Abstract
Skin cancer is the most common cancer in the United States. Exposure to ultraviolet radiation is a known risk factor for skin cancer but is also the principal means by which the body obtains vitamin D. Several studies have suggested that vitamin D plays a protective role in a variety of internal malignancies. With regard to skin cancer, epidemiologic and laboratory studies suggest that vitamin D and its metabolites may have a similar protective effect. These noncalcemic actions of vitamin D have called into question whether the current recommended intake of vitamin D is too low for optimal health and cancer prevention. Part I will review the role of vitamin D in the epidermis; part II will review the role of vitamin D in keratinocyte-derived tumors to help frame the discussion on the possible role of vitamin D in the prevention of skin cancer.
Keywords: 25(OH)D levels, cholecalciferol, supplements, vitamin D, ultraviolet radiation
Vitamin D is a fat-soluble prohormone whose major biologic function is to maintain serum calcium and phosphorous homeostasis. It promotes calcium absorption in the gut and reabsorption from the kidneys and inhibits the secretion of parathyroid hormone. Vitamin D therefore enables the normal mineralization of bone by regulating bone growth and remodeling the activity of osteoblasts and osteoclasts.1 Vitamin D deficiency has significant musculoskeletal consequences, causing rickets in children and osteomalacia and osteoporosis in adults2. In addition to its functions in the endocrine and skeletal systems, vitamin D has roles in modulating the immune, cardiovascular, and inflammatory systems; among other actions, it regulates macrophage phagocytosis3,4 and inhibits macrophage activation.5,6 Many genes encoding proteins that regulate cell proliferation, differentiation, and apoptosis are modulated, in part, by vitamin D.7
Many (but not all) epidemiologic studies have found an association between low levels of vitamin D and all-cause mortality,8–11 cancer mortality,12,13 and cancer survival.14–18 Others have linked lower rates of prostate and ovarian cancer19–25 to residency at lower latitudes. Some studies have shown that cancer patients who undergo treatment in summer have better survival rates than thosewho undergo treatment in winter, suggesting that variation in cancer survival may be associated with seasonal factors, including vitamin D levels.26–30
While the role of vitamin D in visceral cancers is under intensive research, the role of vitamin D in skin cancer is even more controversial. This is because the same spectrum of ultraviolet (UV) light necessary for vitamin D synthesis (290–320 nm) is also the most important environmental risk factor for the development of many skin cancer types. Nevertheless, laboratory studies suggest that vitamin D and its metabolites may reduce the risk of skin cancer by inhibiting the hedgehog signaling pathway,31,32 the pathway underlying the development of basal cell carcinomas and upregulating DNA nucleotide excision repair enzymes.33–35 Mice lacking the vitamin D receptor develop increased numbers of nonmelanoma skin cancers,36 and the addition of vitamin D decreases the growth of nonmelanoma skin cancer and melanoma cells in vitro and in mouse models.31,37–39 In humans, epidemiologic studies have reported mixed findings, with some reporting an association between higher vitamin D levels and increased skin cancer risk, 40,41 others showing a decreased skin cancer risk, 42,43 and still others showing no association. 44–47 Part II of this continuing medical education article will review the role of vitamin D in the skin and cutaneous tumors, review the existing literature, and frame the possible role of vitamin D in skin cancer prevention.
BACKGROUND
Key points
Vitamin D3 (cholecalciferol) is synthesized by keratinocytes in an ultraviolet light–dependent reaction occurring optimally at ultraviolet wavelengths of 290 to 320 nm
Vitamin D2 (ergocalciferol) is obtained only by diet
Individuals with darker skin synthesize less vitamin D3 from sunlight
Vitamin D3 precursors in the skin decrease with age; older individuals have a decreased ability to synthesize cutaneous vitamin D
Vitamin D synthesis
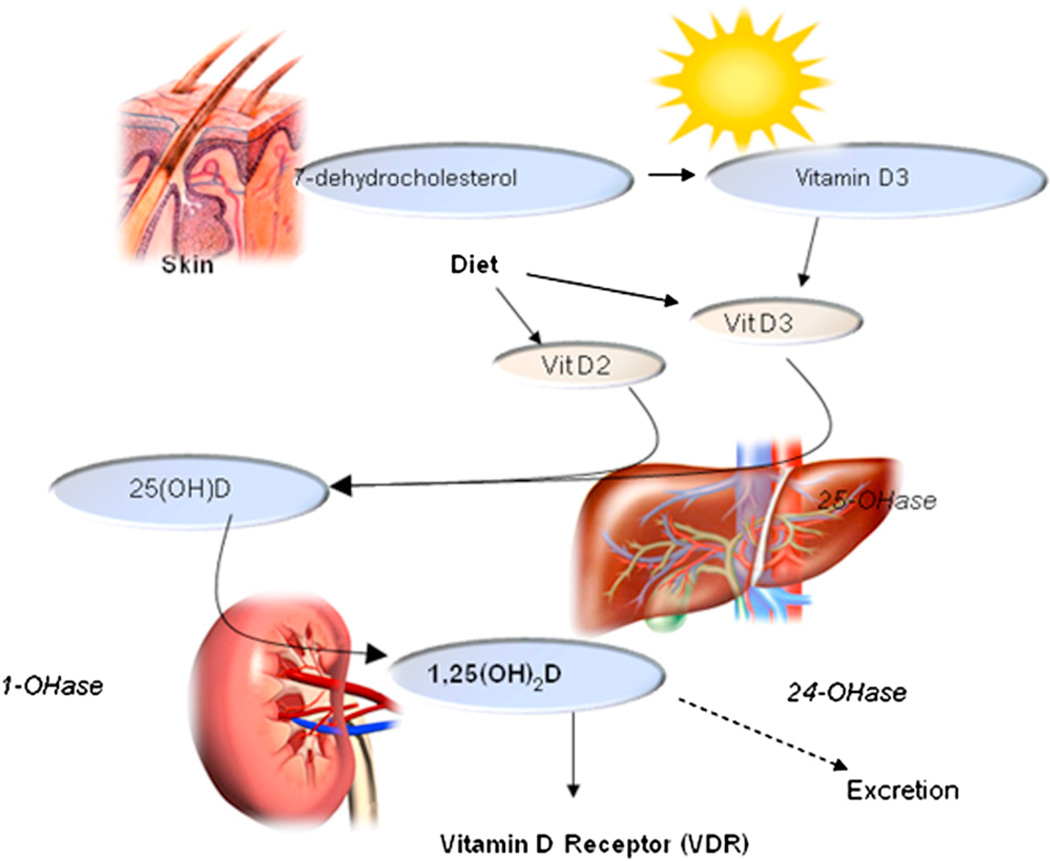
Vitamin D comes in several forms (Table I); the 2 most important are D2 (ergocalciferol, plant-derived) and D3 (cholecalciferol), which is found in select animal products like oily fish (eg, salmon and cod). D3 is also synthesized by keratinocytes, which convert 7-dehydrocholesterol to previtamin D3 in a reaction catalyzed by UV radiation at wavelengths of 290 to 320 nm, 48 with peak synthesis occurring between 295 and 300 nm. 49 Whether obtained from sun exposure, food, or supplements, previtamins D2 and D3 are biologically inert and must undergo 2 hydroxylations in the body for activation (Fig 1). The first occurs in the liver and converts the previtamin to 25-hydroxyvitamin D (25[OH]D), or calcidiol. Because calcidiol has a half-life of several weeks50 and reflects the body’s circulating stores, it is the conventionally measured form of serum vitamin D, and levels typically range between 15 and 80 ng/mL (37.5–200 nmol/L). Most laboratories measure total 25(OH)D without differentiating between D2 and D3 forms. Levels of 25(OH)D are used to evaluate vitamin D status and screen for deficiency.
Table I.
Common forms of vitamin D
| Name | Clinical name | Abbreviation | Source |
|---|---|---|---|
| Ergocalciferol | Vitamin D2 | D2 | Derived from irradiated ergosterol in fungal plants (mushrooms) |
| Cholecalciferol | Vitamin D3 | D3 | Select animal products, such as oil-rich fish (salmon, mackerel, and herring); derived from irradiated 7-dehydrocholesterol from lanolin |
| Calcidiol | 25-hydroxyvitamin D | 25(OH)D | Circulating form of vitamin D, used to evaluate vitamin D status |
| Calcitriol | 1,25-hydroxyvitamin D | 1,25(OH)2D | Hormonally active form of D, binds to the vitamin D receptor |
| Calcipotriene, topical | Calcipotriene | Dovonex | 1,25(OH)2D derivative, topical treatment for psoriasis |
| Calcitriol, topical | Calcitriol | Vectical | Used as treatment for psoriasis |
Fig 1.
Vitamin D pathway.
The second hydroxylation of vitamin D occurs in the kidney and results in the formation of the physiologically active 1,25-dihydroxyvitamin D3 (1,25 [OH]2D), or calcitriol. Serum calcitriol has a half-life of 6 to 8 hours,50 and its production by the kidney is tightly regulated by serum parathyroid, calcium, and phosphate levels. Normal serum levels of calcitriol range between 15 and 45 pg/mL—roughly 1000 times less than levels of 25(OH)D.51 1,25(OH)2D stimulates intestinal calcium and phosphorous absorption, and only 10% of dietary calcium and 60% of phosphorous are absorbed in the absence of vitamin D. Serum 1,25(OH)2D does not reflect vitamin D reserves, and its measurement is not useful for monitoring the vitamin D status of patients. In fact, serum 1,25(OH)2D may be normal or even elevated in those with vitamin D deficiency because of secondary hyperparathyroidism. The measurement of serum 1,25(OH)2D is useful in chronic kidney disease, rickets, and chronic granulomatous diseases, such as sarcoidosis and some lymphomas.52
In addition to its production in the kidney, 1,25(OH)2D is produced within keratinocytes, which also have vitamin D receptors (VDRs).53 The regulation of 1,25(OH)2D production by keratinocytes and other nonrenal cells differs from that of the kidney. However, the amount of keratinocyte-produced 1,25(OH)2D is small and does not contribute to circulating levels under normal circumstances, suggesting the existence of a local autocrine or paracrine regulatory pathway.53 Recent studies show that keratinocyte-synthesized 1,25(OH)2D modulates epidermal cellular proliferation, differentiation, and apoptosis.35,54,55
Vitamin D receptor
After synthesis in the kidney, 1,25(OH)2D is released into the circulatory system, where it binds to the carrier protein vitamin D binding protein (VDBP). Once it reaches its target cells, 1,25(OH)2D dissociates from the VDBP, presumably enters the cell by diffusion, and binds to the cell’s intracellular nuclear VDR to regulate gene transcription. The VDR is present in most tissues and cells in the body, and has a wide range of biologic actions, including decreasing cellular proliferation, inducing terminal differentiation, inhibiting angiogenesis, stimulating insulin production, and stimulating macrophage cathelicidin production.52
The VDR, a member of the steroid nuclear receptor superfamily, is expressed in a wide variety of cells and tumors.56–58 Binding of 1,25(OH)2D to the VDR produces conformational changes, exposing surfaces for coactivating factor binding and dimerization. TheVDR dimerizes with a retinoid receptor, generally the retinoid X receptor, a requisite step for full transactivation of the VDR. Dimerization enables interaction with the target gene promoter at the vitamin D response element (VDRE), where the coactivating proteins carried on the VDR initiate gene transcription of more than 200 genes.59–61 TheseVDREs can be located throughout the gene, even at considerable distances from the transcription start site.
Sources of vitamin D
Diet
A limited number of foods naturally contain vitamin D (Table II). Some mushrooms contain variable amounts of D2; other dietary sources contain the D3 form, including fatty fish like herring, cod, salmon, and fish liver oil. Small amounts of D3 are also found in beef liver and egg yolk. Given the paucity of natural dietary sources of vitamin D, select foods in the United States are fortified with vitamin D, usually in D3 form, providing most of the vitamin D in the American diet. 62 Nearly all milk produced in the United States is fortified as a result of a program instituted in the 1930s to prevent rickets. Select other dairy products, breakfast cereals, some brands of orange juice, margarine, and infant formulas are also fortified (Table II).
Table II.
Dietary sources of vitamin D*
| Source | Serving size | Vitamin D form |
Amount (IU) |
|---|---|---|---|
| Natural | |||
| Herring, cooked | 3.0 OZ | D3 | 1,383 |
| Cod liver oil | 1 tbsp (15 mL) | D3 | 1,360 |
| Salmon, wild | 3.5 oz | D3 | 1,000 |
| Salmon, farmed | 3.5 oz | D3 | 300 |
| Catfish, cooked | 3.0 oz | D3 | 425 |
| Sardines, canned | 1.7 oz | D3 | 250 |
| Mackerel, canned | 3.5 oz | D3 | 345 |
| Tuna, canned | 3.0 oz | D3 | 200 |
| Shiitake mushrooms | |||
| Fresh | 3.5 oz | D2 | 100 |
| Sun-dried | 3.5 oz | D2 | 1,600 |
| Eel, cooked | 3.5 oz | D3 | 200 |
| Egg yolk | 1.0 oz | D3 | 20 |
| Liver (beef) | 3.5 oz | D3 | 15 |
| Supplemented | |||
| Milk | 8.0 oz | D3 | 100 |
| Orange juice | 8.0 oz | D3 | 100–150 |
| Yogurt | 8.0 oz | D3 | 100 |
| Butter | 3.5 oz | D3 | 50 |
| Margarine | 1 tbsp | D3 | 60 |
| Cheese | 3 oz | D3 | 100 |
| Breakfast cereal | 1 serving (0.75–1 cup) | D3 | 40 |
IU, International Unit.
Data taken from the National Institutes of Health web site (http://www.grc.com/health/pdf/NIH_GOV_Dietary_Supplement_Fact_Sheet.pdf") and the US Department of Agriculture’s nutrient database web site (http://www.nal.usda.gov/fnic/foodcomp/search).
Supplements
Intake reference values for nutrients are provided by the Food and Nutrition Board at the Institute of Medicine (IOM) of the National Academies. 63,64 The recommended dietary allowance (RDA) is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97.5%) healthy people. The RDA for vitamin D represents a daily intake sufficient to maintain bone health and normal calcium metabolism in healthy people, assuming that little vitamin D is synthesized from sun exposure (Table III). In November 2010, the IOM reviewed nearly 1000 scientific studies of vitamin D in relation to not only bone health but also many other health outcomes. It concluded that there is clear evidence that vitamin D has bone benefits but that the current research is inconclusive as to whether higher vitamin D intake can reduce the risk for cancer, heart disease, stroke, or other chronic diseases. The IOM recommended 600 international units (IU) per day for people between 1 and 70 years of age and 800 IU per day for those ≥ 71 years of age (an increase from the 400–600 IU/day previously recommended for adults at midlife and older). This 600 IU recommendation is the same for women who are pregnant or lactating. The IOM RDA is based on the benefits of vitamin D to bone because the panel did not find conclusive evidence for nonskeletal actions; however, the report did not specifically exclude the possibility that vitamin D could have a role in cancer prevention.64 This 600 IU RDA is controversial, because several vitamin D investigators have argued that these recommendations are only applicable to healthy individuals and may be too low for patients with disease or for physicians attempting to prevent disease in at-risk populations. The Vitamin D and Omega-3 Trial (VITAL; clinicaltrials.gov identifier NCT01169259) is currently underway and will test in a randomized, controlled trial in 20,000 subjects to determine whether 2000 IU of vitamin D3 will reduce the risk for developing cancer, heart disease, and stroke.
Table III.
Current recommended daily intake of International Units of vitamin D that covers the needs of >97.5% of the US population*
| Age | Children | Adults |
|---|---|---|
| 0–12 mo | 400 | — |
| 1–13 yrs | 600 | — |
| 14–50 yrs | — | 600 |
| 51–70 yrs | — | 600 |
| ≥71 yrs | — | 800 |
Data taken from Ross et al.64
Controversy also exists over which form of vitamin D (D3 or D2) is best for supplementation. Some studies suggest that D3 is more potent than D2,65,66 whereas others suggest that they are bioequivalent.67,68 Trang et al66 found that subjects who took approximately 4000 IU of D3 daily for 14 days had a 1.7-fold increase in serum 25(OH)D levels compared to those who took D2.66 Similarly, Armas et al65 gave 20 healthy male volunteers one 50,000 IU dose of either D2 or D3 and followed serum 25(OH)D levels over 28 days. Although both D2 and D3 produced similar initial rises in serum 25(OH)D, levels fell rapidly in the D2-treated group and were not different from baseline at 14 days, while levels peaked at 14 days and remained high in the D3 group. The authors speculate that D3 has a greater affinity for serum VDBP and therefore greater bioavailability. These differences in binding to VDBP are well known in other animals. The VDBP in fish and fowl, for example, have very low affinity for D269 In contrast, other studies suggest that D2 is as effective as D3 in maintaining circulating concentrations of 25(OH)D.67,68 Holick et al67 compared supplements containing 1000 IU of vitamin D3, 1000 IU of vitamin D2, or 500 IU vitamin D2 and 500 IU vitamin D3 given to healthy adults 18 to 84 years of age daily for 11 weeks in a randomized, placebo-controlled, double-blind study and found that circulating levels of 25(OH)D increased to the same extent in all supplement groups. The disparities in these study results may be attributed to differences in sun exposure or other dietary intake of vitamin D in participants, or differences in the supplement’s stability, because D2 and D3 degrade differently with environmental exposures.70 The Endocrine Society, along with the Canadian Society of Endocrinology and Metabolism and the National Osteoporosis Foundation, published a clinical practice guideline in 2011 titled “Evaluation, Treatment and Prevention of Vitamin D Deficiency.” The Endocrine Society Clinical Guidelines suggests either vitamin D2 or vitamin D3 for the treatment and prevention of vitamin D deficiency.71 The committee also recommended screening of only those individuals who are at high risk for vitamin D deficiency, including patients with osteoporosis, older patients with a history of falls, patients with malabsorption syndrome, chronic renal or hepatic disease, granulomatous diseases (sarcoid), and individuals with darker skin pigmentation (black and Hispanic individuals), obese persons (those with a body mass index [BMI] of >30 kg/m2), and those taking medications known to increase 25(OH)D metabolism, such as anticonvulsants, systemic glucocorticoids, ketoconazole, and HIV medications.
Sun
Ultraviolet B (UVB) light radiation is responsible for cutaneous vitamin D synthesis.52 Interestingly, the portion of the UVB spectrum necessary for vitamin D synthesis coincides with wavelengths implicated in photocarcinogenesis.72 The UVB radiation (290–320 nm) threshold dosage level of 18 to 20 mJ/cm2 required for the conversion of 7-dehydrocholesterol to previtamin D3 in vitro closely mirrors an 18 mJ/cm2 in vivo threshold required for a significant increase in serum 25(OH)D levels in white males with Fitzpatrick type III.73
With full body exposure to sunlight enough to generate 1 minimal erythemal dose, the maximum production level is 10,000 to 25,000 IU per day.74 Studies suggest that clinically significant increases in serum 25(OH)D levels can be seen with UVB doses small enough to produce tanning noticeable only to a colorimeter. Armas et al75 found that subjects’ serum levels of 25(OH)D increased an average of 12 ng/mL after 4 weeks of regular, measured exposure to UVB light. Another study76 estimated that exposure of 25% of body surface to sunlight at noon for 3 to 8 minutes generates about 400 IU of vitamin D in the summer in Boston, Massachusetts and year-round in Miami, Florida. Holick’s recent suggestions77 of biweekly exposures lasting 5 to 30 minutes during midday hours have been the source of much discussion and additional study. Webb et al78 and others examined several factors, including the effects of solar altitudes, air ozone composition, and time of day on cutaneous vitamin D synthesis and found that midday sun, accompanied by high solar altitude, offers the best ratio of vitamin D synthesis–erythema risk. Midday sun exposure corresponding with low solar zenith angles—observed during summer seasons and year-round at equatorial latitudes, and which results in solar rays traveling the shortest distance to the earth—results in optimal vitamin D synthesis.79,80 Greater solar zenith angles occur during the winter season and at more northern latitudes. During these times, more exposure time is needed to achieve adequate vitamin D synthesis. However, one estimate found that very little vitamin D synthesis was possible in the winter in Boston.76 Serum 25(OH)D can be tested in the summer or winter, but should only be done in individuals at risk for vitamin D deficiency as outlined by the Endocrine Society or in individuals who practice extreme photoprotection. There is no evidence showing benefits of screening for vitamin D deficiency in the general population.71
It is uncertain if a “safe” dose of UVB exists (ie, one that maximizes vitamin D synthesis while minimizing DNA damage and photocarcinogenesis). Cutaneous vitamin D3 synthesis after UVB exposure follows an early exponential function.73 With longer exposure to UVB rays, equilibrium is achieved in the skin, and the vitamin begins to degrade as fast as it is generated. Production appears to level off after approximately 10% to 20% of epithelial 7-dehydroxycholesterol stores are used. This maximum level of synthesis is reached after only a sub–minimal erythema dose amount of sunlight. 49,81–83 These data suggest that sunlight exposure follows an inverted J-curve in which an initial amount of beneficial sunlight gives way to harmful effects after a prolonged period. In its recent report, the IOM acknowledged the need for more research on whether the amount of sun exposure needed for cutaneous vitamin D synthesis is associated with an increased risk for skin cancer.
Aside from sunlight exposure timing and duration, other factors affect the amount of vitamin D that can be synthesized by exposure to sunlight alone.
Skin pigmentation
African Americans and those with darker skin types have greater difficulty synthesizing vitamin D from sunlight, because increased melanin in the epidermis absorbs much of the UVB radiation needed for vitamin D production.84,85 In contrast, whites and those with lighter skin types can obtain substantial vitamin D synthesis in as little as 5 minutes of exposure to the hands.85 Serum 25(OH)D levels can differ by skin pigmentation. Most84,86,87 (but not all88) studies suggest that Fitzpatrick skin types I and II have higher levels than darker skin types. Multiple observational studies have shown that African Americans and Hispanics in the United States are at higher risk for vitamin D deficiency than their white counterparts.86,89–92 Research into additional potential genetic factors is ongoing.92 Overall, data suggest that individuals with darker skin types are at increased risk of vitamin D deficiency.
Latitude
Both latitude and season affect the quantity and quality of UVB radiation reaching the earth’s surface. UVB wavelengths of 295 to 300 nm are present in sunlight when the UV index is >3. At this solar intensity, which occurs daily within the tropics and during the spring and summer seasons in temperate regions, adequate amounts of D3 can be made in the skin after only 10 to 15 minutes of twice-weekly sun exposure to the face, arms, hands, or back without sunscreen in most people.76,93,94 At higher latitudes, greater solar zenith angles result in less UVB reaching the earth and lead to less favorable conditions for vitamin D synthesis.76 During winter months, these conditions, coupled with cloud cover and seasonal weather changes, lead to decreased vitamin D synthesis—the so-called “vitamin D winter.”48,68,76 For example, in one study, human skin exposed to sunlight on cloudless days in Boston, Massachusetts (42.2° N) from November through February and Edmonton, Alberta, Canada (52° N) from October through March produced no previtamin D348 There is very little cutaneous vitamin D3 synthesis above or below latitudes of approximately 33° N during the winter.
Obesity
Studies show that vitamin D levels and BMI are negatively correlated in both children and adults.86,95,96 There is growing evidence that in obese individuals (BMI >30 kg/m2), vitamin D is efficiently stored in body fat reserves and consequently loses its bioavailability.97–99 In addition, bariatric patients or patients with malabsorption syndromes are often unable to absorb vitamin D.52
Age
Average skin thickness (both dermal and epidermal) decreases with age.100,101 As a result, cutaneous vitamin D synthesis decreases because of smaller stores of the precursor 7-dehydroxycholesterol.100 The elderly may be at additional risk of deficiency because of decreased mobility and consequently decreased sun exposure.102
Because the cutaneous production of vitamin D is affected by age, skin pigmentation, and latitude of residence along with other factors, such as sunscreen use and sun-avoidant behavior, sunlight exposure may produces variable serum 25(OH)D results. Even living in sunny climates does not guarantee optimum levels of 25(OH)D; one study found that more than half of adults living in Hawaii with an average sun exposure of 30 hours per week still had insufficient serum 25(OH)D levels.103 Other studies conducted in Arizona, Florida, and Chilean populations have all observed that vitamin D deficiency is still noticeable, with darker skinned individuals and those with lower amounts of sun exposure at particular risk.89,103–105
PHOTOPROTECTION AND VITAMIN D LEVELS
Key points
Skin cancer patients and patients who practice extreme photoprotection (systemic lupus, xeroderma pigmentosum, and basal cell nevus syndrome) are at higher risk of vitamin D deficiency
In controlled conditions, sunscreen with a sun protection factor of 15 to 30 can decrease vitamin D synthesis
However, in typical use conditions, sunscreen use has not been shown to appreciably lower vitamin D levels
Frequent shade use and long sleeve use is more likely to cause lower vitamin D levels than sunscreen use
Perhaps the most controversial topic in the debate on vitamin D centers on photoprotection and its effects on the cutaneous synthesis of vitamin D. Patients with systemic lupus erythematosus or individuals in nursing homes (who tend to have little sun exposure) have been shown to be at increased risk for vitamin D deficiency.106,107 Several studies have shown that the regular use of sunscreen correlates with lower circulating levels of 25(OH)D. The use of sunscreens with a sun protection factor (SPF) of 8 inhibits >95% of vitamin D production in the skin.108 After a successful sun safety health campaign encouraging Australians to avoid sun exposure to prevent skin cancer, an increased number of Australians became vitamin D–deficient.109 Similarly, patients with basal cell nevus syndrome, who exhibit higher levels of sun-protective behavior, such as using sunscreen and avoiding the midday sun, have a higher prevalence of vitamin D deficiency than the general population.110 One study even questions the notion that fair-skinned individuals are more likely to have adequate vitamin D levels because they can synthesize the molecule more efficiently than those with darker skin; in this study, fair-skinned subjects were more likely to be deficient, which was attributed to increased sunscreen use and sun-avoiding behavior.88,111 Individuals who practice vigilant sun avoidance and sun protection may contribute to vitamin D deficiency.
In one of the earliest studies on the subject, Matsuoka et al108 found that vitamin D levels are reduced by 95% with uniform, whole-body application of an SPF 30 sunscreen. Contrasting studies have observed a lack of differences in serum 25(OH)D levels between sunscreen and nonsunscreen users.93,112 Other studies acknowledge a decrease of vitamin D levels yet argue that such decreases are not great enough to place sunscreen users at significant risk of vitamin D deficiency.113,114 As discussed by Ness115 and Marks et al,116 these results show that sunscreen use does have appreciable effects on serum levels of vitamin D in either the 25(OH)D or 1,25(OH) 2D3 forms. Sunscreen is generally more effective at blocking the action spectrum of UVB radiation necessary for previtamin D synthesis than it is at blocking the action spectrum for erythema.79
Farrerons et al113 suggests that typical sunscreen use is not nearly as efficient as in Matsuoka et al’s laboratory controlled experiments, with proper application technique and whole-body application being unlikely. Such a lack of adherence to proper sunscreen application technique negatively affects the SPF117–119 Finally, the use of inorganic ingredients (ie, zinc oxide and titanium oxide) in sunscreens that provide a broadband physical barrier to the action spectrum of UV light may mean these formulations reduce total transmitted UV radiation,120 which in turn may affect cutaneous vitamin D synthesis.
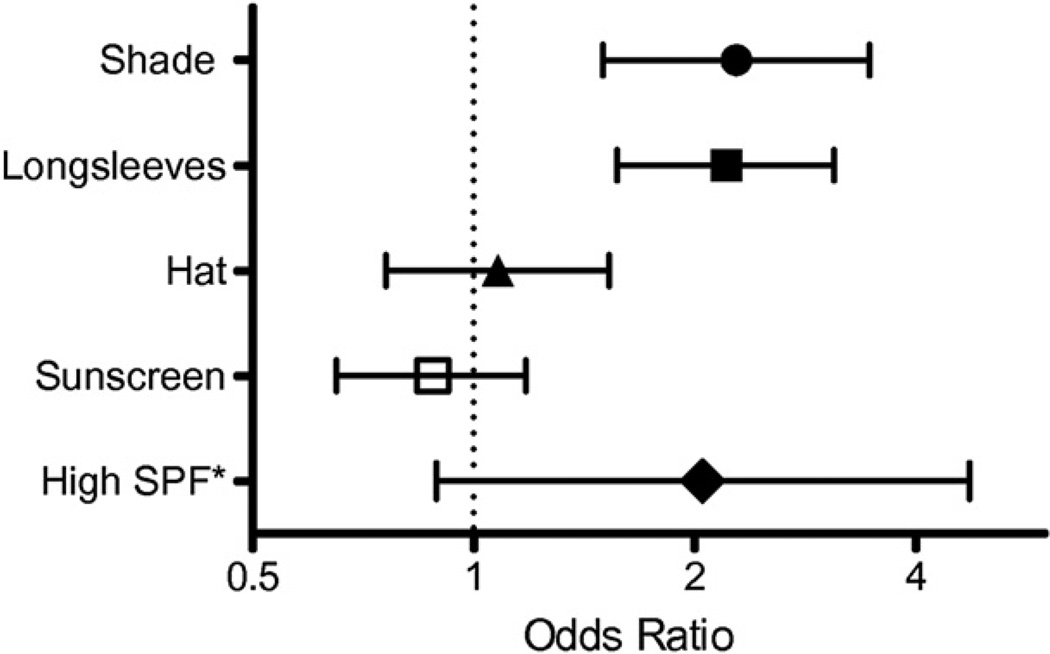
In an attempt to determine the role of sun protection on vitamin D levels at the population level, one study used data from a cross sectional, nationally representative survey of 5920 adults to investigate the relationship between different types of sun protective behaviors and serum 25(OH)D levels in the general US population.121 Overall sun protection was associated with lower 25(OH)D levels in whites, but not in Hispanics or blacks. Staying in the shade and wearing long sleeves were significantly associated with lower 25(OH)D levels and vitamin D deficiency, especially in whites, but wearing a hat and using sunscreen use were not (Fig 2). However, the use of a high SPF sunscreen was associated with a 2-fold increased risk of vitamin D deficiency, although this approached but did not reach statistical significance. The relationships between sun protective behaviors and lower 25(OH)D levels were much weaker among Hispanics and blacks, possibly because of the inherent natural sun protective effect of melanin in darkly pigmented skin. Because the natural pigment in darker skin is a potent UV blocker, any additional sun-protection above and beyond this may have a minimal relative impact. In conclusion, individuals who protect themselves from the sun by seeking shade or wearing long sleeves may have lower 25(OH)D levels and be at risk for vitamin D deficiency.
Fig 2.
Odds ratios of vitamin D deficiency comparing high frequency to low frequency users according to sun protective behaviors among whites in the United States. Data taken from National Health and Nutrition Examination Survey subjects who self-reported high versus low usage of shade, long sleeves, a hat, or sunscreen on a very sunny day (2003–2006). *Multivariate adjusted odds ratio of vitamin D deficiency comparing high usage of sun protection factor >40 sunscreen to sun protection factor <20 sunscreen. The symbols indicate the odds ratios, and the whiskers indicate the 95% confidence intervals.
Despite the fact that sunscreen use may block some cutaneous vitamin D synthesis, it is prudent to continue to recommend sun-cautious behavior given the known hazards of UV exposure. It is likely that in most healthy individuals, a diet that includes vitamin D–rich foods and moderate amounts of supplements combined with a modest amount of everyday sun exposure is enough to maintain adequate serum vitamin D levels, even if the individual photoprotects with sunscreen. What remains unclear is whether the vitamin D produced in the skin has a benefit to the skin that is greater than the equivalent amount of vitamin D ingested as a supplement. Sunlight exposure sufficient to burn the skin is clearly excessive and counterproductive to maintaining the proper balance between vitamin D production and skin health.
CAPSULE SUMMARY.
25-hydroxyvitamin D, or 25(OH)D, is the circulating form of vitamin D used to determine vitamin D status and for screening for vitamin deficiency.
The Institute of Medicine recommends 600 International Units of vitamin D daily for most children and adults.
Both vitamin D2 and vitamin D3 are effective at correcting vitamin D deficiency.
Cutaneous production of vitamin D3 is affected by age, skin pigmentation, latitude, and sun avoidance behaviors
Acknowledgments
Supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases grants K23 AR 051037-01 (Dr Asgari) and K23 AR 056736-01 (Dr Tang) and the Damon Runyon Clinical Investigator Award (Dr Tang).
Abbreviations used
- 1,25(OH)2D
1,25-dihydroxyvitamin D3, the biologically active form of serum vitamin D
- 25(OH)D
25-hydroxyvitamin D
- BMI
body mass index
- IOM
Institute of Medicine
- IU
International Unit
- NMSC
nonmelanoma skin cancer
- RDA
recommended dietary allowance
- SPF
sun protection factor
- UV
ultraviolet
- UVB
ultraviolet B
- VDR
vitamin D receptor
- VDBP
vitamin D binding protein
REFERENCES
- 1.Cranney A, Weiler HA, O’Donnell S, Puil L. Summary of evidence-based review on vitamin D efficacy and safety in relation to bone health. Am J Clin Nutr. 2008;88:513S–519S. doi: 10.1093/ajcn/88.2.513S. [DOI] [PubMed] [Google Scholar]
- 2.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto N, Naraparaju VR. Role of vitamin D3-binding protein in activation of mouse macrophages. J Immunol. 1996;157:1744–1749. [PubMed] [Google Scholar]
- 4.Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, et al. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115–7120. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaler P, Augenlicht L, Klampfer L. Macrophage-derived IL-1beta stimulates Wnt signaling and growth of colon cancer cells: a crosstalk interrupted by vitamin D3. Oncogene. 2009;28:3892–3902. doi: 10.1038/onc.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zehnder D, Quinkler M, Eardley KS, Bland R, Lepenies J, Hughes SV, et al. Reduction of the vitamin D hormonal system in kidney disease is associated with increased renal inflammation. Kidney Int. 2008;74:1343–1353. doi: 10.1038/ki.2008.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandini S, Raimondi S, Gnagnarella P, Dore JF, Maisonneuve P, Testori A. Vitamin D and skin cancer: a meta-analysis. Eur J Cancer. 2009;45:634–641. doi: 10.1016/j.ejca.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 11.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. J Am Med Assoc. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 12.Tretli S, Hernes E, Berg JP, Hestvik UE, Robsahm TE. Association between serum 25(OH)D and death from prostate cancer. Br J Cancer. 2009;100:450–454. doi: 10.1038/sj.bjc.6604865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heist RS, Zhou W, Wang Z, Liu G, Neuberg D, Su L, et al. Circulating 25-hydroxyvitamin D, VDR polymorphisms, and survival in advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:5596–5602. doi: 10.1200/JCO.2008.18.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng K, Wolpin BM, Meyerhardt JA, Wu K, Chan AT, Hollis BW, et al. Prospective study of predictors of vitamin D status and survival in patients with colorectal cancer. Br J Cancer. 2009;101:916–923. doi: 10.1038/sj.bjc.6605262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 2007;32:210–216. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Garland CF, Gorham ED, Mohr SB, Grant WB, Giovannucci EL, Lipkin M, et al. Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol. 2007;103:708–711. doi: 10.1016/j.jsbmb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou W, Heist RS, Liu G, Asomaning K, Neuberg DS, Hollis BW, et al. Circulating 25-hydroxyvitamin D levels predict survival in early-stage non-small-cell lung cancer patients. J Clin Oncol. 2007;25:479–485. doi: 10.1200/JCO.2006.07.5358. [DOI] [PubMed] [Google Scholar]
- 19.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States) Cancer Causes Control. 2005;16:83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 20.Grant WB. Differences in vitamin-D status may explain black-white differences in breast cancer survival rates. J Natl Med Assoc. 2008;100:1040. doi: 10.1016/s0027-9684(15)31441-3. [DOI] [PubMed] [Google Scholar]
- 21.Grant WB, Garland CF. The association of solar ultraviolet B (UVB) with reducing risk of cancer: multifactorial ecologic analysis of geographic variation in age-adjusted cancer mortality rates. Anticancer Res. 2006;26:2687–2699. [PubMed] [Google Scholar]
- 22.Grant WB. Lower vitamin-D production from solar ultraviolet-B irradiance may explain some differences in cancer survival rates. J Natl Med Assoc. 2006;98:357–364. [PMC free article] [PubMed] [Google Scholar]
- 23.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol. 2005;97:179–194. doi: 10.1016/j.jsbmb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz GG, Hanchette CL. UV, latitude, and spatial trends in prostate cancer mortality: all sunlight is not the same (United States) Cancer Causes Control. 2006;17:1091–1101. doi: 10.1007/s10552-006-0050-6. [DOI] [PubMed] [Google Scholar]
- 25.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 26.Zhou W, Suk R, Liu G, Park S, Neuberg DS, Wain JC, et al. Vitamin D is associated with improved survival in early-stage non-small cell lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2005;14:2303–2309. doi: 10.1158/1055-9965.EPI-05-0335. [DOI] [PubMed] [Google Scholar]
- 27.Moan J, Porojnicu A, Lagunova Z, Berg JP, Dahlback A. Colon cancer: prognosis for different latitudes, age groups and seasons in Norway. J Photochem Photobiol B. 2007;89:148–155. doi: 10.1016/j.jphotobiol.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Porojnicu AC, Robsahm TE, Ree AH, Moan J. Season of diagnosis is a prognostic factor in Hodgkin’s lymphoma: a possible role of sun-induced vitamin D. Br J Cancer. 2005;93:571–574. doi: 10.1038/sj.bjc.6602722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porojnicu AC, Lagunova Z, Robsahm TE, Berg JP, Dahlback A, Moan J. Changes in risk of death from breast cancer with season and latitude: sun exposure and breast cancer survival in Norway. Breast Cancer Res Treat. 2007;102:323–328. doi: 10.1007/s10549-006-9331-8. [DOI] [PubMed] [Google Scholar]
- 30.Porojnicu AC, Robsahm TE, Dahlback A, Berg JP, Christiani D, Bruland OS, et al. Seasonal and geographical variations in lung cancer prognosis in Norway. Does Vitamin D from the sun play a role? Lung Cancer. 2007;55:263–270. doi: 10.1016/j.lungcan.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006;4:e232. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang JY, Xiao TZ, Oda Y, Chang KS, Shpall E, Wu A, et al. Vitamin D3 inhibits hedgehog signaling and proliferation in murine Basal cell carcinomas. Cancer Prev Res (Phila) 2011;4:744–751. doi: 10.1158/1940-6207.CAPR-10-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon KM, Deo SS, Wong G, Slater M, Norman AW, Bishop JE, et al. Skin cancer prevention: a possible role of 1,25dihy-droxyvitamin D3 and its analogs. J Steroid Biochem Mol Biol. 2005;97:137–143. doi: 10.1016/j.jsbmb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Mason RS, Sequeira VB, Dixon KM, Gordon-Thomson C, Pobre K, Dilley A, et al. Photoprotection by 1alpha,25-dihydroxyvitamin D and analogs: further studies on mechanisms and implications for UV-damage. J Steroid Biochem Mol Biol. 2010;121:164–168. doi: 10.1016/j.jsbmb.2010.03.082. [DOI] [PubMed] [Google Scholar]
- 35.Moll PR, Sander V, Frischauf AM, Richter K. Expression profiling of vitamin D treated primary human keratinocytes. J Cellular Biochem. 2007;100:574–5792. doi: 10.1002/jcb.21061. [DOI] [PubMed] [Google Scholar]
- 36.Zinser GM, Sundberg JP, Welsh J. Vitamin D(3) receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis. 2002;23:2103–2109. doi: 10.1093/carcin/23.12.2103. [DOI] [PubMed] [Google Scholar]
- 37.Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108:1083–1086. doi: 10.1210/endo-108-3-1083. [DOI] [PubMed] [Google Scholar]
- 38.Seifert M, Rech M, Meineke V, Tilgen W, Reichrath J. Differential biological effects of 1,25-dihydroxyVitamin D3 on melanoma cell lines in vitro. J Steroid Biochem Mol Biol. 2004;89–90:375–379. doi: 10.1016/j.jsbmb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Yudoh K, Matsuno H, Kimura T. 1alpha,25-dihydroxyvitamin D3 inhibits in vitro invasiveness through the extracellular matrix and in vivo pulmonary metastasis of B16 mouse melanoma. J Lab Clin Med. 1999;133:120–128. doi: 10.1016/s0022-2143(99)90004-5. [DOI] [PubMed] [Google Scholar]
- 40.Eide MJ, Johnson DA, Jacobsen GR, Krajenta RJ, Rao DS, Lim HW, et al. Vitamin D and nonmelanoma skin cancer in a health maintenance organization cohort. Arch Dermatol. 2011;147:1379–1384. doi: 10.1001/archdermatol.2011.231. [DOI] [PubMed] [Google Scholar]
- 41.Asgari MM, Tang J, Warton ME, Chren MM, Quesenberry CP, Jr, Bikle D, et al. Association of prediagnostic serum vitamin D levels with the development of basal cell carcinoma. J Invest Dermatol. 2010;130:1438–1443. doi: 10.1038/jid.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang JY, Parimi N, Wu A, Boscardin WJ, Shikany JM, Chren MM, et al. Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men. Cancer Causes Control. 2010;21:387–391. doi: 10.1007/s10552-009-9470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang JY, Fu T, Leblanc E, Manson JE, Feldman D, Linos E, et al. Calcium plus vitamin D supplementation and the risk of nonmelanoma and melanoma skin cancer: post hoc analyses of the women’s health initiative randomized controlled trial. J Clin Oncol. 2011;29:3078–3084. doi: 10.1200/JCO.2011.34.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asgari MM, Maruti SS, Kushi LH, White E. A cohort study of vitamin D intake and melanoma risk. J Invest Dermatol. 2009;129:1675–1680. doi: 10.1038/jid.2008.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinstock MA, Stampfer MJ, Lew RA, Willett WC, Sober AJ. Case-control study of melanoma and dietary vitamin D: implications for advocacy of sun protection and sunscreen use. J Invest Dermatol. 1992;98:809–811. doi: 10.1111/1523-1747.ep12499962. [DOI] [PubMed] [Google Scholar]
- 46.van Dam RM, Huang Z, Giovannucci E, Rimm EB, Hunter DJ, Colditz GA, et al. Diet and basal cell carcinoma of the skin in a prospective cohort of men. The American Journal of Clinical Nutrition. 2000;71:135–141. doi: 10.1093/ajcn/71.1.135. [DOI] [PubMed] [Google Scholar]
- 47.Randerson-Moor JA, Taylor JC, Elliott F, Chang YM, Beswick S, Kukalizch K, et al. Vitamin D receptor gene polymorphisms, serum 25-hydroxyvitamin D levels, and melanoma: UK case-control comparisons and a meta-analysis of published VDR data. Eur J Cancer. 2009;45:3271–3281. doi: 10.1016/j.ejca.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 49.MacLaughlin JA, Anderson RR, Holick MF. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science. 1982;216:1001–1003. doi: 10.1126/science.6281884. [DOI] [PubMed] [Google Scholar]
- 50.Corder EH, Guess HA, Hulka BS, Friedman GD, Sadler M, Vollmer RT, et al. Vitamin D and prostate cancer: a prediagnostic study with stored sera. Cancer Epidemiol Biomarkers Prev. 1993;2:467–472. [PubMed] [Google Scholar]
- 51.Zittermann A, Schleithoff SS, Frisch S, Gotting C, Kuhn J, Koertke H, et al. Circulating calcitriol concentrations and total mortality. Clin Chem. 2009;55:1163–1170. doi: 10.1373/clinchem.2008.120006. [DOI] [PubMed] [Google Scholar]
- 52.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 53.Vantieghem K, Kissmeyer AM, De Haes P, Bouillon R, Segaert S. UVB-induced production of 1,25-dihydroxyvitamin D3 and vitamin D activity in human keratinocytes pretreated with a sterol delta7-reductase inhibitor. J Cellular Biochem. 2006;98:81–92. doi: 10.1002/jcb.20756. [DOI] [PubMed] [Google Scholar]
- 54.Bikle DD. Vitamin D and skin cancer. J Nutr. 2004;134(12 suppl):3472S–3478S. doi: 10.1093/jn/134.12.3472S. [DOI] [PubMed] [Google Scholar]
- 55.De Haes P, Garmyn M, Carmeliet G, Degreef H, Vantieghem K, Bouillon R, et al. Molecular pathways involved in the anti-apoptotic effect of 1,25-dihydroxyvitamin D3 in primary human keratinocytes. J Cellular Biochem. 2004;93:951–967. doi: 10.1002/jcb.20227. [DOI] [PubMed] [Google Scholar]
- 56.Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–5448. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 57.Mocellin S, Nitti D. Vitamin D receptor polymorphisms and the risk of cutaneous melanoma: a systematic review and meta-analysis. Cancer. 2008;113:2398–2407. doi: 10.1002/cncr.23867. [DOI] [PubMed] [Google Scholar]
- 58.Osborne JE, Hutchinson PE. Vitamin D and systemic cancer: is this relevant to malignant melanoma? Br J Dermatol. 2002;147:197–213. doi: 10.1046/j.1365-2133.2002.04960.x. [DOI] [PubMed] [Google Scholar]
- 59.Bell NH. Vitamin D-endocrine system. J Clin Invest. 1985;76:1–6. doi: 10.1172/JCI111930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ross TK, Darwish HM, Moss VE, DeLuca HF. Vitamin D-influenced gene expression via a ligand-independent, receptor-DNA complex intermediate. Proc Natl Acad Sci USA. 1993;90:9257–9260. doi: 10.1073/pnas.90.20.9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88:491S–499S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 62.Calvo MS, Whiting SJ, Barton CN. Vitamin D fortification in the United States and Canada: current status and data needs. Am J Clin Nutr. 2004;80(6 suppl):1710S–1716S. doi: 10.1093/ajcn/80.6.1710S. [DOI] [PubMed] [Google Scholar]
- 63.Vitamin D. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington DC: National Academy Press; 1999. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board of the Institute of Medicine. [Google Scholar]
- 64.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–5391. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 66.Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68:854–858. doi: 10.1093/ajcn/68.4.854. [DOI] [PubMed] [Google Scholar]
- 67.Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677–681. doi: 10.1210/jc.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rapuri PB, Gallagher JC, Haynatzki G. Effect of vitamins D2 and D3 supplement use on serum 250HD concentration in elderly women in summer and winter. Calcif Tissue Int. 2004;74:150–156. doi: 10.1007/s00223-003-0083-8. [DOI] [PubMed] [Google Scholar]
- 69.Hollis BW. Comparison of equilibrium and disequilibrium assay conditions for ergocalciferol, cholecalciferol and their major metabolites. J Steroid Biochem. 1984;21:81–86. doi: 10.1016/0022-4731(84)90063-3. [DOI] [PubMed] [Google Scholar]
- 70.Grady LT, Thakker KD. Stability of solid drugs: degradation of ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3) at high humidities and elevated temperatures. J Pharm Sci. 1980;69:1099–1102. doi: 10.1002/jps.2600690932. [DOI] [PubMed] [Google Scholar]
- 71.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 72.Cole CA, Forbes PD, Davies RE. An action spectrum for UV photocarcinogenesis. Photochem Photobiol. 1986;43:275–284. doi: 10.1111/j.1751-1097.1986.tb05605.x. [DOI] [PubMed] [Google Scholar]
- 73.Matsuoka LY, Wortsman J, Haddad JG, Hollis BW. In vivo threshold for cutaneous synthesis of vitamin D3. J Lab Clin Med. 1989;114:301–305. [PubMed] [Google Scholar]
- 74.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842–856. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 75.Armas LA, Dowell S, Akhter M, Duthuluru S, Huerter C, Hollis BW, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57:588–593. doi: 10.1016/j.jaad.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 76.Terushkin V, Bender A, Psaty EL, Engelsen O, Wang SQ, Halpern AC. Estimated equivalency of vitamin D production from natural sun exposure versus oral vitamin D supplementation across seasons at two US latitudes. J Am Acad Dermatol. 2010;62 doi: 10.1016/j.jaad.2009.07.028. 929.e1-9. [DOI] [PubMed] [Google Scholar]
- 77.Holick MF. Sunlight, UV-radiation, vitamin D and skin cancer: how much sunlight do we need? Adv Exp Med Biol. 2008;624:1–15. doi: 10.1007/978-0-387-77574-6_1. [DOI] [PubMed] [Google Scholar]
- 78.Webb AR, Engelsen O. Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem Photobiol. 2006;82:1697–1703. doi: 10.1562/2005-09-01-RA-670. [DOI] [PubMed] [Google Scholar]
- 79.Sayre RM, Dowdy JC. Darkness at noon: sunscreens and vitamin D3. Photochem Photobiol. 2007;83:459–463. doi: 10.1562/2006-06-29-RC-956. [DOI] [PubMed] [Google Scholar]
- 80.Kimlin MG. The climatology of Vitamin D producing ultraviolet radiation over the United States. J Steroid Biochem Mol Biol. 2004;89–90:479–483. doi: 10.1016/j.jsbmb.2004.03.111. [DOI] [PubMed] [Google Scholar]
- 81.Wolpowitz D, Gilchrest BA. The vitamin D questions: how much do you need and how should you get it? J Am Acad Dermatol. 2006;54:301–317. doi: 10.1016/j.jaad.2005.11.1057. [DOI] [PubMed] [Google Scholar]
- 82.Gilchrest BA. Sun protection and vitamin D: three dimensions of obfuscation. J Steroid Biochem Mol Biol. 2007;103:655–663. doi: 10.1016/j.jsbmb.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 83.Holick MF. The cutaneous photosynthesis of previtamin D3: a unique photoendocrine system. J Invest Dermatol. 1981;77:51–58. doi: 10.1111/1523-1747.ep12479237. [DOI] [PubMed] [Google Scholar]
- 84.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 85.Holick MF, Jenkins M. The UV advantage. iBooks. 2003 [Google Scholar]
- 86.Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics. 2009;124:e362–e370. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen TC, Chimeh F, Lu Z, Mathieu J, Person KS, Zhang A, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460:213–217. doi: 10.1016/j.abb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Glass D, Lens M, Swaminathan R, Spector TD, Bataille V. Pigmentation and vitamin D metabolism in Caucasians: low vitamin D serum levels in fair skin types in the UK. PLoS One. 2009;4:e6477. doi: 10.1371/journal.pone.0006477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jacobs ET, Alberts DS, Foote JA, Green SB, Hollis BW, Yu Z, et al. Vitamin D insufficiency in southern Arizona. Am J Clin Nutr. 2008;87:608–613. doi: 10.1093/ajcn/87.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Egan KM, Signorello LB, Munro HM, Hargreaves MK, Hollis BW, Blot WJ. Vitamin D insufficiency among African-Americans in the southeastern United States: implications for cancer disparities (United States) Cancer Causes Control. 2008;19:527–535. doi: 10.1007/s10552-008-9115-z. [DOI] [PubMed] [Google Scholar]
- 91.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Signorello LB, Williams SM, Zheng W, Smith JR, Long J, Cai Q, et al. Blood vitamin D levels in relation to genetic estimation of African ancestry. Cancer Epidemiol Biomarkers Prev. 2010;19:2325–2331. doi: 10.1158/1055-9965.EPI-10-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marks R, Foley PA, Jolley D, Knight KR, Harrison J, Thompson SC. The effect of regular sunscreen use on vitamin D levels in an Australian population. Results of a randomized controlled trial. Arch Dermatol. 1995;131:415–421. [PubMed] [Google Scholar]
- 94.Davie MW, Lawson DE, Emberson C, Barnes JL, Roberts GE, Barnes ND. Vitamin D from skin: contribution to vitamin D status compared with oral vitamin D in normal and anticonvulsant-treated subjects. Clin Sci (Lond) 1982;63:461–472. doi: 10.1042/cs0630461. [DOI] [PubMed] [Google Scholar]
- 95.Jorde R, Sneve M, Emaus N, Figenschau Y, Grimnes G. Cross-sectional and longitudinal relation between serum 25-hydroxyvitamin D and body mass index: the Tromso study. Eur J Nutr. 2010;49:401–407. doi: 10.1007/s00394-010-0098-7. [DOI] [PubMed] [Google Scholar]
- 96.Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 2010;59:242–248. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 98.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 99.Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009;29:3713–3720. [PubMed] [Google Scholar]
- 100.Need AG, Morris HA, Horowitz M, Nordin C. Effects of skin thickness, age, body fat, and sunlight on serum 25-hydroxyvitamin D. Am J Clin Nutr. 1993;58:882–885. doi: 10.1093/ajcn/58.6.882. [DOI] [PubMed] [Google Scholar]
- 101.Nordin BE, Polley KJ. Metabolic consequences of the menopause. A cross-sectional, longitudinal, and intervention study on 557 normal postmenopausal women. Calcif Tissue Int. 1987;41(Suppl 1):882–885. [PubMed] [Google Scholar]
- 102.Webb AR, Pilbeam C, Hanafin N, Holick MF. An evaluation of the relative contributions of exposure to sunlight and of diet to the circulating concentrations of 25-hydroxyvitamin D in an elderly nursing home population in Boston. Am J Clin Nutr. 1990;51:1075–1081. doi: 10.1093/ajcn/51.6.1075. [DOI] [PubMed] [Google Scholar]
- 103.Binkley N, Novotny R, Krueger D, Kawahara T, Daida YG, Lensmeyer G, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab. 2007;92:2130–2135. doi: 10.1210/jc.2006-2250. [DOI] [PubMed] [Google Scholar]
- 104.Levis S, Gomez A, Jimenez C, Veras L, Ma F, Lai S, et al. Vitamin D deficiency and seasonal variation in an adult South Florida population. J Clin Endocrinol Metab. 2005;90:1557–1562. doi: 10.1210/jc.2004-0746. [DOI] [PubMed] [Google Scholar]
- 105.Gonzalez G, Alvarado JN, Rojas A, Navarrete C, Velasquez CG, Arteaga E. High prevalence of vitamin D deficiency in Chilean healthy postmenopausal women with normal sun exposure: additional evidence for a worldwide concern. Menopause. 2007;14:455–461. doi: 10.1097/GME.0b013e31802c54c0. [DOI] [PubMed] [Google Scholar]
- 106.Muller K, Kriegbaum NJ, Baslund B, Sorensen OH, Thymann M, Bentzen K. Vitamin D3 metabolism in patients with rheumatic diseases: low serum levels of 25-hydroxyvitamin D3 in patients with systemic lupus erythematosus. J Clin Rheumatol. 1995;14:397–400. doi: 10.1007/BF02207671. [DOI] [PubMed] [Google Scholar]
- 107.Liu BA, Gordon M, Labranche JM, Murray TM, Vieth R, Shear NH. Seasonal prevalence of vitamin D deficiency in institutionalized older adults. J Am Geriatr Soc. 1997;45:598–603. doi: 10.1111/j.1532-5415.1997.tb03094.x. [DOI] [PubMed] [Google Scholar]
- 108.Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64:1165–1168. doi: 10.1210/jcem-64-6-1165. [DOI] [PubMed] [Google Scholar]
- 109.Nowson CA, Margerison C. Vitamin D intake and vitamin D status of Australians. Med J Aust. 2002;177:149–152. doi: 10.5694/j.1326-5377.2002.tb04702.x. [DOI] [PubMed] [Google Scholar]
- 110.Tang JY, Wu A, Linos E, Parimi N, Lee W, Aszterbaum M, et al. High prevalence of vitamin D deficiency in patients with basal cell nevus syndrome. Arch Dermatol. 2010;146:1105–1110. doi: 10.1001/archdermatol.2010.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Malvy DJ, Guinot C, Preziosi P, Galan P, Chapuy MC, Maamer M, et al. Relationship between vitamin D status and skin phototype in general adult population. Photochem Photobiol. 2000;71:466–469. doi: 10.1562/0031-8655(2000)071<0466:rbvdsa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 112.Kimlin M, Harrison S, Nowak M, Moore M, Brodie A, Lang C. Does a high UV environment ensure adequate vitamin D status? J Photochem Photobiol B. 2007;89:139–147. doi: 10.1016/j.jphotobiol.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 113.Farrerons J, Barnadas M, Rodriguez J, Renau A, Yoldi B, Lopez-Navidad A, et al. Clinically prescribed sunscreen (sun protection factor 15) does not decrease serum vitamin D concentration sufficiently either to induce changes in parathyroid function or in metabolic markers. Br J Dermatol. 1998;139:422–427. doi: 10.1046/j.1365-2133.1998.02405.x. [DOI] [PubMed] [Google Scholar]
- 114.Sollitto RB, Kraemer KH, DiGiovanna JJ. Normal vitamin D levels can be maintained despite rigorous photoprotection: six years’ experience with xeroderma pigmentosum. J Am Acad Dermatol. 1997;37:942–947. doi: 10.1016/s0190-9622(97)70069-0. [DOI] [PubMed] [Google Scholar]
- 115.Ness AR, Frankel SJ, Gunnell DJ, Smith GD. Are we really dying for a tan? Br Med J. 1999;319:114–116. doi: 10.1136/bmj.319.7202.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marks R. Sunlight and health. Use of sunscreens does not risk vitamin D deficiency. Br Med J. 1999;319:1066. [PMC free article] [PubMed] [Google Scholar]
- 117.Stenberg C, Larko O. Sunscreen application and its importance for the sun protection factor. Arch Dermatol. 1985;121:1400–1402. [PubMed] [Google Scholar]
- 118.Sayre RM, Powell J, Rheins LA. Product application technique alters the sun protection factor. Photodermatol Photoimmunol Photomed. 1991;8:222–224. [PubMed] [Google Scholar]
- 119.Lott DL, Stanfield J, Sayre RM, Dowdy JC. Uniformity of sunscreen product application: a problem in testing, a problem for consumers. Photodermatol Photoimmunol Photomed. 2003;19:17–20. doi: 10.1034/j.1600-0781.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 120.Maier T, Korting HC. Sunscreens—which and what for? Skin Pharmacol Physiol. 2005;18:253–262. doi: 10.1159/000087606. [DOI] [PubMed] [Google Scholar]
- 121.Linos E, Keiser E, Kanzler M, Sainani KL, Lee W, Vittinghoff E, et al. Sun protective behaviors and vitamin D levels in the US population: NHANES 2003–2006. Cancer Causes and Control. 2012;23:133–140. doi: 10.1007/s10552-011-9862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]