Summary
The transcriptional coactivators PGC-1α and PGC-1β are widely thought to be required for mitochondrial biogenesis and fiber typing in skeletal muscle. We show here that mice lacking both PGC-1s in myocytes do indeed have profoundly deficient mitochondrial respiration, but surprisingly have preserved mitochondrial content, isolated muscle contraction capacity, fiber type composition, in-cage ambulation, and voluntary running capacity. Most of these findings are recapitulated in cell culture, and thus cell-autonomous. Functional electron microscopy reveals normal cristae density with decreased cytochrome oxidase activity. These data lead to the following surprising conclusions: that PGC-1s are in fact dispensable for baseline muscle function, mitochondrial content, and fiber typing; that endurance fatigue at low workloads is not limited by muscle mitochondrial capacity; and that mitochondrial content and cristae density can be dissociated from respiratory capacity.
Introduction
Mitochondria in skeletal muscle serve diverse functions and are critical for health. Human mutations in mitochondrial DNA often lead to muscle dystrophy (Seneca et al., 2001; Wallace, 2005). Aberrant mitochondrial function has been proposed to contribute to insulin resistance (Hoeks and Schrauwen, 2012; Szendroedi et al., 2012). Boosting mitochondrial activity in rodents improves exercise capacity (Arany et al., 2007; Calvo et al., 2008; Narkar et al., 2008) and organismal health in numerous models of disease ranging from muscle dystrophy to amyotrophic lateral sclerosis (Da Cruz et al., 2012; Handschin et al., 2007c).
PGC-1α and PGC-1β have emerged as potent transcriptional coactivators that regulate broad programs of mitochondrial biology (Finck and Kelly, 2006; Rowe et al., 2010; Scarpulla, 2008). The PGC-1s also coordinatedly regulate various ancillary programs relevant to mitochondria. In muscle, the PGC-1s co-regulate fatty acid transport and angiogenesis, both processes critical for bringing fuel and oxygen to mitochondria (Arany et al., 2008; Madrazo and Kelly, 2008; Rowe et al., 2011; Vega et al., 2000). The PGC-1s have also been implicated in regulating genes of the neuromuscular junction (Handschin et al., 2007c) and muscle fiber typing (Arany et al., 2007; Lin et al., 2002), processes relevant to mitochondrial biology in skeletal muscle.
There has been controversy, however, to what extent PGC-1α and β normally contribute to these mitochondrial and ancillary programs. While gain-of-function models have been compelling, as outlined above, loss-of-function experiments have been less conclusive. The extensive redundancy that exists between PGC-1α and β complicates interpretations (Lai et al., 2008; St-Pierre et al., 2003). Deletion of either PGC-1α or PGC-1β alone, in the whole body or in skeletal muscle specifically, only mildly affects muscle mitochondria and function (Handschin et al., 2007a; Handschin et al., 2007b; Lai et al., 2008; Leone et al., 2005; Lin et al., 2004; Sonoda et al., 2007; Zechner et al., 2010), while whole-body deletion of both coactivators leads to perinatal lethality due to heart failure (Lai et al., 2008). Evaluation of the role of the PGC-1s in skeletal muscle thus requires deletion of both coactivators specifically in this tissue. Zechner et al. recently generated mice in which PGC-1β is deleted in skeletal muscle, and a hypomorphic allele of PGC-1α is present in all tissues. The authors demonstrated dramatic loss of ETC activity in muscle from these mice, but unchanged fiber types and glucose handling (Zechner et al., 2010). However, whole-body PGC-1α deficiency has profound effects on ambulatory activity, metabolism, and diurnal behavior (Leone et al., 2005; Lin et al., 2004; Liu et al., 2007), all of which are significant confounders to skeletal muscle studies. Moreover, the PGC-1α allele used in these experiments retains intact exons 1–5 (Leone et al., 2005; Zechner et al., 2010), which encode the transactivation and nuclear receptor-binding domains of PGC-1α, and encode for almost all of NT-PGC-1α and PGC-1α4, a naturally occurring splice variant of PGC-1α that is known to retain significant activity (Chang et al., 2012; Chang et al., 2010; Ruas et al., 2012; Zhang et al., 2009). The mice thus likely retained some PGC-1 activity in skeletal muscle. The findings have thus led to controversy (Handschin and Spiegelman, 2011; Zechner et al., 2011).
In light of these controversies, we generated animals that lack both coactivators specifically in skeletal muscle, using complete null alleles of both PGC-1α and β (Lai et al., 2008; Lin et al., 2004), and that retain intact expression of both coactivators in other tissues. We find that ETC activity is severely blunted in these animals. Surprisingly, however, other aspects of mitochondrial biology, including mitochondrial density itself, are intact in both animals and isolated cells. In fact, surprisingly, baseline muscle function is normal, both in vivo and ex vivo. Similarly, fiber types are unchanged. Only forced exercise capacity is impaired. The data thus resolve prior controversies, and indicate that mitochondrial content and ETC capacity are dissociable, that most of ETC capacity in muscle is dispensable for normal function, and that PGC-1 coactivators likely serve to “boost” the system and enable it to withstand stress beyond ordinary activities.
Results
PGC-1α and β are critical for ETC capacity
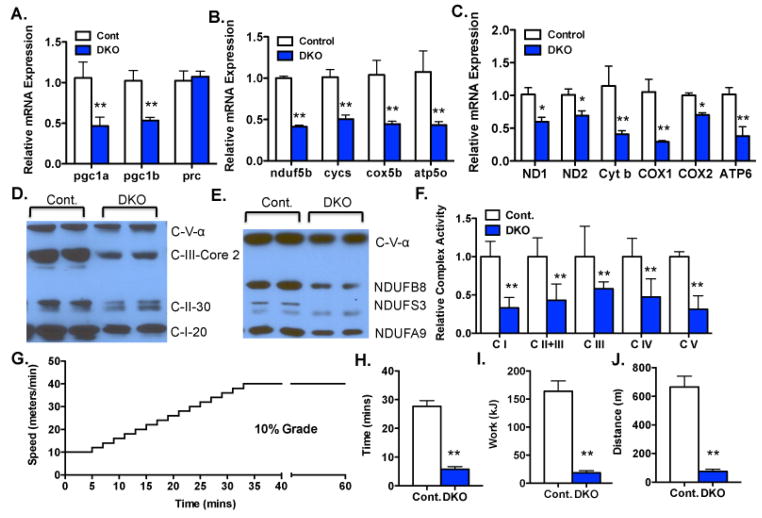
Mice lacking PGC-1α and PGC-1β strictly in skeletal muscle (“double knockout”, or DKO mice) were generated by Cre/Lox recombination. The PGC-1α and β floxed alleles were kindly provided by Dr. Bruce Spiegelman (Lin et al., 2004), and Dr. Daniel Kelly (Lai et al., 2008), respectively. Both alleles are complete nulls after Cre/Lox recombination. Cre recombinase was driven by a modified myogenin promoter containing the MEF2 enhancer (Li et al., 2005), an allele widely used for muscle-specific recombination. DKO mice were born in Mendelian ratios, and appeared grossly indistinguishable from their littermate controls. The mRNA expression of both PGC-1α and β was reduced by 60% in quadriceps from DKO animals (Figure 1A). The residual expression of the PGC-1s likely reflects expression in non-myocytes, in which the Cre recombinase is not expressed. PGC-1 related coactivator (PRC) is a coactivator with weak homology to the PGC-1s (Rowe et al., 2010; Scarpulla, 2008). The expression of PRC was not altered in the DKO animals (Figure 1A). Hematoxylin and eosin staining of transverse sections from the muscles showed no obvious abnormalities (Sup Fig S1A).
Figure 1. PGC-1α and β are critical for ETC capacity.
A–C.) Relative mRNA expression of indicated genes from quadriceps (quad) of Myo-DKO mice (blue bars) and littermate controls (white bars). PGC-1 isoforms (A), nuclear-encoded electron transport chain (ETC) genes (B) and mitochondrial-encoded ETC genes (C). D&E.) Western blot analyses of indicated ETC proteins from quads of Myo-DKO mice F.) Relative activity of the indicated ETC complexes in quad muscles from DKO and control animals. G) Schematic of exercise exhaustion protocol. H–J) Maximal time (H), work (I), and distance (J) achieved on running treadmills prior to exhaustion. Error bars indicate SEM; n > 3 per group in all panels. * - P < 0.05. N.S. – not statistically significant.
The mRNA expressions of nuclear genes encoding components of the ETC and mitochondrial ATPase were severely repressed by 60% in quadriceps from DKO animals (Figure 1B). RNA expression of genes encoded on the mitochondrial genome were similarly repressed (Figure 1C). Soleus and extensor digitorum longus (EDL) muscles revealed similar findings (Supp Fig S1B–C). Western blotting revealed dramatic decreases of proteins of ETC and oxidative phosphorylation (Figure 1D and E). The activities of all 4 complexes of the ETC and the ATPase complex, measured directly in intact quadriceps muscle via state-of-the-art enzymatic spectrophotometric assays(Benit et al., 2006; Rowe et al., 2012; Rustin et al., 1994), were severely depressed, by as much as >60% (Figures S1D and 1F). These marked respiratory defects suggested that the mice would have blunted exercise capacity. Indeed, DKO mice were severely limited in their ability to carry out a treadmill-based exercise stress test (Figures 1G–J). Together, these data indicate that DKO mice have profoundly decreased ETC capacity, which is reflected in marked defects in forced exercise capacity.
ETC capacity is dispensable for baseline activity and muscle function
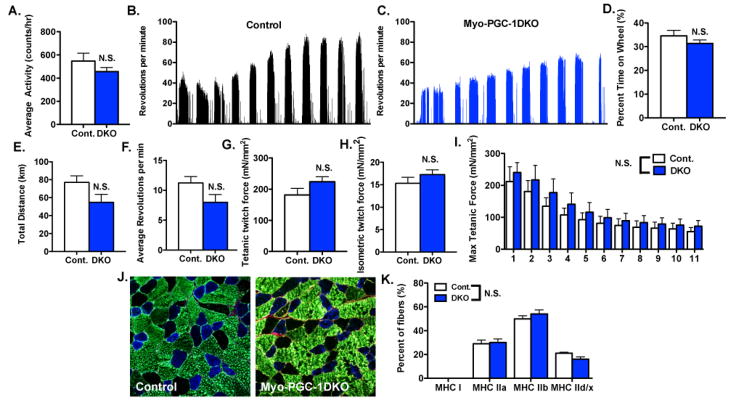
Surprisingly, spontaneous in-cage locomotor activity was intact in DKO mice (Figure 2A). Mice are, however, normally much more active than a standard cage allows them to be. To provide a more opportunity for physical activity, voluntary running wheels were placed in the cages and monitored electronically. Both DKO and control animals voluntarily ran nightly, running approximately 10 hours/day for an approximately calculated total distance of 60–80km over an 11-day period (Figure 2B and C). DKO mice spent the same amount of time on the wheels (Figure 2D) as control animals, and revealed only a mild, statistically non-significant, decrease in total running distance (Figure 2E) or average revolutions/minute (Figure 2F). Mice appeared healthy at all times (not shown).
Figure 2. PGC-1s are dispensable for baseline activity and muscle function.
A.) In-cage locomotor activity over 72hrs of Myo-DKO mice and control littermates. B–C.) Samples of voluntary wheel activity over 11 days. D–F.) Quantification of B–C, represented as percent time spent on wheels (D) total distance run (E) or average revolutions per minute (F). G–I.) Maximal tetanic (G) and isometric twitch forces (H), and contraction-stimulated fatigability (I), of explanted extensor digitorum longus muscle from Myo-DKO and control animals. J–K.) Fiber type composition in plantaris of DKO and control animals. (J) Representative images of immunostaining: MHC Ia (red), MHC IIa (blue) and MHC IIb (green); unstained fibers were counted as MHC IId/x. (K), Quantification of immunostaining. Error bars indicate SEM; n > 6 per group in all panels. N.S. – not statistically significant.
To test muscle function in the absence of systemic organismal effects, EDL muscles were explanted and tested ex vivo. DKO and control muscles achieved nearly indistinguishable maximum tetanic force of approximately 200 mM/mm2 (Figure 2G), isometric twitch force of approximately 15 mN/mm2 (Figure 2H), and rate of fatigability (Figure 2I). Maximum titanic force and fatigability were similarly preserved in soleus muscles from DKO animals (Sup Figure S2A–C). Muscle contractile function and fatigability are dependent, to some extent, on the relative make up of fiber types. The fiber type compositions of DKO and control animals, however, were nearly identical in both plantaris and soleus muscles (Figures 2J, K, and S2D). Together, these data thus indicate that, surprisingly, DKO mice retain nearly intact fiber type composition, baseline muscle activity, and fatigability both in vivo and ex vivo.
Mitochondrial content and ETC capacity are separable
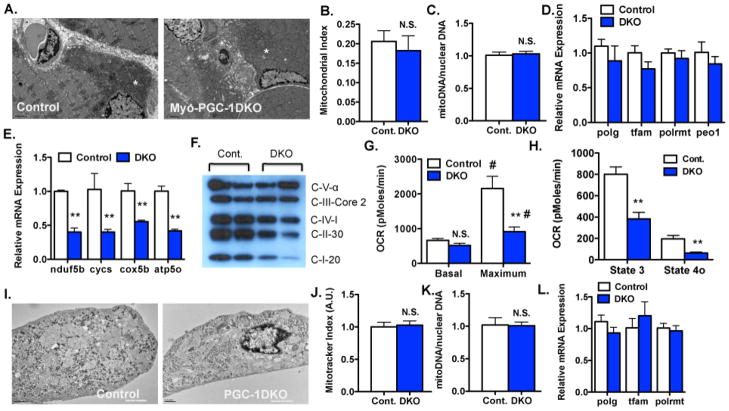
In light of the remarkable loss of respiratory chain capacity in DKO mice (Figure 1), electron microscopy was carried out to evaluate mitochondria in muscle from DKO animals. Surprisingly, the DKO mitochondria appeared entirely normal in number, morphology, and overall content (Figure 3A and B). The content of mitochondrial DNA was also unperturbed in DKO muscles (Figure 3C). Regular intermyofibral mitochondria were seen, as were subsarcolemnal mitochondria. No differences in mitochondrial size or cristae density and morphology were appreciated. Blinded experts in mitochondrial morphology were unable to distinguish the two genotypes.
Figure 3. Mitochondrial content and ETC capacity are separable in DKO muscle and cultured myotubes.
A–B.) Representative images (A) and morphometric quantification of mitochondrial density (B) of transmission electron micrographs of transverse sections of mid-portion of quadriceps (quad) of Myo-DKO mice and control littermates. Sample mitochondria labeled with a white (*). C.) Mitochondrial-to-nuclear DNA content ratio in quad muscles from Myo-DKO and control animals. D.) Relative expression of indicated genes from the quads of the Myo-DKO and control animals. E–L.) Differentiated myotubes from primary myoblasts isolated from DKO (blue) versus control (white) muscles. (E) Relative mRNA expression of indicated genes. (F) Western blot analysis of indicated ETC proteins. (G) Basal and uncoupled respiration rates of DKO and control differentiated myotubes. (H) State 3 and state 4o respiration rates in permeabilized DKO and control differentiated myotubes. (I) TEMs of DKO and control differentiated myotubes. J–K.) Quantification of mitochondrial content by mitotracker (J) and mitochondrial-to-nuclear DNA content ratio (K) in DKO and control cells. L.) Relative expression of indicated genes in primary DKO and control myotubes. n > 4 fields from 6 animals per group. Error bars indicate SEM; n > 3 per group in all panels. * - P < 0.05 compared to control. # - P < 0.05 compared to basal state N.S. – not statistically significant.
These surprising findings indicated that mitochondrial biogenesis is separable from assembly of the respiratory chain. Consistent with this notion, and with the preserved mtDNA content in DKO muscles, the expression of genes involved in replication of mitochondrial DNA was not reduced in DKO animals (Figure 3D). Expression of most genes involved in synthesis of cardiolipin, the predominant structural mitochondrial lipid, was similarly unaffected, as were, for the most part, genes involved in other mitochondrial functions like apoptosis, proteolysis and fusion/fission (Sup Fig S3A–C).
Cell-autonomous separation of mitochondrial content and ETC activity
The observed DKO phenotypes may have had a developmental origin, or have been affected by cues outside the muscle. To investigate the above findings in a cell-autonomous setting, primary muscle myoblasts were isolated from PGC-1α/β homozygous double floxed animals, exposed to adenoviruses expressing Cre recombinase (or LacZ as a control), and then differentiated into myotubes in cell culture. The efficiency of PGC-1α and β deletion was >95% (Sup Figure S3D). Differentiated myotubes appeared grossly normal (Sup Figure S3E), and had normal temporal expression of differentiation markers (Sup Figure S3F), indicating that, interestingly, PGC-1α and β are dispensable for myoblast-to-myotube differentiation in cell culture.
The mRNA expression of nuclear and mitochondrial respiratory chain components were markedly decreased in DKO myotubes (Figure 3E), as was the content of Complex I–V proteins (Figure 3F), recapitulating the findings in vivo.. Maximal oxygen consumption rate (OCR) was reduced by 60% in DKO myotubes, reflecting the marked decreases in components of the ETC (Figure 3G). On the other hand, basal OCR was unaffected in DKO myotubes (Figure 3G), again indicating that PGC-1α and β are dispensable for baseline function. OCR in the presence of palmitate as a primary fuel source was also markedly blunted in DKO myotubes (Sup Figure S3G). Both state 3 and 4o respiration (treated with oligomycin) in permeabilized myotubes provided with ADP and pyruvate/malate as substrates were also markedly reduced (Figure 3H).
Finally, transmission electron microscopy revealed preserved mitochondrial content in DKO myotubes (Figure 3I), again analogous to the findings in vivo. Mitochondrial content, quantified by Mitotracker dye, was identical between DKO and control cells (Figure 3J), as was steady-state mitochondrial DNA amount (Figure 3K), and expression of genes required for mitochondrial DNA replication (Figure 3L). Together, these data indicate that the separation of mitochondrial content and ETC activity is cell-autonomous and independent of developmental processes. Moreover, despite profound effects on respiration capacity, PGC-1α and β are dispensable for baseline respiration, and for myoblast-to-myotube differentiation.
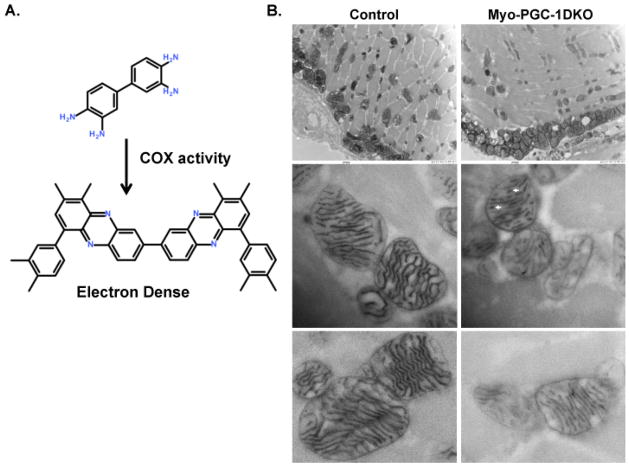
Direct visualization of ETC activity at resolution of cristae
In general, cristae density has been thought to reflect ETC capacity (Giraud et al., 2002; Strauss et al., 2008). Surprisingly, however, cristae density was normal in DKO animals (Figure 3A). We therefore sought to measure ETC activity in situ, at a magnification sufficient to resolve individual cristae. We used a recently redeveloped technique in which an electron dense trace is generated in situ by Complex IV-mediated oxidative polymerization of 3,3′-diaminobenzidine (DAB) (Zsengeller et al., 2012) (Figure 4A). The method thus directly visualizes actively respiring cristae. Images from control animals revealed uniform contrast throughout the cristae and surrounding mitochondrial membrane (presumably the inner membrane) (Figure 4B), consistent with cristae that are packed with ETC components. In sharp contrast, mitochondria from DKO animals revealed numerous cristae with only occasional contrast, in a stippled pattern (white arrow heads) (Figure 4B). Some cristae lacked contrast altogether. No such abnormalities were observed in the control animals. These data thus indicate that cristae in DKO animals are capable of forming, but have reduced Cytochrome c oxidase capacity
Figure 4. Decreased ultrastructural ETC activity in Myo-PGC-1DKO animals.
A.) Schematic of 3,3′-diaminobenzidine (DAB) polymerization. B.) Transmission electron microscopy ultrastructural analysis of mitochondrial cyctochrome c oxidase (COX) enzymatic activity in quadricep muscle of Myo-DKO animals and control littermates. Punctated mitochondrial cristae labeled with white arrowhead.
Discussion
As outlined above, there has been debate about the precise role of the PGC-1 coactivators in skeletal muscle at baseline (Handschin and Spiegelman, 2011; Zechner et al., 2011). Here, we resolve these issues by using alleles of PGC-1α and β that are most likely complete nulls (Handschin et al., 2007b; Lai et al., 2008), and by using a skeletal-muscle specific Cre driver in the context of homozygous floxed alleles, thereby leaving other tissues unaffected. We conclude that: 1) PGC-1α and β are critically required for normal ETC and oxidative capacity; 2) the PGC-1s do not play a role in baseline fiber type composition; 3) the PGC-1s are dispensable for baseline muscle contractile force and fatigability, thus dissociating these processes from oxidative capacity in the muscle; and 4) the PGC-1s are also dispensable for baseline mitochondrial density per se, thus dissociating this process from oxidative capacity.
Our data suggest that PGC-1 coactivators are dispensable for most developmental and hormonal cues that determine muscle differentiation and fiber type composition (Schiaffino and Reggiani, 2011). Consistent with this, deletion of the PGC-1s did not affect myoblast-to-myotube differentiation in cell culture (Sup Figure S3). On the other hand, gain-of-function models convincingly demonstrated the ability of PGC-1s to drive fiber type conversion (Arany et al., 2007; Lin et al., 2002). These observations suggest that the PGC-1s primarily transduce post-natal physiological signals to affect fiber type composition. Exercise, for example, is known to induce PGC-1α and to induce changes in fiber types. Muscle PGC-1α alone is dispensable for exercise-induced fiber type transformation and mitochondrial biogenesis (Geng et al., 2010; Rowe et al., 2012), suggesting that redundancy with PGC-1β may be important in this context. If so, then PGC-1β may be modified by exercise at the post-translational level, since, unlike PGC-1α, levels of PGC-1β transcripts are typically not affected by exercise. Consistent with this notion, PGC-1β is stabilized by cAMP signaling (Shoag et al., 2013).
It is striking that mice lacking >60% of muscle oxidative capacity remain capable of normal locomotion, and indeed of voluntarily running up to 10kms per night (Figure 2). The dissociation of these processes demonstrates that oxidative capacity in skeletal muscle is not primarily rate-limiting for most baseline activities. The data also indicate that the “comfort zone” rate at which mice voluntarily run on in-cage wheels is determined by factors other than oxidative capacity. Fatigue, be it experienced or physiological, is a significant burden faced by patients with myopathies and has a large impact on their perceived health status. The low workload fatigue experienced by these patients is likely mechanistically different from that experienced by higher intensity work such as endurance running. The data presented here suggest that mitochondrial insufficiency in these patients may not account for their fatigue.
DKO cells and muscles contained normal amounts of mitochondria and cristae, despite profound decreases in ETC capacity. The regulation of these two processes is thus separable. Mitochondrial DNA content was also normal in DKO animals and cells, as was the expression of genes involved in mitochondrial genome replication (Figure 3D). The PGC-1s are thus not rate-limiting for mitochondrial DNA content in skeletal muscle. Coordinate regulation of genes involved in cardiolipin biosynthesis was also unaltered in DKO animals and cells. It thus appears that a different threshold of required PGC-1 activity exist for genes of the ETC versus other mitochondrial functions. It will be of future interest to determine if this involves binding to different transcription factors, and/or altered post-translational modifications. PRC, a coactivator with weak homology to the PGC-1s, may play a role, although we did not observe compensatory upregulation of PRC in the DKO animals.
High-resolution visualization of cytochrome oxidase activity intriguingly revealed a stippled pattern of activity along cristae in DKO animals, in contrast to the continuous activity seen in control animals. Assembly of ETC complexes is known to be tightly regulated, and exquisitely stoichiometric(Lenaz and Genova, 2010; Papa et al., 2012). The discontinuous pattern of staining on EM thus suggests that ETC complexes form normally at some sites on the cristae, but not at all at other sites. This pattern may be favored because less dense packing of ETC components along the cristae may not allow proper substrate shuttling (Giraud et al., 2002; Strauss et al., 2008).
In summary, the present study resolves important controversy concerning the role of PGC-1α and β in skeletal muscle, and in the process also demonstrates that oxidative capacity in muscle is surprisingly dissociable from mitochondrial content, from isolated muscle contractility, and from normal organismal activity and fatigability.
Brief Experimental Procedures
All animal experiments were performed according to procedures approved by the Beth Israel Deaconess Medical Center IACUC. PGC-1α floxed mice (Lin et al., 2004), PGC-1β floxed mice (Lai et al., 2008), and Myogenin/MEF2-Cre driver (Li et al., 2005) have been described. Respiratory chain complex activity was measured as previously described(Benit et al., 2006; Rustin et al., 1994). Electron Micrographs were performed by the BIDMC Electron Micrograph Core. Functional COX EMs were performed as previously described (Zsengeller et al., 2012). Oxygen consumption rates (pmol/min) were assessed using a XF Flux Analyzer (Seahorse Biosciences), Data are presented as means ± standard error of the mean (SEM). Statistical analysis was performed with Student’s t-test for all in vitro experiments and ANOVAs for all in vivo experiments. P values of less than 0.05 were considered statistically significant.
Supplementary Material
Highlights.
Muscle deletion of PGC-1α/β dissociates mitochondrial content from ETC activity.
Dissociation of respiration and mitochondrial content is cell autonomous.
PGC-1 coactivators are dispensable for baseline locomotion and muscle function.
PGC-1 α/β muscle DKO animals have normal fiber type composition.
Acknowledgments
This work was supported by grants from the NIH, NIAMS to G.C.R. (AR062128), L.J.G. (AR42238) and NHLBI to Z.A (HL094499).
Footnotes
Author contributions: G.C.R. and Z.A. designed research; G.C.R., I.S.P., N.K., Z.K.Z., R.E., M.O., C.F., S.B., M.F.H. performed research; G.C.R., Z.Y., L.J.G., P.R. and Z.A. analyzed data, G.C.R. and Z.A. wrote the paper.
See Supporting Information for more detailed Experimental Procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 2007;5:35–46. doi: 10.1016/j.cmet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Benit P, Goncalves S, Philippe Dassa E, Briere JJ, Martin G, Rustin P. Three spectrophotometric assays for the measurement of the five respiratory chain complexes in minuscule biological samples. Clin Chim Acta. 2006;374:81–86. doi: 10.1016/j.cca.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, Stevenson SC, Rangwala SM. Muscle-specific expression of PPAR{gamma} coactivator-1{alpha} improves exercise performance and increases peak oxygen uptake. J Appl Physiol. 2008;104:1304–1312. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- Chang JS, Fernand V, Zhang Y, Shin J, Jun HJ, Joshi Y, Gettys TW. NT-PGC-1alpha Protein Is Sufficient to Link beta3-Adrenergic Receptor Activation to Transcriptional and Physiological Components of Adaptive Thermogenesis. J Biol Chem. 2012;287:9100–9111. doi: 10.1074/jbc.M111.320200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JS, Huypens P, Zhang Y, Black C, Kralli A, Gettys TW. Regulation of NT-PGC-1alpha subcellular localization and function by protein kinase A-dependent modulation of nuclear export by CRM1. J Biol Chem. 2010;285:18039–18050. doi: 10.1074/jbc.M109.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Cruz S, Parone PA, Lopes VS, Lillo C, McAlonis-Downes M, Lee SK, Vetto AP, Petrosyan S, Marsala M, Murphy AN, et al. Elevated PGC-1alpha activity sustains mitochondrial biogenesis and muscle function without extending survival in a mouse model of inherited ALS. Cell metabolism. 2012;15:778–786. doi: 10.1016/j.cmet.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng T, Li P, Okutsu M, Yin X, Kwek J, Zhang M, Yan Z. PGC-1alpha plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am J Physiol Cell Physiol. 2010;298:C572–579. doi: 10.1152/ajpcell.00481.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud MF, Paumard P, Soubannier V, Vaillier J, Arselin G, Salin B, Schaeffer J, Brethes D, di Rago JP, Velours J. Is there a relationship between the supramolecular organization of the mitochondrial ATP synthase and the formation of cristae? Biochim Biophys Acta. 2002;1555:174–180. doi: 10.1016/s0005-2728(02)00274-8. [DOI] [PubMed] [Google Scholar]
- Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem. 2007a;282:30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, et al. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest. 2007b;117:3463–3474. doi: 10.1172/JCI31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Kobayashi YM, Chin S, Seale P, Campbell KP, Spiegelman BM. PGC-1alpha regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev. 2007c;21:770–783. doi: 10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. PGC-1 coactivators and the regulation of skeletal muscle fiber-type determination. Cell metabolism. 2011;13:351. doi: 10.1016/j.cmet.2011.03.008. author reply 352. [DOI] [PubMed] [Google Scholar]
- Hoeks J, Schrauwen P. Muscle mitochondria and insulin resistance: a human perspective. Trends Endocrinol Metab. 2012;23:444–450. doi: 10.1016/j.tem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Lai L, Leone TC, Zechner C, Schaeffer PJ, Kelly SM, Flanagan DP, Medeiros DM, Kovacs A, Kelly DP. Transcriptional coactivators PGC-1alpha and PGC-lbeta control overlapping programs required for perinatal maturation of the heart. Genes Dev. 2008;22:1948–1961. doi: 10.1101/gad.1661708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaz G, Genova ML. Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. Antioxidants & redox signaling. 2010;12:961–1008. doi: 10.1089/ars.2009.2704. [DOI] [PubMed] [Google Scholar]
- Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Czubryt MP, McAnally J, Bassel-Duby R, Richardson JA, Wiebel FF, Nordheim A, Olson EN. Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc Natl Acad Sci U S A. 2005;102:1082–1087. doi: 10.1073/pnas.0409103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- Madrazo JA, Kelly DP. The PPAR trio: regulators of myocardial energy metabolism in health and disease. J Mol Cell Cardiol. 2008;44:968–975. doi: 10.1016/j.yjmcc.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa S, Martino PL, Capitanio G, Gaballo A, De Rasmo D, Signorile A, Petruzzella V. The oxidative phosphorylation system in mammalian mitochondria. Advances in experimental medicine and biology. 2012;942:3–37. doi: 10.1007/978-94-007-2869-1_1. [DOI] [PubMed] [Google Scholar]
- Rowe GC, El-Khoury R, Patten IS, Rustin P, Arany Z. PGC-1alpha is Dispensable for Exercise-Induced Mitochondrial Biogenesis in Skeletal Muscle. PLoS One. 2012;7:e41817. doi: 10.1371/journal.pone.0041817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe GC, Jang C, Patten IS, Arany Z. PGC-1beta regulates angiogenesis in skeletal muscle. American journal of physiology Endocrinology and metabolism. 2011;301:E155–163. doi: 10.1152/ajpendo.00681.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe GC, Jiang A, Arany Z. PGC-1 coactivators in cardiac development and disease. Circ Res. 2010;107:825–838. doi: 10.1161/CIRCRESAHA.110.223818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, et al. A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151:1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustin P, Chretien D, Bourgeron T, Gerard B, Rotig A, Saudubray JM, Munnich A. Biochemical and molecular investigations in respiratory chain deficiencies. Clinica chimica acta; international journal of clinical chemistry. 1994;228:35–51. doi: 10.1016/0009-8981(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- Seneca S, Verhelst H, De Meirleir L, Meire F, Ceuterick-De Groote C, Lissens W, Van Coster R. A new mitochondrial point mutation in the transfer RNA(Leu) gene in a patient with a clinical phenotype resembling Kearns-Sayre syndrome. Archives of neurology. 2001;58:1113–1118. doi: 10.1001/archneur.58.7.1113. [DOI] [PubMed] [Google Scholar]
- Shoag J, Haq R, Zhang M, Liu L, Rowe GC, Jiang A, Koulisis N, Farrel C, Amos CI, Wei Q, et al. PGC-1 coactivators regulate MITF and the tanning response. Mol Cell. 2013;49:145–157. doi: 10.1016/j.molcel.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda J, Mehl IR, Chong LW, Nofsinger RR, Evans RM. PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc Natl Acad Sci U S A. 2007;104:5223–5228. doi: 10.1073/pnas.0611623104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol Chem. 2003;278:26597–26603. doi: 10.1074/jbc.M301850200. [DOI] [PubMed] [Google Scholar]
- Strauss M, Hofhaus G, Schroder RR, Kuhlbrandt W. Dimer ribbons of ATP synthase shape the inner mitochondrial membrane. Embo J. 2008;27:1154–1160. doi: 10.1038/emboj.2008.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nature reviews Endocrinology. 2012;8:92–103. doi: 10.1038/nrendo.2011.138. [DOI] [PubMed] [Google Scholar]
- Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol. 2000;20:1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner C, Lai L, Zechner JF, Geng T, Yan Z, Rumsey JW, Collia D, Chen Z, Wozniak DF, Leone TC, et al. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab. 2010;12:633–642. doi: 10.1016/j.cmet.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner C, Leone TC, Kelly DP. Response to Handschin and Spiegelman. Cell metabolism. 2011;13:352. [Google Scholar]
- Zhang Y, Huypens P, Adamson AW, Chang JS, Henagan TM, Boudreau A, Lenard NR, Burk D, Klein J, Perwitz N, et al. Alternative mRNA splicing produces a novel biologically active short isoform of PGC-1alpha. J Biol Chem. 2009;284:32813–32826. doi: 10.1074/jbc.M109.037556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsengeller ZK, Ellezian L, Brown D, Horvath B, Mukhopadhyay P, Kalyanaraman B, Parikh SM, Karumanchi SA, Stillman IE, Pacher P. Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2012;60:521–529. doi: 10.1369/0022155412446227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.