Abstract
Nipah virus, a member of the paramyxovirus family, was first isolated and identified in 1999 when the virus crossed the species barrier from fruit bats to pigs and then infected humans, inducing an encephalitis with up to 40% mortality. At present there is no prophylaxis for Nipah virus. We investigated the possibility of vaccination and passive transfer of antibodies as interventions against this disease. We show that both of the Nipah virus glycoproteins (G and F) when expressed as vaccinia virus recombinants induced an immune response in hamsters which protected against a lethal challenge by Nipah virus. Similarly, passive transfer of antibody induced by either of the glycoproteins protected the animals. In both the active and passive immunization studies, however, the challenge virus was capable of hyperimmunizing the vaccinated animals, suggesting that although the virus replicates under these conditions, the immune system can eventually control the infection.
When viruses participate in a host-parasite interaction in which the pathology induced by the virus is minimal, this can lead to a persistent infection. Although a number of virus-animal models have been studied in the laboratory, little is known to what extent they are operational in nature. In southeast Asia and Australia, pteroid bats (flying foxes) are the natural host for a number of viruses. Due to recent changes in ecological conditions, in particular slash-and-burn agricultural methods, these bats are increasingly coming into contact with humans and domesticated animals. In this situation, the viruses resident in the bats may cross the species barrier and result in a more virulent, even fatal disease.
In recent years, several paramyxoviruses have emerged in this manner. Rubulaviruses, which have been associated with abortions in pigs (10), have been isolated from these fruit bats, both in Australia and in Malaysia (3, 7). In 1995 in Australia, a previously unidentified paramyxovirus, Hendra virus, infected horses and was transmitted to humans, where it induced fatal pulmonary complications (5, 23). In 1998 in Malaysia, a virus closely related to Hendra virus and now designated Nipah virus infected pigs and subsequently humans, where it was responsible for 265 cases of encephalitis, of which about 40% were fatal (8). Molecular biology studies have shown that these two new viruses have a similar genomic structure, but as their genomes contain some 2,000 nucleotides more than previously studied paramyxoviruses (21, 22), the Hendra and Nipah viruses have now been classified into a new genus, Henipaviruses, within the family Paramyxovirinae.
Pteropus species are found in the area covering the western Indian Ocean to southeast Asia and Australia and the southwest Pacific islands. Since the epidemics in Malaysia and Singapore in 1998 to 1999, serum samples analyzed from Bangladesh and northern India in 2001 showed that infected individuals reacted with Nipah virus antigens (14, 15) and the presence of anti-Nipah virus antibody has also been found in fruit bats in Cambodia in 2002 (12). Although Nipah virus was not isolated in these instances, Nipah virus or serologically related viruses may be circulating.
If an efficient program to prevent or treat Nipah virus infection in humans is to be developed, it will be necessary to define the viral antigens which are important in inducing protective responses and to formulate potential immunoprophylactic treatments. In the present study, we expressed the two Nipah virus glycoproteins (G and F) in vaccinia virus recombinants to evaluate their contribution to protection. To do this, we used our hamster animal model, in which the animals die of acute encephalitis following Nipah virus infection (24). Using this model, we show that vaccination with vaccinia virus recombinants expressing either of the two Nipah virus glycoproteins protects the animals from fatal infection. Furthermore, passive transfer of antibody from immunized animals to naive animals protects them from a lethal Nipah virus challenge.
MATERIALS AND METHODS
Cells and viruses.
Vero E6, RK13, and BHK 21 cells were maintained in Dulbecco's modified Eagle's medium (Gibco) containing 10% fetal calf serum. Nipah virus isolated from the cerebrospinal fluid of a patient was received at the Jean Mérieux biosafety level 4 laboratory in Lyon, France, from K. B. Chua and S. K. Lam (University of Malaya, Kuala Lumpur, Malaysia) following two passages in Vero cells. The Nipah virus isolated and characterized by Harcourt et al. (6) was isolated from the same biological material as the Nipah virus described in the present publication. A virus stock was made (under P4 conditions) following a third passage on Vero cells: the supernatant was harvested 2 days after infection when the Vero cells showed fusion and syncytium formation. The virus stock was titrated in six-well plates by incubating 200 μl of serial 10-fold dilutions of supernatant in each well (containing 106 Vero cells per well) for 1 h at 37°C. The cells in each well were then washed twice with Dulbecco's modified Eagle's medium and 2 ml of 1.6% carboxymethylcellulose in Dulbecco's modified Eagle's medium containing 2% fetal calf serum was added to each well. The plates were incubated for 5 days at 37°C, and the wells were washed with phosphate-buffered saline (pH 7.4), fixed with 10% formalin for 20 min, washed, and stained with methylene blue. After infecting Vero cells at a multiplicity of infection of 0.01 PFU/cell, virus titers reached 2 × 107 PFU/ml. This stock of Nipah virus at the third Vero cell passage level was used for challenge studies in hamsters.
Stocks of vaccinia virus and recombinant viruses were grown in BHK21 cells. Cells were infected at 0.01 PFU/cell, and the cells were harvested 3 days later, sonicated, and stored at −80°C. Virus was titrated in Vero cells.
Cloning of Nipah virus glycoprotein genes and construction of vaccinia virus recombinants.
To clone the Nipah virus genes coding for the two viral glycoproteins, Vero E6 cells infected with Nipah virus were extracted with RNA Now according to the manufacturer's instructions and subjected to reverse transcription-PCR. The 5′ and 3′ primers used for the G protein were 5′-CGCGGATCCAGTCATAACAATTCAAG-3′ and 5′-CGCGGATCCGAGGTTGATTTTTATG-3′, respectively. Those for the F protein were 5′-CGCAGGATCGAAGCTCTTGCCTCG-3′ and 5′-CATCAATCTGGATCCACTATGTCCC-3′, respectively. The resulting cDNA was cloned into plasmid pT-Adv with the Clontech Advantage PCR cloning kit according to the manufacturer's instructions.
DNA was sequenced using a cycle sequencing reaction with the ABI Prism Big Dye terminator cycle sequencing ready reaction kit (PE Biosystems). The reaction products were analyzed on an ABI Prism automatic sequencer (Perkin-Elmer). Nucleic acid sequence analysis revealed that, compared to the published nucleic acid sequence analysis for Nipah virus (sequence NC002728 in GenBank), there was a single nucleotide difference in the Nipah virus G gene at position 683 (A to G), but this change is silent as far as the primary sequence is concerned. Vaccinia virus recombinants were prepared with the host range selection system described by Perkus et al. (13). Briefly, the genes to be expressed were subcloned by excising the inserts from the pT-Adv plasmids with BamHI and cloned into the BamHI site of plasmid pCOPAK H6 (13), which also contains the K1L vaccinia virus gene. Vero cells were infected with the NYVAC strain of vaccinia virus (20) and transfected with the pCOPAK plasmid. The vaccinia virus recombinants were selected on RK13 cells.
Antibodies.
cDNAs encoding Nipah virus G and F were subcloned into the BglII site of plasmid pV1J (11). Six-week-old BALB/c female mice (IFFA-CREDO, France) were immunized with 2 μg of pV1J-G or pV1J-F by epidermal gene gun (Bio-Rad, Ivry sur Seine, France) delivery, as previously described (1). A second immunization was given 6 weeks later, and the mice were bled after a total of 12 weeks. The sera were used for FACScan analysis at a dilution of 1:50 (F) or 1:100 (G).
Antibody determinations.
Sera from hamsters were tested individually by enzyme-linked immunosorbent assay (ELISA) for the presence of Nipah virus antibodies. Crude extracts of Nipah virus antigens were prepared from Vero cells infected at a multiplicity of infection of 0.01 PFU/cell for 24 h. The cells were washed with phosphate-buffered saline and lysed in phosphate-buffered saline containing 1% Triton X-100 (107 cells/ml) at 4°C for 10 min. The cell lysate was sonicated twice for 30 s each to full cell destruction and centrifuged at 5,000 rpm at 4°C for 10 min. The supernatant was frozen at −80°C. Noninfected Vero cells were similarly treated to prepare control antigen. Cross-titration of the Nipah virus antigens was performed with serum from a convalescent, Nipah virus-infected patient to determine the antigen titer corresponding to the dilution showing the highest optical density reading.
Neutralizing antibody titers were determined in Vero cells. Serum dilutions in phosphate-buffered saline starting with 1:20 were mixed with 50 PFU of Nipah virus in 96-well plates and incubated for 1 h at 37°C, and then 20,000 Vero cells were added. The plates were read after 5 days, and the dilution of serum reducing 50% of the virus titer was recorded.
Primers and TaqMan probes.
The conditions used are those described by Guillaume et al. (unpublished data). Briefly, the primers and probe were designed with the program Primer Express (Perkin-Elmer, Applied Biosystems) following the recommended criteria. A target region in the NP gene was selected. The forward primer (NiV.NP1209, 5′-GCAAGAGAGTAATGTTCAGGCTAGAG-3′) and the reverse primer (NiV.NP1314, 5′-CTGTTCTATAGGTTCTTCCCCTTCAT-3′) amplify 105 bp of the Nipah virus NP gene. The fluorescent probe (NiV.NP1248Fam, 5′-TGCAGGAGGTGTGCTCATTGGAGG-3′) is designed to anneal to a sequence internal to the PCR primers. The fluorescent reporter dye 6-carboxyfluorescein (FAM) was located at the 5′ end of the probe, and the quencher, 6-carboxytetramethylrhodamine (TAMRA), was located at the 3′ end.
Quantitative reverse transcription-PCR assays were performed with the ABI Prism 7700 TaqMan sequence detector. The one-step reverse transcription-PCR system (TaqMan one-step PCR master mix reagents kit, Applied Biosystems) was used for uninterrupted thermal cycling. A master mix reaction was prepared and dispensed in 20-μl aliquots or 22.5-μl aliquots into thin-walled MicroAmp optical tubes (ABI Prism, Applied Biosystems), allowing continuous monitoring of the amount of RNA. Then 5 μl of RNA extract from serum or 2.5 μl of RNA transcript was added to each tube. The final reaction mixture contained 900 nM each primer and 200 nM probe. Prior to amplification, the RNA was reverse transcribed at 50°C for 30 min. This was followed by one cycle of denaturation at 94°C for 5 min. PCR amplification then proceeded with 45 cycles at 94°C for 15 s and 60°C for 1 min.
Immunization of hamsters.
For protection studies, inbred golden hamsters (Janvier, Le Fenest St. Isles, France) were vaccinated twice (1 month apart) with 107 PFU of vaccinia virus recombinants expressing either the G or F Nipah virus glycoprotein or with 5 × 106 of each of the recombinants when they were used for coimmunization. The animals were challenged 3 months after the last immunization.
To prepare polyclonal monospecific serum against the F and G glycoproteins, hamsters were immunized on days 0 and 14 with 107 PFU of the vaccinia virus recombinants followed by sonicated vaccinia virus recombinant-infected BHK21 cells (with Freund's complete adjuvant) at 28 days and the same antigen (with Freund's incomplete adjuvant) at 42 days. The animals were bled 14 days after the last immunization, and the antibodies were determined by ELISA and neutralization.
RESULTS
Expression of Nipah virus glycoproteins in vaccinia virus.
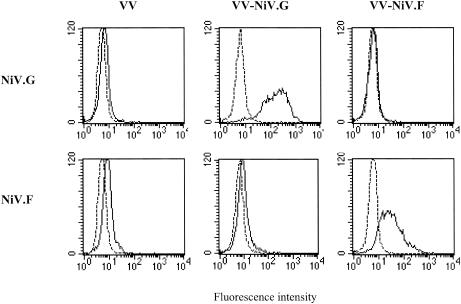
The Nipah virus G and F proteins expressed from vaccinia virus were tested in vitro for the expression of biologically active proteins. HeLa cells infected with vaccinia virus recombinants expressing the Nipah virus G or F glycoprotein (VV-NiV.G and VV-NiV.F, respectively) were examined by FACScan analysis for expression of the Nipah virus proteins at the plasma membrane. Both viral glycoproteins were expressed at the cell surface (Fig. 1). When HeLa cells were infected with both vaccinia virus recombinants, cell fusion (syncytium formation) was induced (Fig. 2).
FIG. 1.
FACScan analysis of HeLa cells infected with vaccinia virus (VV) recombinants expressing either the G or F glycoprotein of Nipah virus (NiV). HeLa cells were infected with either VV-NiV.G or VV-NiV.F or a control vaccinia virus at a multiplicity of infection of 0.1 PFU/cell for 16 h, and the expression of the glycoproteins was measured at the surface of the cells with a polyclonal monospecific antiserum to either the G or F glycoprotein.
FIG. 2.
Induction of fusion by coexpression of the Nipah virus G and F glycoproteins. HeLa cells were infected with vaccinia virus-Nipah virus recombinants expressing either the G or F glycoprotein or doubly infected with both as shown in Fig. 1. The cells were then examined for viral expression by immunofluorescence and also the induction of fusion.
Immunization of hamsters with vaccinia virus recombinants expressing G or F protects against a lethal infection.
Hamsters were immunized subcutaneously with either 107 PFU of VV-NiV.G or VV-NiV.F or with the two combined (5 × 106 PFU of each recombinant). One month later, the animals were boosted with the same dose of vaccinia virus recombinant. In the animal model we developed for Nipah virus, intraperitoneal inoculation of hamsters with our Nipah virus isolate induces a fatal encephalitis 7 to 10 days later (24). When the VV-NiV.G-, VV-NiV.F-, or VV-NiV.F+G-vaccinated animals were challenged with Nipah virus 3 months after the last immunization (1,000 PFU/animal intraperitoneally), there was complete protection against mortality (Fig. 3). After challenge, the levels of both neutralizing antibodies and antibodies as measured by ELISA increased in all vaccinated animals (Fig. 4). Further studies on sera from the hamsters showed that the presence of virus could only be detected at a late stage of infection (day 5 to 6) in control nonimmunized animals. No virus was detected in the vaccinated animals (Tables 1 and 2).
FIG. 3.
Protection of hamsters from a lethal challenge with Nipah virus by vaccination with vaccinia virus recombinants expressing the Nipah virus G and/or F glycoprotein. Hamsters were vaccinated twice at a 1-month interval with either VV-NiV.G or VV-NiV.F or both and challenged with Nipah virus 3 months after the last immunization (seven or eight animals per group). Animals were examined daily.
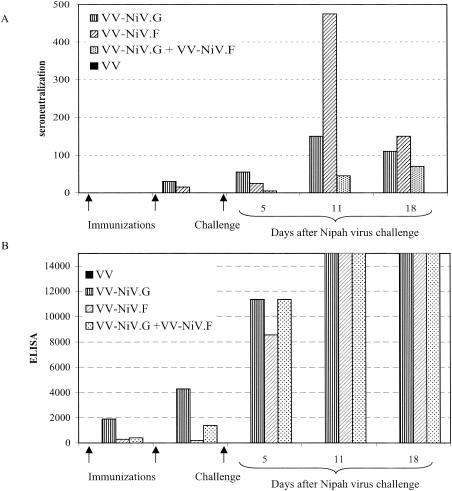
FIG. 4.
Antibody responses after vaccination with vaccinia virus recombinants and challenge with Nipah virus The hamsters were bled after immunization and also at periods after challenge with Nipah virus. Antibody levels were measured by (A) neutralization and (B) ELISA.
TABLE 1.
Detection of viral RNAa
| Test | Nipah virus RNA detected by TaqMan assay (no. of hamsters tested)
|
|||
|---|---|---|---|---|
| VV-NiV.G | VV-NiV.F | VV-NiV.G/VV-NiV.F | Control | |
| J1 | − (4) | − | − | − |
| J2 | − | − | − | − |
| J3 | − (4) | − | − | − |
| J4 | − | − | − | − |
| J5 | − (4) | − | − | 4 (5) |
| J6 | − | − | − | 2 (3) |
| J7 | − (4) | − | − | |
| J8 | − | − | − | |
Five animals were tested each day for each vaccination test except as indicated.
TABLE 2.
Viral RNA quantitation
| Test | Hamster no. | RNA detected by RT-PCR TaqMan for controls (103 copies/ml) |
|---|---|---|
| J5 | H1 | 274 |
| H2 | ||
| H3 | 209 | |
| H4 | 404 | |
| H5 | 456 | |
| J6 | H6 | 2,217 |
| H9 | ||
| H10 | 667 |
Serum from VV-NiV.G and VV-NiV.F recombinant-immunized hamsters passively protects naive hamsters against a lethal Nipah virus challenge.
To dissect the importance of the humoral immune response in protection, hamsters were hyperimmunized with the vaccinia virus recombinants (see Materials and Methods), and the animals with sera containing the highest levels of neutralizing antibody to Nipah virus were pooled (160 neutralizing units/ml). Hamsters were given 0.2 ml of antiserum directed against either the G or F Nipah virus glycoprotein or a mixture of the two by intraperitoneal injection. One hour later, the animals were challenged with virus, and 24 h later 0.2 ml of serum was again passively transferred. The hamsters were observed for clinical signs for 2 months. Animals receiving either of the antisera (monospecific polyclonal G or F) or the mixture of the two were protected from a lethal Nipah virus infection (Fig. 5). After infection, serum antibody levels against Nipah virus were strongly induced (Fig. 6).
FIG. 5.
Passive protection of hamsters against a lethal Nipah virus infection. Antibody was raised in hamsters against the vaccinia virus recombinants expressing either G or F, and pooled serum either against the individual glycoproteins or an equal mixture of each was inoculated intraperitoneally (0.2 ml/animal) 2 h prior to challenge with Nipah virus. A second inoculation of antiserum (0.2 ml) was given 24 h later. The animals were challenged with Nipah virus and observed for 43 days.
FIG. 6.
Immune response of hamsters challenged with Nipah virus in the presence of passively administered polyclonal monospecific anti-Nipah virus serum. The hamsters from Fig. 5 were bled at intervals, and the serum was examined for anti-Nipah virus antibodies by ELISA.
DISCUSSION
In nature, paramyxoviruses can infect both humans and animals. Often, viruses preferentially infect one species and grow poorly in a second. Thus, a virus that grows poorly in humans can be used to create a Jenner type vaccine. In the same manner, by the use of modern biotechnology, the antigens of a virus that is a human pathogen can be expressed from an equivalent animal virus in order to induce protective responses (16, 27). In certain cases when paramyxoviruses cross the species barrier to infect humans, they become more virulent. The natural host of Hendra and Nipah viruses is probably the fruit bat (3, 5, 26), but in 1994 in Australia, horses became infected by Hendra virus, and in 1998 in Malaysia Nipah virus infected pigs. In both cases, virus was amplified in the second animal species, and this led to human infection. The severity of the disease caused by Nipah virus in pigs (more than a million culled) and in humans (40% fatality) had great economic and social consequences. Ribavirin was tried on some patients but with no significant results (2, 17). No Nipah virus-specific antivirals were available to combat the epidemic, and their production remains a priority if effective measures are to be taken when future epidemics occur.
The present study investigated the immunological parameters which may play a role in protection against Nipah virus infection. We examined the possibility of inducing active protection with the Nipah virus glycoproteins expressed from vaccinia virus recombinants and also that of passively protecting animals with hyperimmune serum. Paramyxoviruses, including Nipah virus, have two glycoproteins at the virus surface, G and F. The G glycoprotein is responsible for attachment to the cellular receptor, whereas the F glycoprotein induces fusion between the viral and cellular membranes. G and F act in concert to bring about fusion. In our studies, we confirmed this for the vaccinia virus-expressed Nipah virus proteins, showing that only coinfection with both G and F induced fusion.
If antibodies are to block infection, they should presumably block attachment of G to its receptor or the interaction of F with the cell membrane. Sera from hamsters immunized with either of the vaccinia virus recombinants induced high antibody levels but relatively low neutralizing antibodies. Studies by Tamin et al. (18) with the WR strain of vaccinia virus obtained much higher levels of neutralizing antibodies in mice. However, the WR virus is much more virulent and grows to high titers in several organs. In contrast, the NYVAC strain that we used is highly attenuated, and infectious virus is difficult to detect in the immunized animals. In other paramyxoviruses, the response to the attachment protein often tends to be dominant, but we found that the antibody responses to Nipah virus F and Nipah virus G were of the same order, confirming studies in mice (18).
Hamsters vaccinated with either VV-NiV.G or VV-NiV.F were completely protected from a lethal infection. Confirming the contribution of the humoral response in this process, naive animals were also shown to be protected by hyperimmune serum passively transferred prior to challenge. Thus, with our animal model, we were able to show that it is possible to protect both actively and passively against lethal Nipah virus infections. However, in both active and passive immunization, the antibody response to Nipah virus was strongly stimulated, suggesting that the virus replicated in the vaccinated animals. However, attempts to detect virus in the serum were unsuccessful. In control nonimmunized animals, virus could only be detected in the serum of moribund animals. It is probable, as observed in several other paramyxovirus infections, that the virus is mainly cell associated.
In humans, both relapsing and late-onset cases of infection have been observed (9, 19, 25). In these situations, the immunobiology of the infection is unknown. We did not observe any of these late pathologies in our challenged immunized animals up to 5 months postchallenge. Similarly, in the passively protected animals, no late disease was observed. However, we have not determined the lower limits of antibody protection in vivo or the effect of passively immunizing the animals once the infection has been initiated. This should be determined not only for the contribution to protection from acute disease but also from late-onset symptoms.
Acknowledgments
These studies were supported by a grant from the Direction Générale de l'Armée no. 01.34.027.00.470.75.01. V.G. was supported by a scholarship from the Direction Générale de l'Armée. R.B. is a CNRS scientist.
REFERENCES
- 1.Cardoso, A. I., N. Sixt, A. Vallier, J. Fayolle, R. Buckland, and T. F. Wild. 1998. Measles virus DNA vaccination: antibody isotype is determined by the method of immunization and by the nature of both the antigen and the coimmunized antigen. J. Virol. 72:2516-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chong, H. T., A. Kamarulzaman, C. T. Tan, K. J. Goh, T. Thayaparan, S. R. Kunjapan, N. K. Chew, K. B. Chua, and S. K. Lam. 2001. Treatment of acute Nipah virus encephalitis with ribavirin. Ann. Neurol. 49:810-813. [DOI] [PubMed] [Google Scholar]
- 3.Chua, K. B., C. L. Koh, P. S. Hooi, K. F. Wee, J. H. Khong, B. H. Chua, Y. P. Chan, M. E. Lim, and S. K. Lam. 2002. Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes Infect. 4:145-151. [DOI] [PubMed] [Google Scholar]
- 4.Chua, K. B., L. F. Wang, S. K. Lam, and B. T. Eaton. 2002. Full-length genome sequence of Tioman virus, a novel paramyxovirus in the genus Rubulavirus isolated from fruit bats in Malaysia. Arch. Virol. 147:1323-1348. [DOI] [PubMed] [Google Scholar]
- 5.Field, H., P. Young, J. M. Yob, J. Mills, L. Hall, and J. Mackenzie. 2001. The natural history of Hendra and Nipah viruses. Microbes Infect. 3:307-314. [DOI] [PubMed] [Google Scholar]
- 6.Harcourt, B. H., A. Tamin, K. Halpin, T. G. Ksiazek, P. E. Rollin, W. J. Bellini, and P. A. Rota. 2001. Molecular characterization of the pollymerase gene and genomic termini of Nipah virus Virology 287:192-201. [DOI] [PubMed] [Google Scholar]
- 7.Kirkland, P. D., P. W. Daniels, M. N. Nor, R. J. Love, A. W. Philbey, and A. D. Ross. 2002. Menangle and Nipah virus infections of pigs. Vet. Clin. N. Am. Food Anim. Pract. 18:557-571. [DOI] [PubMed] [Google Scholar]
- 8.Lam, S. K., and K. B. Chua. 2002. Nipah virus encephalitis outbreak in Malaysia. Clin. Infect. Dis. 34:S48-51. [DOI] [PubMed] [Google Scholar]
- 9.Lim, C. C., W. L. Lee, Y. S. Leo, K. E. Lee, K. P. Chan, A. E. Ling, H. Oh, A. P. Auchus, N. I. Paton, F. Hui, and P. A. Tambyah. 2003. Late clinical and magnetic resonance imaging follow up of Nipah virus infection. J. Neurol. Neurosurg. Psychiatr. 74:131-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Love, R. J., A. W. Philbey, P. D. Kirkland, A. D. Ross, R. J. Davis, C. Morrissey, and P. W. Daniels. 2001. Reproductive disease and congenital malformations caused by Menangle virus in pigs. Aust. Vet. J. 79:192-198. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery, D. I., J. W. Shiver, K. R. Leandrer, H. C. Perry, A. Friedman, D. Martinez, J. B. Ulmer, J. J. Donnelly, and E. Liu. 1993. Heterologous and homologous protection against influenza A: optimization of DNA vectors. DNA Cell Biol. 12:777-783. [DOI] [PubMed] [Google Scholar]
- 12.Olson, J. G., C. Rupprecht, P. E. Rollin, U. S. An, M. Niezgoda, T. Clemins, J. Walston, and T. G. Ksiazek. 2002. Antibodies to Nipah virus-like virus in bats (Pteropus lylei), Cambodia. Emerg. Infect. Dis. 8:987-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perkus, M. E., K. Limbach, and E. Paoletti. 1989. Cloning and expression of foreign genes in vaccinia virus, with a host range selection system. J. Virol. 63:3829-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ProMed. 2002. Nipah virus-like virus—Bangladesh (2001). Archive number 20020830.5187. ProMed, Geneva, Switzerland.
- 15.ProMed. 2003. Nipah virus-like virus—India (North Bengal) 2001. Archive number 20030106.0050. ProMed, Geneva, Switzerland.
- 16.Schmidt, A. C., D. R. Wenzke, J. M. McAuliffe, M. St. Claire, W. R. Elkins, B. R. Murphy, and P. L. Collins. 2002. Mucosal immunization of rhesus monkeys against respiratory syncytial virus subgroups A and B and human parainfluenza virus type 3 by using a live cDNA-derived vaccine based on a host range-attenuated bovine parainfluenza virus type 3 vector backbone. J. Virol. 76:1089-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snell, N. J. 2001. Ribavirin—current status of a broad spectrum antiviral agent. Expert Opin. Pharmacother. 2:1317-13124. [DOI] [PubMed] [Google Scholar]
- 18.Tamin, A., B. H., Harcourt, T. G. Ksiazek, P. E. Rollin, W. J. Bellini, and P. A. Rota. 2002. Functional properties of the fusion and attachment glycoproteins of Nipah virus. Virology 296:190-200. [DOI] [PubMed] [Google Scholar]
- 19.Tan, C. T., K. J. Goh, K. T. Wong, S. A. Sarji, K. B. Chua, N. K. Chew, P. Murugasu, Y. L. Loh, H. T. Chong, K. S. Tan, T. Thayaparan, S. Kumar, and M. R. Jusoh. 2002. Relapsed and late-onset Nipah virus encephalitis. Ann. Neurol. 51:703-708. [DOI] [PubMed] [Google Scholar]
- 20.Tartaglia, J., M. E. Perkus, J. Taylor, E. K. Norton, J. C. Audonnet, W. I. Cox, S. W. Davies, J. van der Hoeven, B. Meignier, M. Rivière, B. Languet, and E. Paoletti. 1992. NYVAC: a highly attenuated strain of vaccinia virus. Virology 188:217-232. [DOI] [PubMed] [Google Scholar]
- 21.Wang, L. F., M. Yu, E. Hansson, L. I. Pritchard, W. P. Michalski, and B. T. Eaton. 2000. The exceptionally large genome of Hendra virus: support for the creation of a new genus within the family Paramyxoviridae. J. Virol. 74: 9972-9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L. F., B. H. Harcourt, M. Yu, A. Tamin, P. A. Rota, W. J. Bellini, and B. T. Eaton. 2001. Molecular biology of Hendra and Nipah viruses. Microbes Infect. 3:279-287. [DOI] [PubMed] [Google Scholar]
- 23.Westbury, H. A. 2000. Hendra virus disease in horses. Rev. Sci. Technol. 19:151-159. [DOI] [PubMed] [Google Scholar]
- 24.Wong, K. T., I. Grosjean, C. Brisson, B. Blanquier, M. Fevre-Montange, A. Bernard, P. Loth, M.-C. Georges-Courbot, M. Chevallier, H. Akaoka, P. Marianneau, S. K. Lam, T. F. Wild, and V. Deubel. A golden hamster model for human acute Nipah virus infection. Am. J. Pathol., in press. [DOI] [PMC free article] [PubMed]
- 25.Wong, S. C., M. H. Ooi, M. N. Wong, P. H. Tio, T. Solomon, and M. J. Cardosa. 2001. Late presentation of Nipah virus encephalitis and kinetics of the humoral immune response. J. Neurol. Neurosurg. Psychiatr. 71:552-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yob, J. M., H. Field, A. M. Rashdi, C. Morrissy, B. van der Heide, P. Rota, A. bin Adzhar, J. White, P. Daniels, A. Jamaluddin, and T. Ksiazek. 2001. Nipah virus infection in bats (order Chiroptera) in peninsular Malaysia. Emerg. Infect. Dis. 7:439-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yunus, A. S., S. Krishnamurthy, M. K. Pastey, Z. Huang, S. K. Khattar, P. L. Collins, and S. K. Samal. 1999. Rescue of a bovine respiratory syncytial virus genomic RNA analog by bovine, human and ovine respiratory syncytial viruses confirms the “functional integrity” and “cross-recognition” of BRSV cis-acting elements by HRSV and ORSV. Arch. Virol. 144:1977-1990. [DOI] [PubMed] [Google Scholar]