Abstract
Synthesis of the relaxed-circular (RC) genome of hepadnaviruses is a multistep process that requires template switching during reverse transcription. Studies of duck hepatitis B virus indicated the presence of cis-acting sequences, distinct from the donor and acceptor sequences for the template switches, which contribute to the synthesis of RC DNA. However, knowledge about cis-acting requirements distinct from the donor and acceptor sites for human hepatitis B virus (HBV) was lacking. In this study, we searched for cis-acting sequences for synthesis of HBV RC DNA by analyzing a set of deletion variants that collectively represent most of the HBV genome. Sequences of epsilon, DR1, DR2, 5′r, and 3′r were not analyzed in the study. Results from Southern blotting showed that multiple cis-acting sequences were involved in the synthesis of HBV RC DNA. Analysis of several HBV/woodchuck hepatitis virus chimeras corroborated the findings from the analysis of deletion variants. This study represents a comprehensive and quantitative analysis of cis-acting sequences that contribute to the synthesis of HBV RC DNA.
Hepadnaviruses are a family of enveloped viruses with circular double-stranded DNA genomes of 3.0 to 3.3 kb. Members of the family include human hepatitis B virus (HBV), woodchuck hepatitis virus (WHV), ground squirrel hepatitis virus, and duck hepatitis B virus (DHBV) (for reviews, see references 4 and 27). Hepadnaviruses infect the livers of their hosts and can cause acute and chronic liver diseases. HBV is the leading cause of primary hepatocellular carcinoma. More than 350 million people are chronically infected with HBV worldwide (10). Chronic HBV infection increases the risk for hepatocellular carcinoma 100-fold (3). Since viral replication is necessary for maintaining viral persistence, the study of HBV DNA replication is crucial for understanding and ultimately controlling HBV pathogenesis.
Hepadnaviruses replicate their genomes by reverse transcription of an RNA intermediate, the pregenomic RNA (pgRNA) (for a review, see reference 4). Reverse transcription is a complex multistep process. DHBV is an invaluable model in elucidating the mechanism by which hepadnaviruses replicate. DHBV and HBV share little, if any, nucleotide sequence similarity; however, they have similar genome organizations and are believed to use similar mechanisms for reverse transcription. Our present understanding of hepadnavirus reverse transcription is derived largely from the study of DHBV.
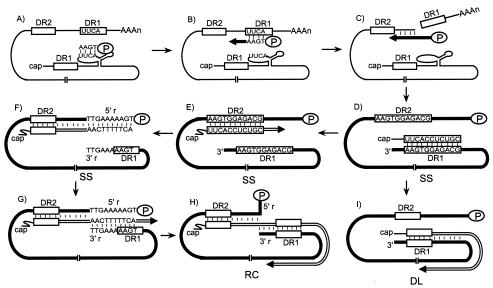
Reverse transcription of hepadnaviruses takes place in the cytoplasmic nucleocapsids of infected cells (29). The first two steps are encapsidation of pgRNA and initiation of minus-strand DNA synthesis. Hepadnavirus P protein binds an RNA stem-loop at the 5′ end of the pgRNA, termed epsilon, to initiate encapsidation of pgRNA (1, 2, 8, 24). P protein, acting as primer and reverse transcriptase, initiates minus-strand DNA synthesis using nucleotides within epsilon as a template (Fig. 1A) (24, 33-35). After the synthesis of three or four nucleotides at epsilon, the nascent minus-strand DNA switches templates to a complementary sequence at direct repeat 1 (DR1), which is near the 3′ end of the pgRNA (Fig. 1B) (21, 32, 33). Minus-strand DNA synthesis resumes from this position. Following elongation, the RNase H activity of the P protein degrades the pgRNA template that has been copied (Fig. 1C) (25, 29). Minus-strand DNA synthesis proceeds to the 5′ end of pgRNA. This synthesis results in a full-length minus-strand DNA with P protein still covalently attached to its 5′ end (Fig. 1D) (12, 26, 36). The final RNase H cleavage product is a short RNA fragment of 17 or 18 nucleotides (nt) that is the primer for the initiation of the synthesis of plus-strand DNA (Fig. 1D) (11, 36). The 3′ end of the primer contains the sequence of DR1. For the synthesis of the most abundant form of the mature genome, relaxed-circular (RC) DNA, two template switches are required during plus-strand DNA synthesis. First, the plus-strand primer switches templates to anneal to DR2, which is near the 5′ end of the minus-strand DNA, and initiates plus-strand DNA synthesis from DR2 (11, 28). This template switch is called primer translocation (Fig. 1E). Synthesis of plus-strand DNA proceeds to the 5′ end of the minus-strand DNA (Fig. 1F). The nascent plus-strand DNA undergoes another template switch, called circularization, to permit elongation with the 3′ end of minus-strand DNA as a template (11). The minus-strand DNA is terminally redundant for 9 or 10 nt (5′r and 3′r) (12). The nascent plus-strand DNA, after copying the 5′r sequence, anneals to 3′r (Fig. 1G). Elongation of the plus-strand DNA from 3′r ultimately generates the RC DNA form (Fig. 1H). There is a second pathway for the synthesis of plus-strand DNA. This pathway generates a duplex-linear (DL) DNA because the RNA primer initiates plus-strand DNA synthesis from DR1 on the minus strand. No template switch is involved during the synthesis of the plus strand of DL DNA (Fig. 1I) (28). This type of plus-strand synthesis is termed in situ priming.
FIG. 1.
Model of HBV reverse transcription. (A) Initiation of minus-strand DNA synthesis. The pgRNA (thin line) is the template for the synthesis of minus-strand DNA. P protein (oval), being the primer and the reverse transcriptase, initiates minus-strand DNA synthesis at the bulge of the epsilon near the 5′ end of the pgRNA. Three or four nucleotides are synthesized with the epsilon sequence used as the template. (B) Minus-strand template switch. The tetranucleotide covalently linked to P protein is moved from epsilon to the UUCA sequence of DR1 near the 3′ end of the pgRNA, and minus-strand DNA synthesis resumes from this position. Minus-strand DNA is indicated by a thick black line, and an arrow indicates the direction of elongation. (C) Elongation of minus-strand DNA. As the minus-strand DNA is synthesized, the RNase H activity of P protein degrades the pgRNA template. (D) Completion of minus-strand DNA synthesis and generation of the plus-strand primer. Completion of minus-strand DNA synthesis results in a genome-length minus-strand DNA. The primer for plus-strand DNA initiation is generated by the final RNase H cleavage. This short RNA fragment contains a DR1 sequence at its 3′ end. (E) Primer translocation. In this template switch, the plus-strand primer moves from DR1 to base pair with DR2 and initiates plus-strand DNA synthesis from DR2. Plus-strand DNA is represented by a double line, with an arrow indicating the direction of its synthesis. (F) Elongation of plus-strand DNA from DR2. Plus-strand DNA initiated at DR2 elongates to the 5′ end of the minus-strand DNA, where it runs out of the template. The sequence of the terminal redundancy, 5′r and 3′r, on the minus-strand DNA and the complementary sequence on plus-strand DNA are shown. (G) Circularization. In this template switch, plus-strand DNA anneals to the complementary 3′r. Plus-strand DNA synthesis resumes from the 3′r of the minus-strand template. (H) Generation of RC DNA. Elongation of plus-strand DNA after circularization results in a RC form of the genome. (I) In situ priming. In this process, the plus-strand primer initiates plus-strand DNA synthesis from the DR1 sequence on the minus-strand template. This results in a DL form of the genome.
cis-acting requirements for plus-strand DNA synthesis have been studied more in DHBV than other hepadnaviruses. The role of sequence identity between DR1 and DR2 and between 5′r and 3′r in plus-strand DNA synthesis has been examined in DHBV (15-18, 28). Moreover, three additional cis-acting sequences that make significant contributions to the synthesis of RC DNA were identified in DHBV (6, 7). Although there have been several studies describing cis-acting sequences for HBV replication (22, 31), a comprehensive and quantitative analysis of cis-acting sequences for HBV replication is lacking. For the present study, we asked whether HBV has additional cis-acting requirements outside of DR1, DR2, 5′r, and 3′r for the synthesis of RC DNA. We analyzed a series of deletion mutants which collectively represent the entire HBV genome except for sequences of epsilon, DR1, DR2, 5′r, and 3′r. We found that HBV does contain additional cis-acting sequences that make positive contributions to RC DNA synthesis. Removal of these sequences led to defects of different magnitudes. Substitutions of these sequences with corresponding WHV sequences also led to cis-acting defects in RC DNA synthesis.
MATERIALS AND METHODS
Molecular clones.
All HBV molecular clones were derived from subtype ayw (GenBank accession number V01460). WHV sequences were from the WHV2 molecular clone (GenBank accession number M11082). Nucleotide position 1 of HBV subtype ayw is the C of the EcoRI site (GAATTC).
The wild-type (WT) HBV reference virus, LJ196, expresses HBV pgRNA under the control of the cytomegalovirus (CMV) immediate early promoter and does not express P, C, or envelope proteins. LJ196 was derived from parental clone pCH-9/3091 (20) (kindly provided by Michael Nassal) with the following modifications. (i) Based on primer extension analysis, plasmid pCH-9/3091 contained an extra pgRNA transcription start site (data not shown). Therefore, two nucleotides were deleted, between the CMV promoter and HBV sequences, to ensure that only the authentic start sites of pgRNA were used. (ii) Two substitutions were introduced to prevent the expression of the envelope proteins. The start codon of the S open reading frame (ORF) was changed from ATG to ACG, and the termination codon TAA was introduced into the sixth codon of the S ORF by changing the C at nt 169 to A. A BamHI site was generated by a mutation at nt 175 (T to C). (iii) To eliminate the expression of P protein, the termination codon TAG was introduced into the 13th codon of the P ORF by mutating the T at nt 2342 to A. (iv) To prevent the expression of C protein, the termination codon TAA was introduced into the fourth codon of the C ORF by mutating the G at nt 1908 to T and the C at nt 1910 A. nt 1911 was also mutated, which resulted in the introduction of the HindIII site.
The expression plasmid for HBV P and C proteins, LJ96, was derived from parental clone pCH3143 (9) (kindly provided by Michael Nassal) with the same mutations to prevent the expression of the envelope proteins as described above for LJ196. Plasmid pCH3143 was defective for pgRNA encapsidation due to the deletion of part of epsilon (9).
All the deletion mutants were made in the LJ196 background, except for deletion mutant 1833-1844. The names of the deletion mutants reflect the deleted nucleotides (inclusive) of the HBV sequences. Deletion mutant 1833-1844 was constructed from a plasmid that was similar to LJ196 but lacking the P or C mutations. Deletion mutants were constructed either by removing restriction fragments or by oligonucleotide-directed mutagenesis. All deletion junctions and the entire cloned PCR fragments were verified by DNA sequencing.
HBV/WHV chimeric clones (HW1 to HW5) were also made in the LJ196 background. PCR fragments were generated from the plasmid pSPWHV5.12× (a gift from Jesse Summers, University of New Mexico), a clone containing two full-length copies of the WHV2 genome. These fragments were generated with primers that contained a WHV2 sequence at their 3′ ends and an HBV restriction site at their 5′ ends. These fragments were then ligated into analogous restriction sites in LJ196. The construction of any molecular clone used in our analysis will be provided upon request.
Cell culture and transfection.
Human hepatoblastoma cell line HepG2 was grown at 37°C in 5% CO2 in RPMI 1640 medium supplemented with 10% fetal bovine serum, 0.01 M HEPES, and 0.1 mM minimal essential medium nonessential amino acids. HepG2 cells were approximately 60 to 70% confluent at the time of transfection. Two hours before transfection, the cells were transferred to Dulbecco minimal essential medium (DMEM) with the same supplementation. Transfections of plasmids were performed on 60-mm-diameter plates by calcium phosphate precipitation as previously described (13). The cells were grown in DMEM until harvest, and fresh medium was added to the cells daily. The kinetics of accumulation of intracellular viral DNA of the WT reference virus was analyzed in HepG2 cells from day 3 to day 7 posttransfection. Southern blotting showed that RC DNA reached a peak level and remained stable after day 4, and total viral DNA accumulated to the highest level on day 5 posttransfection (data not shown). Therefore, for the analysis of the WT reference and variant viruses, HepG2 cells were harvested on day 5 posttransfection.
Human hepatoma cell line Huh7 was grown in DMEM-F12 medium supplemented with 10% fetal bovine serum at 37°C in 5% CO2. Huh7 cells were approximately 80% confluent at the time of transfection. Transfections were performed on 60-mm-diameter plates as described previously (13). The kinetics of accumulation of intracellular viral DNA of the WT reference virus was analyzed in Huh7 cells from day 2 to day 10. Southern blotting showed that the amount of RC DNA reached a maximum after day 6, whereas the amount of total viral DNA increased from day 2 to day 7 and started to decrease after day 7 (data not shown). Therefore, in this study, Huh7 cells were harvested on day 6 posttransfection.
Isolation of viral DNA and Southern blotting.
To harvest both HepG2 and Huh7 cells, plates were frozen at −70°C after being washed with HEPES-buffered saline plus EGTA buffer (2 mM HEPES, 150 mM NaCl, 0.5 mM EGTA [pH 7.45]). After being thawed to room temperature, the cells were treated with lysis buffer containing 50 mM Tris-HCl (pH 8.0), 1 mM EDTA, and 0.2% NP-40. Isolation of intracellular replicative intermediates was performed by micrococcal nuclease-sodium dodecyl sulfate-pronase treatment as described previously (14). The concentration of 0.2% NP-40 in the lysis buffer was chosen based on the following analysis. Cells transfected with the WT reference virus were treated with lysis buffer containing NP-40 at concentrations of 0.2, 0.4, 1, 2, and 5%. In both cell lines, Southern blotting of viral DNA isolated from these lysates showed a decrease in the amount of viral DNA with increasing concentrations of NP-40 (data not shown). Therefore, 0.2% NP-40 was used in the lysis buffer to ensure the highest yield of DNA.
Viral DNA was resolved on 1.25% agarose-1× Tris-borate-EDTA gels. Southern blotting analysis was performed as described previously (13). A genome-length, minus-strand-specific HBV subtype ayw RNA probe was used to detect the minus-strand DNA.
Statistical analysis.
In the Southern blotting analyses, we wanted to compare the proportion of RC DNA of the variants to that of the WT reference virus. Using statistical methods, we tested the null hypothesis, H0, against the alternative hypothesis, H1, where H0 and H1 were defined as follows: for H0, the proportion of RC DNA of the variant is the same as that of the WT reference; for H1, the proportion of RC DNA of the variant is different from that of the WT. We then used a two-sided Wilcoxon signed rank test (using Mstat 3.21) to decide whether to accept H0 or H1 (14). In general, if P is <0.05, we reject H0 and accept H1 and consider the difference in levels of RC DNA synthesis to be statistically significant.
RESULTS
Aim and experimental design.
Our aim was to identify cis-acting sequences, distinct from those of DR1, DR2, 5′r, and 3′r, which contribute to the synthesis of HBV RC DNA. To search for these sequences in the genome, we analyzed a set of deletion variants, which collectively represent all of the HBV genome except for sequences of DR1, DR2, 5′r, 3′r, and epsilon (Fig. 2). The names of the deletion variants reflected the nucleotides (inclusive) of the HBV sequences that were deleted. In our studies, the molecular clone that expressed HBV subtype ayw WT pgRNA with no P, C, or envelope protein expression was referred to as the WT reference or standard virus. We analyzed viral DNA synthesis in transfected cell cultures. Huh7 and HepG2 cells were cotransfected with two plasmids. One plasmid expressed WT pgRNA or its derivatives and served as the template for reverse transcription but did not express envelope, P, or C proteins. The second plasmid expressed P and C proteins but not envelope proteins, and its pgRNA was defective for encapsidation. This strategy ensured that any mutant phenotype observed was due to a disruption of a cis-acting sequence instead of the expression of a mutant form of a viral protein. Because neither plasmid expressed the envelope proteins, viral cytoplasmic capsids were not secreted as virions. For the isolation of viral DNA from cytoplasmic capsids, HepG2 cells were harvested on day 5 posttransfection and Huh7 cells were harvested on day 6 posttransfection. These time points reflected the times when total viral DNA and RC DNA reached their peak levels (data not shown). Conditions for the isolation of viral DNA were optimized for both HepG2 and Huh7 cultures (see Materials and Methods). Viral DNA was analyzed by Southern blotting.
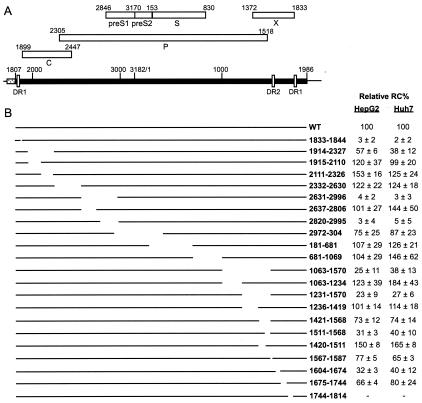
FIG. 2.
Schematic representation of HBV genome organization and the deletion variants. (A) HBV genome organization in the plasmid expressing HBV pgRNA. A black rectangle represents the HBV sequence (nt 1807 to 3182 and 1 to 1986), which is transcribed from the CMV immediate early promoter (dotted box) in the plasmid expressing HBV pgRNA. The positions of DR1 (nt 1833 to 1844) and DR2 (nt 1588 to 1598) are indicated. Locations of the ORFs for viral proteins are shown as white boxes. The plasmids expressing WT and variant pgRNAs did not express C, P, or envelope proteins. C and P proteins were provided by an expression plasmid during transfection (see Materials and Methods). (B) Representation of the locations of the HBV sequences deleted in the variants. The names of the variants indicate the first and last nucleotides of the HBV sequence being deleted (inclusive). On the right panel are the mean values and the standard deviations of the relative RC DNA proportion of each variant compared to that of the WT. The values are an average of measurements made from multiple independent transfections (at least five for HepG2 and at least six for Huh7).
Multiple cis-acting sequences are required for the synthesis of RC DNA.
Southern blot analysis of WT replicative intermediates reveals a characteristic pattern of DNA forms that included RC, DL, and single-stranded (SS) DNA and other minor DNA species (Fig. 3A and B, lanes 1). RC DNA was assumed to be the DNA form that had the lowest mobility in the gel. The identity of DL DNA was determined based on its comigration with the 3.2-kb linear DNA marker (data not shown). The identity of SS DNA was determined based on its comigration with the SS DNA marker (data not shown). The identity of other minor DNA species on Southern blots is not yet known. For the deletion variants, the identities of DL DNA and SS DNA were determined by their corresponding linear DNA and SS DNA markers (data not shown). We used the proportion of RC DNA in the total DNA intermediates that contain mature minus-strand DNA as the measurement of the efficiency of RC DNA synthesis. In HepG2 cells, the average proportion of RC DNA for the WT reference virus was 20.7% ± 2.7%. In Huh7 cells, this value was 11.7% ± 2.5%.
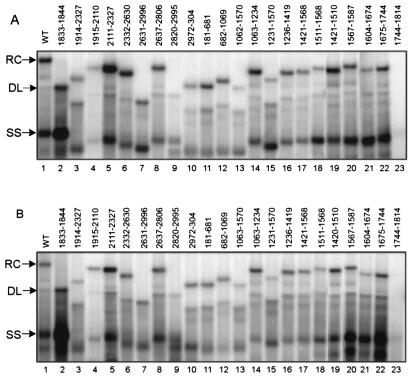
FIG. 3.
Replication of the WT standard virus and deletion variants in HepG2 (A) and Huh7 (B) cells. Replicative intermediates of WT standard and deletion variants were isolated from cytoplasmic viral capsids in HepG2 (A) and Huh7 (B) cells and analyzed by Southern blotting. A genome-length, minus-strand-specific RNA probe was used to detect viral DNA. The positions of RC, DL, and SS DNA of the WT standard virus are indicated.
We asked whether each of the deletion variants synthesized RC DNA to the same degree as the WT reference did. Therefore, we compared the proportion of RC DNA of each of the deletion variants with that of the WT reference and expressed the relative RC DNA proportion of the variants to that of the WT. The values reported in Fig. 2 represent averages of the relative RC DNA proportions derived from measurements made with viral DNA isolated from multiple independent transfections (at least five for HepG2 and at least six for Huh7). Representative Southern blots of these variants in both HepG2 and Huh7 cells are shown in Fig. 3.
All deletion variants except one accumulated viral DNA when analyzed by Southern blotting. Variant 1744-1814 did not accumulate detectable levels of DNA in HepG2 or Huh7 cells (Fig. 3A and B, lanes 23). This indicated that the sequence within nt 1744 to 1814 is required for one or more steps prior to the synthesis of plus-strand DNA. Because deletion variant 1744-1814 did not accumulate minus-strand DNA, further analysis of this region was not carried out.
For the remainder of the variants, we placed them into four groups based on their ability to synthesize RC DNA compared to that of the WT reference. Placing variants into four groups was done to simplify the description of their phenotypes and was not meant to imply similarities in the mechanisms that are affected by the mutations.
Group I: deletion variants that had severe defects in synthesizing RC DNA.
Variants in group I synthesized RC DNA at proportions less than 5% of that of the WT reference. Group I included variants from two regions of the genome (Fig. 2 and 3). The first region, represented solely by variant 1833-1844, synthesized only 3% of the WT level of RC DNA in HepG2 cells (P < 0.043) and 2% in Huh7 cells (P < 0.043). The decrease in the proportion of RC DNA was accompanied by the increased accumulation of both SS DNA and DL DNA. The 12 nt deleted in mutant 1833-1844 lie between the 5′ copy of DR1 and epsilon. The second region was initially mapped within nt 2631 and 2996. This deletion variant, variant 2631-2996, had only 4% of the WT level of RC DNA in HepG2 cells (P < 0.028) and 3% in Huh7 cells (P < 0.028). To further locate the cis-acting sequences in this region, we analyzed two more deletion variants, variants 2637-2806 and 2820-2995, which bisect the original deletion. Variant 2637-2806 synthesized WT levels of RC DNA, whereas deletion variant 2820-2995 had ≤3% of the WT RC level in both cell lines. Therefore, sequences within nt 2820 and 2995 and within nt 1833 and 1844 make large contributions to the synthesis of HBV RC DNA.
Group II: deletion variants that had modest defects in synthesizing RC DNA.
Variants in group II synthesized detectable levels of RC DNA that were lower than those of the WT reference. The difference in the RC DNA proportions among variants in this group and the WT reference was statistically significant (P < 0.05). This set included two regions as shown in Fig. 2 and 3. The first region included sequences surrounding DR2. Variant 1063-1570 synthesized less than 40% of WT RC DNA (P < 0.028 for both cell lines). Further mapping of the cis-acting sequences in this region showed that deletion variants 1231-1570, 1421-1568, and 1511-1568 all synthesized significantly less RC DNA than did the WT reference, while variants 1063-1234, 1236-1419, and 1420-1510 synthesized RC DNA at proportions similar to or higher than those of the WT reference (see below). Therefore, we conclude that sequence between nt 1511 and 1568 was necessary for efficient synthesis of RC DNA. Interestingly, sequences downstream of nt 1511 to 1568 also contributed to the synthesis of RC DNA. Variant 1567-1587 synthesized 77% of WT RC DNA in HepG2 cells (P < 0.05) and 65% of WT RC DNA in Huh7 cells (P < 0.05). Variant 1604-1674, which was immediately downstream of DR2, was also partially defective in the synthesis of RC DNA in both cell lines (P < 0.028 for HepG2 and P < 0.012 for Huh7).
The second region in group II was adjacent to epsilon. Variant 1914-2327 was partially defective in synthesizing RC DNA in both cell lines (57% of the WT level in HepG2 cells, P < 0.028; 38% in Huh7 cells, P < 0.018). However, an attempt to further locate the cis-acting sequences by using two smaller deletion variants bisecting nt 1914 to 2327 failed to uncover a mutant phenotype. Deletion variants 1915-2110 and 2111-2327 were able to synthesize RC DNA to a level close to or higher than that of the WT reference in both cell lines. Since variant 1914-2327 was 413 nt shorter than the WT genome, we asked whether its mutant phenotype was a result of changing the genome size rather than removing a cis-acting sequence from this region. To this end, we analyzed two more variants in which nt 1914 to 2327 were replaced with lacZ sequences (9). Both substitution mutants had dramatic defects in synthesizing RC DNA in HepG2 and Huh7 cells (they synthesized less than 10% of the WT level, P < 0.02; data not shown). This result suggested that the phenotype of deletion variant 1914-2327 was not due to a smaller genome size or to the creation of novel sequences at the junction that had a negative influence.
In addition, variant 1675-1744 had a slight defect in RC DNA synthesis in HepG2 cells (66% of WT RC DNA, P < 0.045) but had no significant defect in synthesizing RC DNA in Huh7 cells (80% of WT RC DNA, P < 0.12). Another variant with different phenotypes in the two cell lines was variant 2972-304. In HepG2 cells, RC DNA of variant 2972-304 was 75% of that of the WT level (P < 0.004). However, in Huh7 cells, the RC DNA proportion was not significantly different from that of the WT reference (P < 0.25).
Group III: deletion variants that synthesized RC DNA at proportions higher than those for the WT reference virus.
Variants in group III synthesized RC DNA at proportions higher those synthesized by the WT reference, and the difference was statistically significant (P < 0.05). In HepG2 cells, this group included deletion variants 1915-2110, 2111-2327, and 1420-1510 (Fig. 2). In Huh7 cells, this set included deletion variants 2111-2327, 2332-2630, 181-681, 1063-1234, 1236-1419, and 1420-1510 (Fig. 2). At least two possibilities might explain this phenomenon. The first possibility is that the sequences deleted in these mutants played an inhibitory role in the synthesis of RC DNA. Removing these nucleotides therefore led to increased proportions of RC DNA. The second possibility is that a smaller genome might carry out plus-strand DNA synthesis more rapidly and accumulate higher proportions of RC DNA.
Group IV: deletion variants that synthesized RC DNA to proportions not significantly different from those of the WT reference virus.
Deletion variants in group IV synthesized RC DNA to proportions that were not significantly different from those synthesized by the WT reference, based on statistical analysis (P > 0.05), although the mean values for the relative RC DNA proportions for some variants were greater than 100 (Fig. 2). This group included variants 2332-2630, 2637-2806, 281-681, 682-1069, 1063-1234, and 1236-1419 for HepG2 cells and variants 1914-2110, 2637-2806, 2972-304, 682-1069, and 1675-1744 for Huh7 cells (Fig. 2 and 3).
In summary, analyses of the deletion mutants spanning the genome in both cell lines revealed multiple cis-acting sequences that make positive contributions to the synthesis of HBV RC DNA. The significance of the deletion variants that synthesized higher-than-WT levels of RC DNA is not apparent and awaits further analysis. In general, results with HepG2 cells were consistent with results with Huh7 cells except for those for variants 1915-2110, 2972-304, 181-681, and 1675-1744 (Fig. 2).
HBV/WHV chimeras confirmed the cis-acting sequences that contribute to HBV RC DNA synthesis.
The analysis of variants with deletions indicated the presence of multiple cis-acting sequences that make positive contributions to the synthesis of HBV RC DNA. As a complementary and corroborating approach, we analyzed several HBV variants in which regions containing cis-acting sequences were replaced with heterologous sequences. A mutant phenotype of a substitution variant would corroborate our findings of the corresponding deletion variant and reinforce the conclusion that a cis-acting sequence was present in that region of the HBV genome.
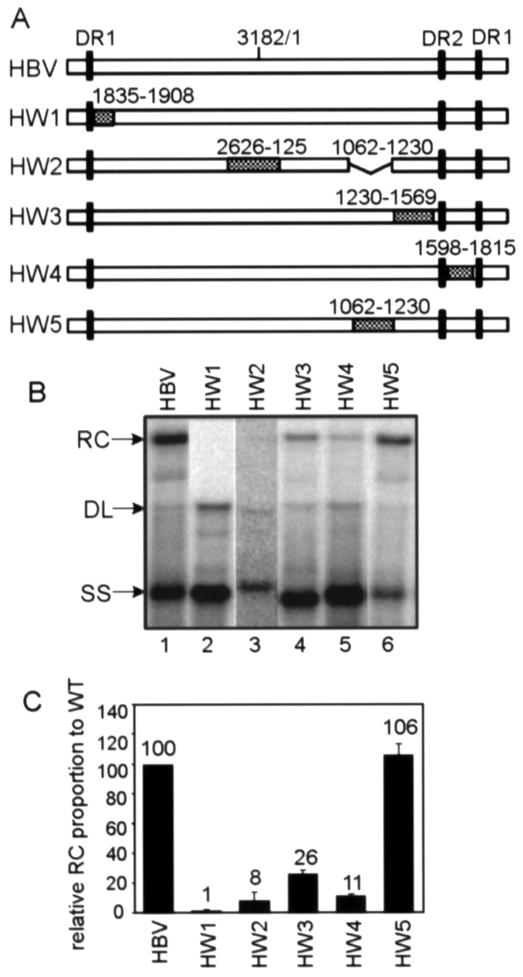
We targeted four HBV cis-acting sequences that make positive contributions to RC DNA synthesis: nt 1833 to 1844, 2820 to 2995, 1230 to 1569, and 1604 to 1674 (Fig. 2 and 3). We constructed HBV/WHV chimera variants (HW1 to HW4) (Fig. 4A) in which the selected HBV fragments were replaced by the corresponding WHV sequences (30). We substituted relatively large sections of sequences when constructing these chimeras (Fig. 4A). In variant HW2, the substituted WHV fragment was 138 bp longer than the original HBV sequence due to the WHV genome being larger than the HBV genome (5). To keep the genome size unchanged, sequences from nt 1062 to 1230 were deleted in HW2. Deletion of nt 1062 to 1230 does not alter the synthesis of RC DNA (Fig. 3A and B, lanes 14). The ability of these chimeras to synthesize RC DNA was analyzed in HepG2 cells and evaluated by Southern blotting (Fig. 4B and C). All four chimeras analyzed were defective for RC DNA synthesis. Similar to deletion variants 1833-1844 and 2631-2995, HW1 and HW2 chimeras had severe defects in synthesizing RC DNA (P < 0.01 for HW1 and P < 0.005 for HW2). The HW3 chimera had partial defects in synthesizing RC DNA (P < 0.005), which was also consistent with the deletion results. Interestingly, chimera HW4 accumulated comparable levels of minus-strand-containing DNA, in contrast to the phenotype of deletion variant 1744-1814 (Fig. 2 and 3) and deletion variant 1604-1814 (data not shown), which did not accumulate minus-strand DNA. This indicated that the substituted WHV sequence was functional in accumulating minus-strand DNA in the context of the HBV genome. For HW4, its RC DNA synthesis was also partially defective (P < 0.005). Therefore, results for HW1, HW2, HW3, and HW4 corroborated the findings from our deletion analysis.
FIG. 4.
Replication of HBV/WHV chimera variants in HepG2 cells. (A) WT HBV and HBV/WHV chimera variants (HW1 to HW5). The white boxes represent HBV sequences, and the hatched boxes represent the substituted WHV sequences. The black vertical bars indicate the positions of DR1 and DR2 sequences. In the HW2 construct, HBV sequences from nt 1062 to 1230 were deleted to maintain the genome size. (B) Southern blotting of WT HBV and HBV/WHV chimeras. Replicative intermediates were isolated from the cytoplasmic viral capsids in HepG2 cells and analyzed by Southern blotting. A genome-length, minus-strand-specific RNA probe was used to detect viral DNA. The positions of RC DNA, DL DNA, and SS DNA are indicated. (C) Proportions of RC DNA of the chimeras relative to that of the WT standard. The proportion of RC DNA is the level of RC DNA among the total intermediates containing mature minus-strand DNA on Southern blots. In each transfection, the RC DNA proportion of each of the chimeras was normalized to that of the WT standard, which gives the value for the relative RC DNA proportion. The mean values of the relative RC DNA proportion from at least six independent transfections were plotted on the histogram. The numbers above each bar indicate the mean values of the relative RC proportion. The error bars indicate the standard deviations.
As a control, we analyzed a chimera variant in which a region shown not to be important for HBV RC DNA synthesis was replaced with the corresponding WHV sequence. We asked whether this variant synthesized RC DNA at a level similar to that of the WT standard. Chimera HW5, which had sequences from nt 1062 to 1230 replaced by the corresponding WHV sequence, was able to replicate RC DNA at a level similar to that of the WT standard (Fig. 4).These chimeras were also analyzed in Huh7 cells. Results obtained with Huh7 cells were very similar to the results with HepG2 cells (data not shown).
DISCUSSION
We analyzed multiple deletion mutants that as a set represent the entire HBV genome except for sequences of DR1, DR2, 5′r, 3′r, and epsilon. We found evidence for the presence of multiple cis-acting sequences that make positive contributions to the synthesis of RC DNA. Analysis of several HBV/WHV chimeras corroborated these findings.
Until the present study, little was known about the cis-acting requirements for HBV RC DNA synthesis. Perri and Ganem identified a sequence near the 5′ terminus of pgRNA of the adw2 subtype, called UBS, which is important for the synthesis of the plus-strand DNA (22, 23). In another study, a cis-acting sequence designated Φ (F) located upstream of the 3′ copy of DR1 on pgRNA of the ayw subtype was shown to be important for efficient viral replication (31). Our study represents a comprehensive and quantitative analysis of the cis-acting sequences for the synthesis of HBV RC DNA. The sequence comprising nt 1833 to 1844 overlaps the 5′ UBS sequence of adw2 (22). Consistent with the Φ sequence, which is from nt 1769 to 1791, being important for efficient viral replication, we found the sequence comprising nt 1744 to 1814 necessary for one or more steps prior to plus-strand DNA synthesis (31). More importantly, based on our quantitative analysis, we identified multiple cis-acting sequences for RC DNA synthesis that had not previously been reported.
The mechanisms by which these cis-acting sequences contribute to the synthesis of HBV RC DNA are unknown. In DHBV, three cis-acting sequences, 3E, M, and 5E, which are distinct from the acceptor and donor sites, are necessary for the synthesis of RC DNA (6, 7, 19). It was demonstrated that base pairing among 3E, M, and 5E contributes to plus-strand template switches, possibly by placing the minus-strand DNA into such a conformation that both ends are juxtaposed (14). Although DHBV and HBV share very little, if any, nucleotide sequence similarity, a comparison of our findings with those for the DHBV cis-acting sequences reveal interesting similarities, at least superficially. The locations of sequences comprising nt 1833 to 1844, 2829 to 2995, and 1511 to 1587 on the HBV genome are similar to the locations of 3E, M, and 5E on the DHBV genome. Both nt 1833 to 1844 and DHBV 3E are located between DR1 and epsilon. The sequence comprising nt 2829 to 2995 is in the middle of the genome and contains part of the pre-S1 ORF, and the DHBV M sequence is also located in the middle of the genome and contains part of the preS-1 ORF. The sequence comprising nt 1511 to 1587 and DHBV 5E are both located upstream of the DR2 sequence. In terms of the magnitude of defects of the HBV deletion mutants, variants 1833-1844 and 2829-2995 have severe defects in synthesizing RC DNA, whereas variant 1511-1587 has a modest defect. These observations appear to correspond to the phenotypes of the 3E, M, and 5E deletion mutants of DHBV, respectively (6, 7). These similarities between DHBV and HBV cis-acting sequences raise the question of whether HBV also uses base pairing between these cis-acting sequences to contribute to plus-strand DNA synthesis, similar to that of DHBV. Although there appear to be similarities between the cis-acting sequences of HBV and DHBV, HBV appears to have more cis-acting requirements than DHBV for the synthesis of RC DNA. Sequences within nt 1614 to 1744, 1914 to 2327, and 2972 to 304 also contribute to efficient RC DNA synthesis. Therefore, it is likely that the mechanisms of HBV plus-strand DNA synthesis are not identical to those of DHBV. The HBV cis-acting sequences may also function by affecting the kinetics of RC DNA synthesis. Further analysis needs to be performed to understand exactly how the HBV cis-acting sequences function to contribute to RC DNA synthesis.
Throughout our analysis, we measured the accumulation of RC DNA in transfected cells by Southern blotting as a way to evaluate its synthesis. The accumulation of RC DNA is determined by the balance of its synthesis and loss. The loss of DNA-containing capsids could occur through their release into the media or by intracellular degradation. We think the selective loss of RC DNA-containing capsids does not contribute significantly to the lack of accumulation of RC DNA in our analysis. The normal exit pathway for RC DNA-containing capsids from cells is dependent on HBV envelope proteins. In our experimental system, envelope proteins are not present. Although a small portion of capsids containing replicative intermediates at all stages of DNA synthesis was released into the media in our system, the majority of the WT replicative intermediates remain cytoplasmic (data not shown). However, for the mutants, it is possible that they have accelerated selective release of RC DNA-containing capsids (but not DL DNA- or SS DNA-containing capsids) into the media in an envelope protein-independent manner or accelerated selective degradation of RC DNA-containing capsids intracellularly. Although to our knowledge there is no precedence for such mechanisms, our analysis cannot exclude these possibilities.
In our study, we used deletion variants to identify cis-acting sequences for the synthesis of RC DNA. It is possible that instead of removing cis-acting sequences that contribute directly to the synthesis of plus-strand DNA, the deletions indirectly affected plus-strand synthesis, for example, by fortuitously affecting the conformation of the minus-strand DNA template. However, the observations that not all deletion variants have mutant phenotypes and that most of the cis-acting sequences identified can be further mapped to smaller sequences make this possibility less likely. Additionally, the fact that the WHV substitution mutants had phenotypes similar to those of the original HBV deletion mutants appears inconsistent with the possibility that deletions fortuitously created a bad DNA template. The resolution of this issue awaits a mechanistic understanding of the deletion variants.
For DL DNA, plus-strand DNA is initiated from DR1, and no template switch is involved in its synthesis (28). We measured the levels of DL DNA of the deletion variants by Southern blotting (data not shown). Most of the deletion mutants had proportions of DL DNA similar to those of the WT reference. However, variants 1833-1844, 1914-2327, 2631-2996, 1063-1570, and 181-681 synthesized two- to threefold more DL DNA than did the WT in both HepG2 and Huh7 cells. It is interesting that the increase in DL DNA in the first four variants mentioned above was accompanied by the defects in RC DNA synthesis. It could be speculated that the increase in DL DNA in these deletion variants was a result of inhibition in priming from DR2 (primer translocation). To make the analysis of in situ priming more compelling, an alternative assay such as a primer extension needs to be performed.
Acknowledgments
We thank Michael Nassal (University Hospital, Freiburg, Germany) for the parental HBV clones and Jesse Summers (University of New Mexico) for the WHV clone. We thank Alan McLachlan (Scripps Institute) for advice on culturing HepG2 cells. We thank Paul Ahlquist and Jesse Summers for critical review of the manuscript.
This work was supported by NIH grants P01 CA22443, P30 CA07175, and P30 CA14520.
REFERENCES
- 1.Bartenschlager, R., M. Junker-Niepmann, and H. Schaller. 1990. The P gene product of hepatitis B virus is required as a structural component for genomic RNA encapsidation. J. Virol. 64:5324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartenschlager, R., and H. Schaller. 1992. Hepadnaviral assembly is initiated by polymerase binding to the encapsidation signal in the viral RNA genome. EMBO J. 11:3413-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beasley, R. P., L. Y. Hwang, C. C. Lin, and C. S. Chien. 1981. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22,707 men in Taiwan. Lancet ii:1129-1133. [DOI] [PubMed] [Google Scholar]
- 4.Ganem, D., and R. Schneider. 2001. Hepadnaviridae: the viruses and their replication, p. 2923-2969. In D. Knipe, P. Howley, D. Griffin, R. Lamb, M. Martin, B. Roizman, and S. Strauss (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 5.Girones, R., P. J. Cote, W. E. Hornbuckle, B. C. Tennant, J. L. Gerin, R. H. Purcell, and R. H. Miller. 1989. Complete nucleotide sequence of a molecular clone of woodchuck hepatitis virus that is infectious in the natural host. Proc. Natl. Acad. Sci. USA 86:1846-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Havert, M. B., L. Ji, and D. D. Loeb. 2002. Analysis of duck hepatitis B virus reverse transcription indicates a common mechanism for the two template switches during plus-strand DNA synthesis. J. Virol. 76:2763-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Havert, M. B., and D. D. Loeb. 1997. cis-Acting sequences in addition to donor and acceptor sites are required for template switching during synthesis of plus-strand DNA for duck hepatitis B virus. J. Virol. 71:5336-5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch, R. C., J. E. Lavine, L. J. Chang, H. E. Varmus, and D. Ganem. 1990. Polymerase gene products of hepatitis B viruses are required for genomic RNA packaging as well as for reverse transcription. Nature 344:552-555. [DOI] [PubMed] [Google Scholar]
- 9.Junker-Niepmann, M., R. Bartenschlager, and H. Schaller. 1990. A short cis-acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO J. 9:3389-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kane, M. A. 1998. Status of hepatitis B immunization programmes in 1998. Vaccine 16(Suppl.):S104-S108. [DOI] [PubMed] [Google Scholar]
- 11.Lien, J.-M., C. E. Aldrich, and W. S. Mason. 1986. Evidence that a capped oligoribonucleotide is the primer for duck hepatitis B virus plus-strand DNA synthesis. J. Virol. 57:229-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lien, J.-M., D. J. Petcu, C. E. Aldrich, and W. S. Mason. 1987. Initiation and termination of duck hepatitis B virus DNA synthesis during virus maturation. J. Virol. 61:3832-3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu, N., K. M. Ostrow, and D. D. Loeb. 2002. Identification and characterization of a novel replicative intermediate of heron hepatitis B virus. Virology 295:348-359. [DOI] [PubMed] [Google Scholar]
- 14.Liu, N., R. Tian, and D. D. Loeb. 2003. Base pairing among three cis-acting sequences contributes to template switching during hepadnavirus reverse transcription. Proc. Natl. Acad. Sci. USA 100:1984-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loeb, D. D., K. J. Gulya, and R. Tian. 1997. Sequence identity of the terminal redundancies on the minus-strand DNA template is necessary but not sufficient for the template switch during hepadnavirus plus-strand DNA synthesis. J. Virol. 71:152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loeb, D. D., and R. Tian. 2001. Mutations that increase in situ priming also decrease circularization for duck hepatitis B virus. J. Virol. 75:6492-6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loeb, D. D., R. Tian, and K. J. Gulya. 1996. Mutations within DR2 independently reduce the amount of both minus- and plus-strand DNA synthesized during duck hepatitis B virus replication. J. Virol. 70:8684-8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loeb, D. D., R. Tian, K. J. Gulya, and A. E. Qualey. 1998. Changing the site of initiation of plus-strand DNA synthesis inhibits the subsequent template switch during replication of a hepadnavirus. J. Virol. 72:6565-6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller-Hill, K., and D. D. Loeb. 2002. cis-Acting sequences 5E, M, and 3E interact to contribute to primer translocation and circularization during reverse transcription of avian hepadnavirus DNA. J. Virol. 76:4260-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nassal, M. 1992. The arginine-rich domain of the hepatitis B virus core protein is required for pregenome encapsidation and productive viral positive-strand DNA synthesis but not for virus assembly. J. Virol. 66:4107-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nassal, M., and A. Rieger. 1996. A bulged region of the hepatitis B virus RNA encapsidation signal contains the replication origin for discontinuous first-strand DNA synthesis. J. Virol. 70:2764-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perri, S., and D. Ganem. 1997. Effects of mutations within and adjacent to the terminal repeats of hepatitis B virus pregenomic RNA on viral DNA synthesis. J. Virol. 71:8448-8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perri, S., and D. Ganem. 1996. A host factor that binds near the termini of hepatitis B virus pregenomic RNA. J. Virol. 70:6803-6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollack, J. R., and D. Ganem. 1994. Site-specific RNA binding by a hepatitis B virus reverse transcriptase initiates two distinct reactions: RNA packaging and DNA synthesis. J. Virol. 68:5579-5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radziwill, G., W. Tucker, and H. Schaller. 1990. Mutational analysis of the hepatitis B virus P gene product: domain structure and RNase H activity. J. Virol. 64:613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seeger, C., D. Ganem, and H. Varmus. 1986. Biochemical and genetic evidence for the hepatitis B virus replication strategy. Science 232:477-484. [DOI] [PubMed] [Google Scholar]
- 27.Seeger, C., and W. S. Mason. 2000. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 64:51-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staprans, S., D. D. Loeb, and D. Ganem. 1991. Mutations affecting hepadnavirus plus-strand DNA synthesis dissociate primer cleavage from translocation and reveal the origin of linear viral DNA. J. Virol. 65:1255-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Summers, J., and W. S. Mason. 1982. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell 29:403-415. [DOI] [PubMed] [Google Scholar]
- 30.Summers, J., J. Smolec, and R. Snyder. 1978. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc. Natl. Acad. Sci. USA 75:4533-4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang, H., and A. McLachlan. 2002. A pregenomic RNA sequence adjacent to DR1 and complementary to epsilon influences hepatitis B virus replication efficiency. Virology 303:199-210. [DOI] [PubMed] [Google Scholar]
- 32.Tavis, J. E., and D. Ganem. 1995. RNA sequences controlling the initiation and transfer of duck hepatitis B virus minus-strand DNA. J. Virol. 69:4283-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tavis, J. E., S. Perri, and D. Ganem. 1994. Hepadnavirus reverse transcription initiates within the stem-loop of the RNA packaging signal and employs a novel strand transfer. J. Virol. 68:3536-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, G.-H., and C. Seeger. 1993. Novel mechanism for reverse transcription in hepatitis B viruses. J. Virol. 67:6507-6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, G. H., and C. Seeger. 1992. The reverse transcriptase of hepatitis B virus acts as a protein primer for viral DNA synthesis. Cell 71:663-670. [DOI] [PubMed] [Google Scholar]
- 36.Will, H., W. Reiser, T. Weimer, E. Pfaff, M. Büscher, R. Sprengel, R. Cattaneo, and H. Schaller. 1987. Replication strategy of human hepatitis B virus. J. Virol. 61:904-911. [DOI] [PMC free article] [PubMed] [Google Scholar]