbergensis. J Bacteriol. 1983 Oct;156(1):301–307. doi: 10.1128/jb.156.1.301-307.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascón S., Neumann N. P., Lampen J. O. Comparative study of the properties of the purified internal and external invertases from yeast. J Biol Chem. 1968 Apr 10;243(7):1573–1577. [PubMed] [Google Scholar]
- Görts C. P. Effect of glucose on the activity and the kinetics of the maltose-uptake system and of alpha-glucosidase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1969 Jul 30;184(2):299–305. doi: 10.1016/0304-4165(69)90032-4. [DOI] [PubMed] [Google Scholar]
- Ibrahim N. G., Stuchell R. N., Beattie D. S. Formation of the yeast mitochondrial membrane. 2. Effects of glucose repression on mitochondrial protein synthesis. Eur J Biochem. 1973 Jul 16;36(2):519–527. doi: 10.1111/j.1432-1033.1973.tb02938.x. [DOI] [PubMed] [Google Scholar]
- Lustig A., Levens D., Rabinowitz M. The biogenesis and regulation of yeast mitochondria RNA polymerase. J Biol Chem. 1982 May 25;257(10):5800–5808. [PubMed] [Google Scholar]
- Lustig A., Padmanaban G., Rabinowitz M. Regulation of the nuclear-coded peptides of yeast cytochrome c oxidase. Biochemistry. 1982 Jan 19;21(2):309–316. doi: 10.1021/bi00531a017. [DOI] [PubMed] [Google Scholar]
- O'Malley K., Pratt P., Robertson J., Lilly M., Douglas M. G. Selection of the nuclear gene for the mitochondrial adenine nucleotide translocator by genetic complementation of the op1 mutation in yeast. J Biol Chem. 1982 Feb 25;257(4):2097–2103. [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. Distinct repressible mRNAs for cytoplasmic and secreted yeast invertase are encoded by a single gene. Cell. 1981 Aug;25(2):525–536. doi: 10.1016/0092-8674(81)90071-4. [DOI] [PubMed] [Google Scholar]
- Perlman P. S., Mahler H. R. Derepression of mitochondria and their enzymes in yeast: regulatory aspects. Arch Biochem Biophys. 1974 May;162(1):248–271. doi: 10.1016/0003-9861(74)90125-8. [DOI] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem J. 1965 Oct;97(1):284–297. doi: 10.1042/bj0970284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saltzgaber-Muller J., Kunapuli S. P., Douglas M. G. Nuclear genes coding the yeast mitochondrial adenosine triphosphatase complex. Isolation of ATP2 coding the F1-ATPase beta subunit. J Biol Chem. 1983 Oct 10;258(19):11465–11470. [PubMed] [Google Scholar]
- Sherman F., Stewart J. W., Parker J. H., Inhaber E., Shipman N. A., Putterman G. J., Gardisky R. L., Margoliash E. The mutational alteration of the primary structure of yeast iso-1-cytochrome c. J Biol Chem. 1968 Oct 25;243(20):5446–5456. [PubMed] [Google Scholar]
- St John T. P., Davis R. W. The organization and transcription of the galactose gene cluster of Saccharomyces. J Mol Biol. 1981 Oct 25;152(2):285–315. doi: 10.1016/0022-2836(81)90244-8. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A., Akai A., Needleman R. B. Assembly of the mitochondrial membrane system: isolation of nuclear and cytoplasmic mutants of Saccharomyces cerevisiae with specific defects in mitochondrial functions. J Bacteriol. 1975 Jun;122(3):826–831. doi: 10.1128/jb.122.3.826-831.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A. Assembly of the mitochondrial membrane system. II. Synthesis of the mitochondrial adenosine triphosphatase. F1. J Biol Chem. 1969 Sep 25;244(18):5027–5033. [PubMed] [Google Scholar]
- Zitomer R. S., Montgomery D. L., Nichols D. L., Hall B. D. Transcriptional regulation of the yeast cytochrome c gene. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3627–3631. doi: 10.1073/pnas.76.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon A. P., de Groot R. J., van Eyk E., van der Horst G. T., Grivell L. A. Isolation and characterization of nuclear genes coding for subunits of the yeast ubiquinol-cytochrome c reductase complex. Gene. 1982 Dec;20(3):323–337. doi: 10.1016/0378-1119(82)90201-3. [DOI] [PubMed] [Google Scholar]