Abstract
The co-operation of specialized organ systems in complex multicellular organisms depends on effective chemical communication. Thus, body fluids (like blood, lymph or intraspinal fluid) contain myriads of signaling mediators apart from metabolites. Moreover, these fluids are also of crucial importance for immune and wound responses. Compositional analyses of human body fluids are therefore of paramount diagnostic importance. Further improving their comprehensiveness should increase our understanding of inter-organ communication. In arthropods, which have trachea for gas exchange and an open circulatory system, the single dominating interstitial fluid is the hemolymph. Accordingly, a detailed analysis of hemolymph composition should provide an especially comprehensive picture of chemical communication and defense in animals. Therefore we used an extensive protein fractionation workflow in combination with a discovery-driven proteomic approach to map out the detectable protein composition of hemolymph isolated from Drosophila larvae. Combined mass spectrometric analysis revealed more than 700 proteins extending far beyond the previously known Drosophila hemolymph proteome. Moreover, by comparing hemolymph isolated from either fed or starved larvae, we provide initial provisional insights concerning compositional changes in response to nutritional state. Storage proteins in particular were observed to be strongly reduced by starvation. Our hemolymph proteome catalog provides a rich basis for data mining, as exemplified by our identification of potential novel cytokines, as well as for future quantitative analyses by targeted proteomics.
Introduction
Extracellular body fluids, for example blood plasma or interstitial fluid, serve as transport systems for metabolites, nutrients, hormones or oxygen in virtually all animals. Insects have only one extracellular fluid called hemolymph that is usually kept in circulation by an open heart within the body cavity. The hemolymph is in direct contact with all internal organs. It delivers necessary substances such as nutrients to the cells and it transports metabolic waste products away from those same cells. It contains hemocytes, most of which are phagocytic cells [1]. Moreover, it contains proteins that provide hemostatic responses to wounding [2]. Many additional hemolymph proteins help to protect the insect against invading microorganisms [3]. Hormones that regulate developmental timing, metamorphosis, metabolism, growth, reproduction and associated behavior are secreted and circulated in the hemolymph [4], [5], [6], [7], [8].
Despite its importance for development and physiology, there is only limited information about insect hemolymph composition. Initial biochemical analyses have focused on low-molecular-weight compounds such as inorganic salts, amino acids, organic acids, lipids and sugars [9], but not much is known about the protein composition of hemolymph. Mass spectrometry-based proteomics (MS) has emerged as a powerful tool for monitoring protein composition in body fluids in different states. Studies in several insect species have identified hemolymph proteins after separation by one- and two-dimensional gel electrophoresis in conjunction with MS [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]. More comprehensive proteomic analysis including in-solution digestion of hemolymph proteins followed by liquid chromatography-mass spectrometry (LC-MS) has been carried out for the honey bee [24] but is prominently missing for the fruit fly Drosophila melanogaster, the insect model system most widely used in cell and developmental biology that has provided invaluable insights of very general significance for eukaryotes including humans.
Elegant co-culture experiments with isolated organs from Drosophila larvae have strongly suggested that starvation affects not just the metabolite [25], [26], [27] but also the signaling factor composition of hemolymph [28], and recent genetic analyses have clearly identified secreted signaling proteins that are thought to have a variable hemolymph concentration regulated by the nutritional status [29], [30], [31]. Therefore, a comparison of hemolymph samples from fed and starved larvae might in principle also point to novel candidate signaling factors although their often very low effective concentrations represents a great challenge.
Here we present a deep shotgun proteomic analysis of hemolymph samples from third-instar Drosophila larvae. The overall aim of this study was to establish a comprehensive proteome map [32] of the Drosophila larval hemolymph. In addition, we describe an initial step towards an understanding of the impact of nutrient conditions on hemolymph protein composition. Our results extend the number of known hemolymph proteins by almost an order of magnitude and demonstrate dramatic starvation effects on storage proteins.
Materials and Methods
Drosophila Culture and Hemolymph Isolation
Flies of the Oregon R (OreR) wild-type strain were cultivated at 25°C and 45% relative humidity on standard food (100 g/l yeast, 75 g/l sucrose, 55 g/l cornmeal, 10 g/l wheat flour, 8 g/l agar, 0.45 ml/l nipagine, 0.9 ml/l propyl paraben). To generate larvae for hemolymph collection, an initial egg collection for the elimination of overaged eggs was performed during 1 hour in fresh fly bottles with standard food. Thereafter, flies were transferred to another set of fresh fly bottles with standard food and eggs were collected for 2 hours. Flies were discarded and the bottles with the eggs were incubated at 25°C. After incubation for 64 hours, larvae were washed out from the food and transferred to either bottles with fresh standard food (fed) or to bottles with filter paper soaked with 20% sucrose (starved). Hemolymph isolation was started after incubation for another 24 hours at 25°C. Immediately before hemolymph collection, larvae were surface sterilized in 70% ethanol. Excess fluid was blotted off on filter paper. Batches of 10–15 larvae were dipped in halocarbon oil of high viscosity (Halocarbon-oil 1000N, Solvadis Chemag, #102780) and opened by gently pulling the epidermis apart with forceps to start hemolymph bleeding. The hemolymph accumulated in a drop around the larvae was collected with a fine glass pipette, transferred into an Eppendorf tube and immediately frozen in liquid nitrogen. We emphasize that our isolation procedure did not include removal of hemocytes in order to minimize the time available for potential proteolytic and chemical modifications of hemolymph proteins during isolation that harm subsequent proteomic analyses. The complete sampling procedure until freezing took less than two minutes and resulted in 3–4 µl of hemolymph per aliquot. Between 30–40 aliquots, collected from three independent batches of larvae, were pooled for the MS analyses that resulted in the data described in Table S1. Pools of around 20 additional aliquots collected from independent batches of larvae were used in an initial pilot MS analysis. Developmental stages of larvae were assigned based on mouth hook morphology [33].
Sample Preparation for Mass Spectrometry
Pooled hemolymph aliquots were briefly centrifuged for removal of insoluble material and lysed in 50 mM (NH4)HCO3 containing 0.2% RapiGest (Waters). The protein concentration was determined in a Qubit fluorometer (Invitrogen). 1 mg of the total protein lysate was reduced with 5 mM Tris(2-carboxyethyl)phosphine hydrochloride (TCEP) and treated with 10 mM iodoacetamide to modify cysteine residues. Tryptic digestion was carried out overnight using 20 µg trypsin (Promega) per sample and a concentration of RapiGest of 0.1%. The samples were purified by reverse phase C-18 chromatography (Sep-PaK, Waters). For sample fractionation, isoelectric focusing of peptides was performed (OFFGEL fractionator 3100, Agilent). A 24 well strip with a linear pH gradient ranging from 3–10 was used (GE Healthcare). The offgel (OG) fractionation was performed as described [34]. In short, the OG fractionation was started after dispensing 150 µl of the peptide solution in each well. The potential was fixed for the first hour at 500 V, then set to a maximum of 8000 V and after finishing the separation kept at 500 V (total of 50 kVh; total run time ∼18 h). The current limit was set at 100 µA and the temperature was maintained at 20°C. After OG fractionation, the 24 peptide fractions were cleaned by reverse phase C-18 chromatography (MicrospinColumns, SEM SS18V, The Nest Group, Inc).
Mass Spectrometry Analysis
For mass spectrometry analysis samples were resuspended in 50 µl of buffer A (5% acetonitrile, 0.2% formic acid). From each sample, 1 µl of material was loaded on a LTQ-Orbitrap XL ETD (Thermo Fisher Scientific). The instrument was coupled to an Eksigent nano-LC system. Samples were automatically injected into a 10-µl sample loop and loaded onto an analytical column that was packed in-house with Magic C18 AQ beads (3 µm, 100 Å, Microm) 9 cm in length × 75 µm (internal diameter). Peptide mixtures were delivered to the analytical column at a flow rate of 500 nl/minute (3% acetonitrile, 0.2% formic acid) for 16 minutes and then eluted using a gradient of acetonitrile (3%–35%; 0.53%/minute) with 0.2% formic acid at a flow rate of 250 nl/minute. The samples were measured in a survey scan from 300 to 2,000 a.m.u., followed by 6 data-dependent MS/MS scans with dynamic exclusion (isolation width 2 a.m.u., repeat count 1, exclusion list size 500, dynamic exclusion duration 60 s). In a second survey scan, the same settings were applied with the addition of a static exclusion list of all peptides monitored in the first survey scan. The static exclusion list contained all MS1 spectra with an assigned MS/MS from the first survey run. Subsequently, the first two survey scans were used to generate a third survey scan with inclusion lists for the MS1 features which had not been analyzed by either the first or the second MS run. The inclusion lists for all OG fractions were generated with the Progenesis software tool (Non Linear Dynamics, New Castle upon Tyne, UK Version 4.0). Manually, seeding vectors (4–7) were set over the whole retention time followed by automatic alignment of the feature maps with a sensitivity threshold of 3. Filters for features with MS/MS were applied to remove those. The remaining MS1 features were exported with a retention time window of 2.5 min to an Xcalibur compatible inclusion list. The generated inclusion list was used to perform a third survey scan on the respective OG fraction. In total, 144 measurements were performed.
Database Search and Protein Identification
Raw data were converted into the open format mzXML. Using the Sequest algorithm [35], fragment mass spectra were searched against a protein sequence database containing 21,317 D. melanogaster proteins (FlyBase version 2008_10) and 256 common contaminants (keratins, trypsin, etc.). Spectra were searched for a match to fully-tryptic and semi-tryptic peptides with up to two missed cleavage sites with a mass tolerance of 0.04 Da. Carbamidomethylation (+57.021464 Da) was set as fixed modification for all Cysteines and oxidation (+15.994915 Da) was considered as optional modification for Methionines. Search results were post-processed using Peptide Prophet (TPP version 4.5.0) [36] to model correct versus incorrect peptide spectrum matches (PSMs). Based on the target-decoy search strategy [37] a stringent score cutoff was determined that resulted in an estimated false discovery rate (FDR) of less than 0.2% at the PSM level. PSMs above this cutoff were classified with the PeptideClassifier software [38].
A minimal list of unambiguous protein identifications (based on class 1a, 1b, or 3a peptides) and protein group identifications that imply one gene model (based on class 2a, 2b peptides) was generated (Table 1). For class 3b peptides, which imply distinct proteins encoded by different gene models, the minimal possible number of protein groups not identified by peptides of higher information content was determined. For a protein identification, we required at least two independent PSMs. This resulted in a final estimated protein-level FDR of 1.3%. Raw data from the proteomic experiments will be made available at PRIDE (http://www.ebi.ac.uk/pride/).
Table 1. Summary of identified spectra, peptides, proteins and estimated FDR levels.
| Evidence classa) | No. of spectra | No. of distinct peptides | No. of distinct proteinsb) |
| Class 1a | 51,359 | 4,129 | 429 |
| Class 1b | 5,005 | 784 | 117 |
| Class 2a | 1,282 | 170 | 25 |
| Class 2b | 8,053 | 1,377 | 120 |
| Class 3a | 159 | 23 | 6 |
| Class 3b | 3,631 | 251 | 28c) |
| target DB | 69,489 | 6,734 | 725 |
| decoy DB | 128 | 55 | 10 |
| estimated FDRd) | <0.2% | <0.8% | <1.4% |
According to our peptide classification scheme [38], [46], class 1a peptides unambiguously identify a single unique protein sequence encoded by a unique transcript. Class 1b peptides also unambiguously identify a unique protein sequence encoded by several transcripts of the same gene model with identical coding region and differences in the 5′ and/or 3′ untranslated regions. Class 2a peptides identify a subset and class 2b peptides all protein sequences encoded by a gene model. Class 3a peptides unambiguously identify one protein sequence, but this sequence could be encoded by several gene models from distinct loci (e.g. histones). Finally, class 3b peptides can be derived from different protein sequences encoded by several gene models from distinct loci and have the least information content.
For protein groups identified by class 2a or 2b peptides (a gene model identification) all possible protein accessions are listed in Table S1.
The minimal number of additional protein identifications by 3b peptides is shown.
Based on the total hits in target and decoy databases (DB), the FDR was estimated at the spectra, peptide and protein level.
For prediction of globular proteins we used Globplot 2.3 (http://globplot.embl.de/) [39], for prediction of signal peptides SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/) [40].
Differential Protein Expression Analysis
Using the decoy-search hits, peptide-spectrum matches (PSMs) were stringently filtered to a FDR of less than 0.2%. Corresponding peptides were classified with the PeptideClassifier software [38]. A minimal list of unambiguous protein identifications (based on class 1a, 1b, or 3a peptides) and protein group identifications that imply one gene model (based on class 2a, 2b peptides) was generated (Table 1). For class 3b peptides, which imply distinct proteins encoded by different gene models, the minimal possible number of protein groups not identified by peptides of higher information content was determined.
Differential protein expression analysis was carried out with the R package DESeq (version 1.6.1) [41]) using the spectral count data as input. Based on normalized count data, DESeq modeled gene/protein expression with a negative binomial distribution and generated a list of genes/proteins ranked according to statistical significance. Default parameters were chosen as described in the DESeq package vignette (a “local” fit was used to estimate dispersion).
Results and Discussion
Hemolymph was collected from larvae of the Oregon R wild-type strain. A first batch was isolated from larvae obtained after egg collection for 2 hours and ageing for an additional 89 hours at 25°C in the presence of unlimited standard Drosophila food (Fig. 1A). At the time of hemolymph isolation these larvae were therefore expected to be in mid L3 stage. Scoring of larval mouth hook morphology, which allows accurate larval stage assignment, clearly confirmed that the larvae had all reached the L3 stage (n = 50). Mid L3 was chosen for hemolymph collection as this stage is accompanied by the most extensive growth of all Drosophila development [33]. For comparison, we also analyzed hemolymph from larvae of identical age after exposure to starvation conditions (Fig. 1A). During the last 24 hours, this second batch of larvae was aged in the presence of 20% sucrose, i.e. without a source of amino acids and other non-carbohydrate metabolites. At the onset of starvation, the majority of larvae were still in the L2 stage according to mouth hook morphology (62.9% in L2, 5.6% during L2/L3 molt, 31.5% in L3; n = 54). Later, at the time of hemolymph isolation, all the starved larvae had reached the L3 stage (n = 50) but they were clearly smaller than the fed larvae (Fig. 1B). Moreover, larvae that were kept further under starvation condition instead of being sacrificed for hemolymph collection did not pupariate like the fed larvae (Fig. 1C). Pupariation was either blocked (in ∼30%) or delayed (in ∼70%). The pupae formed by the starved larvae were smaller than those of fed larvae (Fig. 1D). These results confirm that starvation was initiated at a time when the majority of the larvae had not yet reached the so-called critical weight. Starvation before attainment of the critical weight is known to delay metamorphosis onset, while later starvation no longer causes delays [42].
Figure 1. Starvation protocol and developmental effects.
(A) At 65 hours after egg deposition (AED), half of the larvae were transferred to starvation medium (20% sucrose). Twenty-four hours later, hemolymph from fed and starved larvae was collected for deep shotgun proteomics. Developmental timing of ecdysone titer, larval stages L2 and L3, acquisition of critical weight, wandering behavior and pupation under optimal conditions is indicated as well. Numbers indicate time in hours AED. (B) Size of fed and starved larvae at time of hemolymph collection. (C) At 65 hours AED, larvae were either shifted to starvation medium or further maintained on rich medium followed by analysis of the fraction of pupae over time (n = 278 fed and 141 starved) (D) Size of pupae formed by either fed or starved larvae. Bars = 0.5 mm.
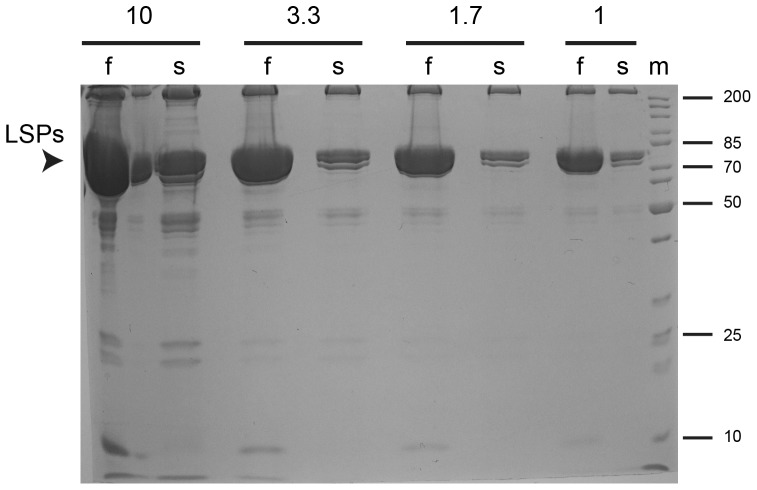
For the isolation of hemolymph, larvae were gently opened with forceps to release undiluted hemolymph that was quickly isolated without removal of hemocytes. Compared to fed larvae, protein concentration in hemolymph isolated from starved larvae was found to be about twofold lower in two independent experiments. Analysis by SDS-PAGE revealed that hemolymph of starved larvae contained far lower levels of the predominant hemolymph proteins with apparent molecular weights around 80 kDa (Fig. 2). These larval serum proteins (Lsp1α, Lsp1β, Lsp1γ, and Lsp2) are strongly up-regulated during the L3 stage. Their amount in hemolymph of third instar wandering stage larvae grown in rich medium corresponds to up to 70% of the total hemolymph protein [43], [44], [45].
Figure 2. Abundance of larval serum proteins.
Hemolymph was isolated from fed (f) and starved (s) larvae (see Fig. 1). Proteins in samples of 10, 3.3, 1.7 or 1 µl hemolymph were resolved by SDS-PAGE and stained with Coomassie Blue. The position of the major larval serum proteins (LSPs) is indicated by an arrowhead. Position and size (kDa) of molecular weight markers (m) are indicated on the right side.
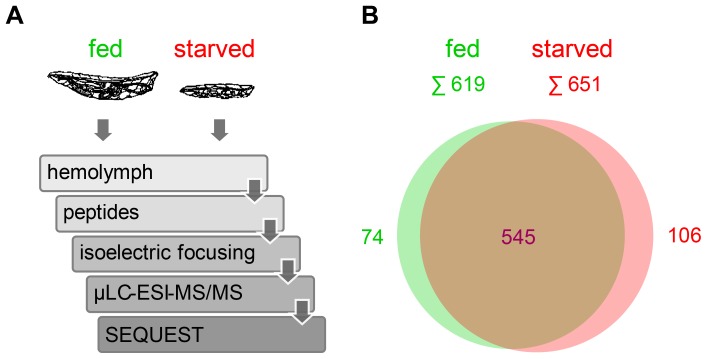
Hemolymph samples were analyzed on an Orbitrap XL mass spectrometer (Fig. 3A). Peptides were classified using a deterministic classification scheme [38], [46] (Table S1). Within the two samples, we identified in total 6734 unique peptides corresponding to protein products from 725 different gene models with a FDR of 1% (Table 1, Fig. 3B). 75% (545 gene models) were detected in hemolymph from both fed and starved larvae. 10% (74 gene models) were only detected in hemolymph from fed larvae, while 25% (106 gene models) were only observed in hemolymph from starved larvae in which also a higher total number of different gene models were detected (651 versus 619). Previous analyses of the Drosophila hemolymph proteome [11], [12], [14], [16], [17], [18], [47] have been considerably less comprehensive. Overall these earlier studies have detected only 13% of the gene models identified in our analysis. 90% of the previously identified hemolymph proteins were also detected in our study. The large majority of these previously described proteins are very abundant hemolymph components as inferred from spectral counting [48] (Table S2). Similarly, previous analyses of the hemolymph proteome in other insects (including the bee Apis mellifera, the silkworm Bombyx mori, and the tobacco hornworm Manduca sexta) have been of comparatively limited scope, revealing primarily abundant constituents [10], [15], [19], [24], [49], [50], [51], [52], [53], [54].
Figure 3. Characterization of the larval hemolymph proteome.
(A) Workflow of the analyses. Hemolymph samples from fed and starved larvae were digested in solution. Tryptic peptides were separated by isoelectric focusing for complexity reduction. Peptides were analyzed using microcapillary liquid chromatography–electrospray ionization–tandem MS (µLC-ESI-MS/MS). SEQUEST spectral search was performed for peptide spectrum matching. (B) Venn diagram illustrating the number of gene models detected in hemolymph from fed and starved larvae, respectively.
The substantial increase in the number of identified hemolymph proteins resulting from our analysis in Drosophila larvae brings the complexity of this proteome far closer to the range described for the extensively analyzed human plasma proteome. The human plasma proteome project has detected a highly non-redundant set of 1929 protein sequences at 1% FDR [55]. In both, human plasma and Drosophila larval hemolymph, protein abundances vary over a very wide range. The concentration (∼40 mg/ml) of the most abundant protein in human plasma, serum albumin, is comparable to that of the most abundant component of Drosophila larval hemolymph, the major apolipoprotein Rfabg [56], [57]. By intense shotgun proteomics, proteins with a concentration more than 6.5 orders of magnitudes lower have been identified in human plasma. Nevertheless, shotgun proteomics has clear limitations especially in case of low abundance proteins. Some human plasma proteins are known to have concentrations that are more than 10 orders of magnitude lower than the most abundant components, and in general, the known low abundance proteins have escaped detection by shotgun proteomics [55], [58]. Moreover, in this approach low abundance is just one of several limiting factors with protein size and absence of suitable tryptic cleavage sites being among the additional crucial detection determinants. Thus our protein catalog of Drosophila larval hemolymph cannot be expected to be complete and an absence of some known hemolymph constituents is clearly evident. For example, we have not detected insulin-like peptides encoded by the dilp genes. Dilps 2, 3 and 5 are released into the hemolymph from specialized neurosecretory cells within the larval brain in response to nutrient uptake and presumably act at nanomolar concentrations [30], [31]. Similarly, we did not detect Upd2/Leptin that is secreted from the fat body in response to nutrient uptake and triggers Dilp 2/5 release from the brain neurosecretory cells [29]. Detection and quantification of very low abundance components will require different and targeted approaches [32], [59], [60], [61]. Moreover, depletion of quantitatively dominating components is an additional strategy allowing deeper sampling. As Drosophila Lsp null mutants are viable and fertile [45], analyses of their hemolymph might further increase overall proteome coverage in future studies.
In contrast to Dilps and Upd2, we have readily detected other proteins that have been proposed to function as growth factors. For example, we have clearly observed the products from all of the six Drosophila members of the family of Imaginal Disc Growth Factors genes (Idgf1-5, CG5210). In fact, our data suggests that these chitinase-related proteins are abundant hemolymph components (among top 10%) in fed and in starved larvae. IDGFs were originally identified in conditioned medium because of their growth-promoting activity on Drosophila cl8 cells [62], [63]. Moreover, we also detected Adenosine deaminase-related growth factor A (Adgf-A) in hemolymph. Adgf-A is the main regulator of extra-cellular adenosine during larval stages and has been shown to play important roles in the control of hemocyte proliferation [64], [65], [66].
Our hemolymph proteome contains several proteins that have not been detected in previous shotgun analyses but were recently shown to be hemolymph proteins after an initial identification by genetic approaches while our work was ongoing. The minor apolipoproteins apoLTP/CG15828 and Cv-d/CG31150 [57], [67] belong to this group for example.
To illustrate the potential of our hemolymph protein catalog for data mining and future functional analyses, we generated a list of potential novel cytokines (Table S2). For this list, we filtered out all CG numbers that were predicted to encode a globular secreted protein smaller than 400 amino acid residues. Moreover, we retained only those that have not yet been reported to be hemolymph components according to our knowledge. The resulting list comprised 30 entries that might deserve further analysis. Two among this list (CG15201 and CG31997) are SVC family proteins that have a motif initially proposed to be related to insulin-like growth factor (IGF) but more recently classified as more similar to the C-domain of von Willebrand factor (VWC) [68].
As our hemolymph isolation procedure did not include hemocyte removal, detection of some cytosolic and nuclear proteins was expected. Moreover, as larval wounding was involved in our hemolymph isolation procedure, tissue damage and consequential rupture of crystal cells might have augmented a release of non-secreted cellular proteins like histones and ribosomal proteins into the hemolymph [69], [70], [71]. To what extent such release occurs even during unperturbed development of Drosophila larvae is not known. Non-secreted cellular proteins detected in our work might therefore have originated from the included intact hemocytes, as well as from lysis of hemocytes and other cells before or during hemolymph isolation. Reliable clarification of the origin of non-secreted cellular proteins will require additional experiments and will depend on methods with detection sensitivity higher than shotgun proteomics in particular in case of those revealed by only one or a few peptides. A provisional estimate based on our histone peptide counts and PaxDb data concerning humans [72] suggested that tissue leakage into our hemolymph samples has occurred to a comparable extent as apparent in case of human plasma. Moreover, assuming that our 24 hour starvation period did not have significant effects on cellular levels of ribosomal proteins, the numerical comparison of all unequivocal peptides derived from ribosomal proteins (297 in fed, 264 in starved) suggests that the release of non-secreted cellular proteins into our two samples has occurred to a comparable extent.
To identify proteins with different abundance in hemolymph from fed and starved larvae, respectively, we compared spectral counts using DESeq [41] (Fig. 4, Table S3). Spectral counting is only an approximate measure of abundance. Moreover, an interpretation of our spectral counts needs to take into account that the total protein concentration in hemolymph from fed and starved larvae is not identical. As indicated above, protein content of hemolymph from starved larvae is twofold lower compared to fed larvae primarily because of the absence of larval serum proteins in starved larvae. As we have analyzed the same amount of total protein for the fed and the starved sample, normalization is not trivial. We emphasize that the differences in protein abundance suggested by our data may not necessarily reflect the reality, in particular in case of proteins with low spectral counts, where sampling bias and contingencies as well as normalization problems might have caused distortions. As a consequence, we restrict our following comments to cases with putative concentration differences that were far more extensive than twofold and also apparent in an independent biological replicate, our initial smaller pilot experiment. For these proteins statistical support for differential abundance was very strong. The top 10% of the differentially regulated proteins resulting from 40 genes are compiled in Table 2 (for complete data set see Table S3).
Figure 4. Effects of starvation on hemolymph proteome.
The magnitude versus amplitude (MA) plot shows the log2 fold change of the expression of the identified D. melanogaster proteins in the starved versus fed condition against the mean normalized spectral count. The top 10% differentially expressed proteins are highlighted, including 50 up-regulated proteins (red dots) and 22 down-regulated proteins (green dots). Protein identifiers are shown for selected proteins discussed in the text. Unambiguous protein identifications by class 1a, 1b, and 3a peptides are shown as full circles. Protein groups identified by class 2a or 2b peptides (which unambiguously imply a gene model) are shown as open circles, ambiguous identifications by 3b peptides are shown as open diamonds (the respective identifiers are listed in Table S2).
Table 2. Starvation-associated protein abundance changes in larval hemolymph.
| gene symbol | after starvation | p-value | total counts | log2 starved/fed | transcript dev. expr.a) | comment |
| Yp3 | Down | 1.04E-04 | 143 | -Inf | −3.59694 | yolk protein, female specific |
| Fbp1 | Down | 2.37E-04 | 112 | -Inf | −14.7243 | fat body protein 1 |
| Fbp2 | Down | 0.004945 | 38 | -Inf | −9.69436 | fat body protein 2 |
| CG7320 | Down | 0.024777 | 19 | -Inf | −6.07039 | hexamerin related |
| CG3264 | Down | 0.027552 | 18 | -Inf | 0.058894 | putative alkaline phosphatase |
| CG31075 | Down | 0.044311 | 14 | -Inf | −1.07039 | putative mito. aldehyde dehydrogenase |
| Npc2h | Down | 0.096736 | 9 | -Inf | −0.926 | Niemann-Pick Type C-2h |
| Lsp1α | Down | 4.55E-05 | 2958 | −8.48 | −5.40939 | Hexamerin |
| Lsp2 | Down | 0.006818 | 1827 | −4.67 | −7.6886 | Hexamerin |
| CG31769 | Down | 0.123496 | 14 | −3.82 | 0.321928 | |
| Obp99b | Down | 0.02253 | 857 | −3.80 | −8.02791 | odorant binding protein |
| Lsp1β | Down | 0.032916 | 6144 | −3.65 | −3.88753 | Hexamerin |
| Pxn | Down | 0.053156 | 53 | −3.38 | −0.1375 | Peroxidasin, extracellular matrix |
| Irc | Down | 0.067196 | 41 | −3.33 | −0.76553 | Immune-regulated catalase |
| CG13962 | Down | 0.147981 | 43 | −2.48 | −1.20163 | |
| Lsp1γ | Down | 0.146536 | 7195 | −2.38 | −2.90689 | Hexamerin |
| Tsf1 | Down | 0.16602 | 99 | −2.10 | −1.43296 | Transferrin 1 |
| Lcp3 | Up | 0.013845 | 29 | Inf | −2.26303 | Larval cuticle protein 3 |
| CG6180 | Up | 0.102422 | 10 | Inf | 0.321928 | putative phosp.ethanolamine bdg. prot. |
| sPLA2 | Up | 0.102422 | 10 | Inf | −2 | secretory Phospholipase A2 |
| CG13227 | Up | 0.102422 | 10 | Inf | 1.888969 | |
| CG30457 | Up | 0.102422 | 10 | Inf | 3.836501 | |
| Gs2 | Up | 0.102422 | 10 | Inf | −0.48543 | Glutamine synthetase 2 |
| CG6206 | Up | 0.141877 | 8 | Inf | −0.54597 | Lysosomal α-mannosidase |
| CG6673 | Up | 0.141877 | 8 | Inf | 0.915936 | Glutathione S transferase O2 |
| Spn55B | Up | 0.141877 | 8 | Inf | −0.28911 | Serpin |
| CG15043 | Up | 0.141877 | 8 | Inf | 0.168123 | |
| Vago | Up | 0.169725 | 7 | Inf | −1.66448 | single VWC domain protein |
| Sema-1b | Up | 0.169725 | 7 | Inf | 0.304006 | Semaphorin-1b |
| CG17278 | Up | 0.169725 | 7 | Inf | 0.514573 | |
| Sap-r | Up | 0.169725 | 7 | Inf | −1.65992 | Saposin-related |
| Sp7 | Up | 0.016934 | 42 | 5.24 | −0.1375 | Serine protease 7, melanization |
| CG6045 | Up | 0.043504 | 28 | 4.64 | −0.18903 | |
| CG10031 | Up | 0.05937 | 24 | 4.40 | 0.321928 | |
| CG15117 | Up | 0.09185 | 19 | 4.05 | 1.434403 | putative glucuronidase |
| Cpr51A | Up | 0.175735 | 13 | 3.47 | 3.321928 | Cuticular protein 51A |
| GNBP3 | Up | 0.12615 | 41 | 2.73 | −1.63691 | Gram-negative bacteria bdg. prot. 3 |
| Obp56d | Up | 0.076917 | 134 | 2.66 | −0.22651 | Odorant-binding protein 56d |
| Spn77Ba | Up | 0.100789 | 193 | 2.38 | −0.31034 | Serpin 77Ba |
| PO45 | Up | 0.1351 | 1537 | 2.34 | −2.09085 | prophenoloxidase 45 |
Change in transcript levels during development in rich medium was estimated based on expression profiling data from [77]. For transcript levels around the time when starvation was started (early) the values observed at L2 and L3/12hours were averaged. For transcript levels around the time of hemolymph collection (late) the values at L3/puff stage 1–2 were used. The given values correspond to log2(early/late).
The lowest p value (p<4.55E-05) resulted in case of Lsp1α where almost 3000 spectra were detected in the hemolymph from fed and only 9 spectra in the sample from starved larvae (Table S3). Strong differences were also observed in case of the closely related Lsp1β, Lsp1γ, and Lsp2. This count data concurs with our independent evidence from the analysis of hemolymph samples by SDS-PAGE and Coomassie Blue staining (Fig. 2). It also agrees entirely with predictions based on the demonstrated developmental delay induced by our starvation protocol (Fig. 1A) and the known developmental regulation of these major Lsps during development in rich medium [44], [45]. Because at the time of hemolymph collection, the starved larvae in contrast to the fed cohort had not yet reached the stage where Lsp expression is maximal, the level of major Lsps is expected to be reduced in the starved larvae. We conclude that in case of the Lsps, spectral counting with our data provided reliable information on abundance.
The Drosophila Lsps were originally identified because of their high abundance in hemolymph of third instar wandering stage larvae [73], [74], [75]. They have been shown to serve as storage proteins that are metabolized during the nonfeeding larval wandering and pupal stages (for reviews see [75], [76], [77]. Homologs are found throughout insects and are generally designated as hexamerins since they form homo- and heteromeric hexamers. In preparation for the nonfeeding stages, expression of the Drosophila Lsps is strongly induced in the fat body of mid third instar larvae by the raising ecdysone titer. Beyond the traditional focus of attention on this impressive peak of Lsp expression before the nonfeeding late larval and pupal stages recent evidence has suggested that Lsps are also expressed during other stages although at considerably lower levels. Several microarray experiments have clearly demonstrated the presence of in particular Lsp1β and Lsp2 transcripts in adult flies. Moreover, these two genes were among those most strongly and consistently downregulated after 24 hour starvation of adult flies [78], [79]. We propose therefore that these Lsp genes are used for nutrient storage in anticipation of upcoming starvation, not just before the nonfeeding developmental stages where they are regulated by ecdysone, but also during adult life where they appear to be controlled by nutrient availability during cycles of feast or famine. The strongly decreased Lsp abundance in hemolymph of starved larvae might therefore not just reflect an indirect effect of starvation on development but also a more direct non-developmental regulation by nutrients.
Five gene models (Yp3, Fbp1, Fbp2, CG7320, Obp99b) were identified with characteristic similarities to the major Lsp genes (Lsp1α, β, γ and Lsp2) (Table 2). The products of these gene models were also absent or dramatically lower in hemolymph of starved larvae. Moreover, according to expression profiling during development in rich medium [80], their transcript levels are strongly upregulated in late third instar larvae coincident with upregulation of Lsp gene expression. Therefore, the strongly reduced hemolymph concentration of the corresponding proteins presumably reflects at least in part the inhibitory effect of starvation on development. However, we point out that transcripts of Obp99b were also found to be downregulated strongly and consistently in response to starvation in adults [78], [79], as in case of Lsp1β and Lsp2. We suggest that the product of Obp99b, which is characterized by a developmental transcript profile quite distinct from other related odorant binding proteins [80], might function as a storage protein. In support of this proposal, most of the other gene models (CG7320, Fbp1, Fbp2, and Yp3), which with regard to developmental expression profile in rich medium and dependence of protein abundance in hemolymph on larval feeding behave like Obp99b and Lsp genes, have close functional connections to storage proteins. CG7320 encodes an uncharacterized minor hexamerin-related protein. Fat body protein 1 (Fbp1) serves as a receptor for hexamerin re-import into the fat body for production of protein storage granules [81]. Yp3 is a yolk protein known to be used for storage in preparation before the nonfeeding stage of embryogenesis [82].
The other half of the gene models coding for protein products that were absent or decreased in hemolymph of starved larvae (Table 2) did not belong to the group with an Lsp-like strong transcriptional upregulation during the third larval instar. Their reduced abundance in starved hemolymph is therefore not a reflection of the inhibitory effect of starvation on development. Three of these gene models (Npc2h, Tsf1, Pxn) were previously found to be downregulated by starvation in adults according to transcriptomic analyses [78].
Apart from the 17 gene models characterized by reduced abundance of protein products in hemolymph of starved larvae, we detected 23 gene models with an opposite behavior (Table 2 and S2) using the stringent criteria described above. Judging from their developmental transcript profiles [80], increased product abundance in hemolymph from starved larvae in these cases is unlikely to be a secondary consequence of the inhibitory effect of starvation on development, with two possible exceptions, Cpr51A and CG30457. Moreover, in contrast to the proteins decreased in starved hemolymph, where a clear correlation was apparent with transcriptomics data from starvation experiments with adults [78], [79], this was not the case with proteins enriched in starved hemolymph. While starvation in adults was found to be accompanied by transcriptional downregulation of defense and immune response genes [78], our proteomics data from larval hemolymph did not reveal this same response. Several of the proteins enriched in starved hemolymph have actually been implicated in defense and immunity (Sp7, PO45, GNBP3, Spn55B, Spn77Ba). Apart from Spn55B and 77Ba, all other detected serpins (Spn4, Spn5, Spn27a, Spn88Eb, Spn43Ab, Spn28D, Spn1, Spn42E) except Spn1 appeared to be enriched in hemolymph from starved larvae as well, although with weaker statistical support. Serpins superfamily proteins are involved in the regulation of many different rapid physiological responses often by functioning as protease inhibitors [83]. However, given the small number of cases with robust statistical support, general conclusions concerning the effect of starvation onto defense and immune or any other process in larvae are impossible. Gene ontology analyses also failed to reveal statistically significant differences between the hemolymph proteomes of starved and fed larvae.
We would like to point out that our data should also be of considerable interest for further improvement of the Drosophila genome annotation, which so far is largely based on transcript analyses and a bias for long open reading frames.
Conclusions
For high and middle abundance proteins, our study provides the first comprehensive picture of the composition of the hemolymph proteome in the Drosophila larva. Our data propel the known compositional complexity of Drosophila hemolymph more closely towards the state of the extensively characterized human plasma proteome. Our hemolymph proteome will support future data mining. The peptide catalogue (Table S1) can instruct future quantitative comparisons of the levels of hemolymph proteins in different developmental stages and physiological conditions using targeted proteomics approaches. Our initial comparison of hemolymph from fed and starved larvae by spectral counting indicated that the level of at least some proteins in Drosophila larval hemolymph is influenced dramatically by the nutritional status. Known storage proteins were far more abundant in hemolymph from fed compared to starved larvae. As the inhibitory effect of starvation on larval development appears to augment the primary response to nutritional status, our comparison points effectively to novel candidate storage proteins.
Supporting Information
List of all identified peptides including characteristic properties and classification [38] .
(XLS)
Complete data set (including identification of predicted small secreted globular proteins and comparison with previously published proteomic analyses of larval hemolymph).
(XLS)
A listing of all identified Drosophila protein-groups (with their evidence class), ranked by differential expression (p-value calculated by DESeq). Gene symbols and FlyBase IDs (“FBgn#”) are based on the mapping table from flybase.org. The spectral counts are shown for both conditions separately and for the combined total, followed by the DESeq normalized spectral counts that were used for the MA-plot (Figure 4). The log2-fold change is calculated based on the normalized spectral counts and the significance of differential expression is indicated by the DESeq p-value.
(XLS)
Acknowledgments
We thank János Szabad for help with hemolymph preparation.
Funding Statement
This work was supported by grants from SystemsX.ch WingX), the University Research Priority Program Systems Biology/Functional Genomics of the University of Zurich, and the Swiss National Science Foundation. CHA and EB are members of the Quantitative Model Organism Proteomics Initiative, which is funded in part by the University Research Priority Program Systems Biology/Functional Genomics of the University of Zurich. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Krzemien J, Crozatier M, Vincent A (2010) Ontogeny of the Drosophila larval hematopoietic organ, hemocyte homeostasis and the dedicated cellular immune response to parasitism. Int J Dev Biol 54: 1117–1125. [DOI] [PubMed] [Google Scholar]
- 2. Dushay MS (2009) Insect hemolymph clotting. Cell Mol Life Sci 66: 2643–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lemaitre B, Hoffmann J (2007) The host defense of Drosophila melanogaster. Annu Rev Immunol 25: 697–743. [DOI] [PubMed] [Google Scholar]
- 4. Gade G (2004) Regulation of intermediary metabolism and water balance of insects by neuropeptides. Annu Rev Entomol 49: 93–113. [DOI] [PubMed] [Google Scholar]
- 5. Leopold P, Perrimon N (2007) Drosophila and the genetics of the internal milieu. Nature 450: 186–188. [DOI] [PubMed] [Google Scholar]
- 6. Mirth CK, Riddiford LM (2007) Size assessment and growth control: how adult size is determined in insects. Bioessays 29: 344–355. [DOI] [PubMed] [Google Scholar]
- 7. Hietakangas V, Cohen SM (2009) Regulation of tissue growth through nutrient sensing. Annu Rev Genet 43: 389–410. [DOI] [PubMed] [Google Scholar]
- 8. Toivonen JM, Walker GA, Martinez-Diaz P, Bjedov I, Driege Y, et al. (2007) No influence of Indy on lifespan in Drosophila after correction for genetic and cytoplasmic background effects. PLoS Genet 3: e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wyatt GR (1961) The biochemistry of insect hemolymph. Annual Review of Entomology 6: 75–102. [Google Scholar]
- 10. Cardoen D, Ernst UR, Van Vaerenbergh M, Boerjan B, de Graaf DC, et al. (2011) Differential proteomics in dequeened honeybee colonies reveals lower viral load in hemolymph of fertile worker bees. PLoS One 6: e20043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Morais Guedes S, Vitorino R, Domingues R, Tomer K, Correia AJ, et al. (2005) Proteomics of immune-challenged Drosophila melanogaster larvae hemolymph. Biochem Biophys Res Commun 328: 106–115. [DOI] [PubMed] [Google Scholar]
- 12. Guedes Sde M, Vitorino R, Tomer K, Domingues MR, Correia AJ, et al. (2003) Drosophila melanogaster larval hemolymph protein mapping. Biochem Biophys Res Commun 312: 545–554. [DOI] [PubMed] [Google Scholar]
- 13. Han YS, Chun J, Schwartz A, Nelson S, Paskewitz SM (1999) Induction of mosquito hemolymph proteins in response to immune challenge and wounding. Dev Comp Immunol 23: 553–562. [DOI] [PubMed] [Google Scholar]
- 14. Karlsson C, Korayem AM, Scherfer C, Loseva O, Dushay MS, et al. (2004) Proteomic analysis of the Drosophila larval hemolymph clot. J Biol Chem 279: 52033–52041. [DOI] [PubMed] [Google Scholar]
- 15. Paskewitz SM, Shi L (2005) The hemolymph proteome of Anopheles gambiae. Insect Biochem Mol Biol 35: 815–824. [DOI] [PubMed] [Google Scholar]
- 16. Vierstraete E, Cerstiaens A, Baggerman G, Van den Bergh G, De Loof A, et al. (2003) Proteomics in Drosophila melanogaster: first 2D database of larval hemolymph proteins. Biochem Biophys Res Commun 304: 831–838. [DOI] [PubMed] [Google Scholar]
- 17. Vierstraete E, Verleyen P, Baggerman G, D’Hertog W, Van den Bergh G, et al. (2004) A proteomic approach for the analysis of instantly released wound and immune proteins in Drosophila melanogaster hemolymph. Proc Natl Acad Sci U S A 101: 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vierstraete E, Verleyen P, Sas F, Van den Bergh G, De Loof A, et al. (2004) The instantly released Drosophila immune proteome is infection-specific. Biochem Biophys Res Commun 317: 1052–1060. [DOI] [PubMed] [Google Scholar]
- 19. Hou Y, Zou Y, Wang F, Gong J, Zhong X, et al. (2010) Comparative analysis of proteome maps of silkworm hemolymph during different developmental stages. Proteome Sci 8: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levy F, Rabel D, Charlet M, Bulet P, Hoffmann JA, et al. (2004) Peptidomic and proteomic analyses of the systemic immune response of Drosophila. Biochimie 86: 607–616. [DOI] [PubMed] [Google Scholar]
- 21. Lai R, Lomas LO, Jonczy J, Turner PC, Rees HH (2004) Two novel non-cationic defensin-like antimicrobial peptides from haemolymph of the female tick, Amblyomma hebraeum. Biochem J 379: 681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vierstraete E, Verleyen P, De Loof A, Schoofs L (2005) Differential proteomics for studying Drosophila immunity. Ann N Y Acad Sci 1040: 504–507. [DOI] [PubMed] [Google Scholar]
- 23. Zhao XF, He HJ, Dong DJ, Wang JX (2006) Identification of differentially expressed proteins during larval molting of Helicoverpa armigera. J Proteome Res 5: 164–169. [DOI] [PubMed] [Google Scholar]
- 24. Chan QW, Howes CG, Foster LJ (2006) Quantitative comparison of caste differences in honeybee hemolymph. Mol Cell Proteomics 5: 2252–2262. [DOI] [PubMed] [Google Scholar]
- 25. Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, et al. (2005) Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci U S A 102: 3105–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chown SL, Nicolson SW (2004) Insect physiological ecology: Oxford University Press.
- 27. Lee G, Park JH (2004) Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 167: 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Britton JS, Edgar BA (1998) Environmental control of the cell cycle in Drosophila: nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development 125: 2149–2158. [DOI] [PubMed] [Google Scholar]
- 29. Rajan A, Perrimon N (2012) Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell 151: 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ikeya T, Galic M, Belawat P, Nairz K, Hafen E (2002) Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol 12: 1293–1300. [DOI] [PubMed] [Google Scholar]
- 31. Geminard C, Rulifson EJ, Leopold P (2009) Remote control of insulin secretion by fat cells in Drosophila. Cell Metab 10: 199–207. [DOI] [PubMed] [Google Scholar]
- 32. Ahrens CH, Brunner E, Qeli E, Basler K, Aebersold R (2010) Generating and navigating proteome maps using mass spectrometry. Nat Rev Mol Cell Biol 11: 789–801. [DOI] [PubMed] [Google Scholar]
- 33.Ashburner M (1989) Drosophila: A Laboratory Handbook: Cold Spring Harbor Laboratory Press.
- 34. Heller M, Michel PE, Morier P, Crettaz D, Wenz C, et al. (2005) Two-stage Off-Gel isoelectric focusing: protein followed by peptide fractionation and application to proteome analysis of human plasma. Electrophoresis 26: 1174–1188. [DOI] [PubMed] [Google Scholar]
- 35. Yates JR III, Eng JK, McCormack AL, Schieltz D (1995) Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Anal Chem 67: 1426–1436. [DOI] [PubMed] [Google Scholar]
- 36. Keller A, Nesvizhskii AI, Kolker E, Aebersold R (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74: 5383–5392. [DOI] [PubMed] [Google Scholar]
- 37. Elias JE, Gygi SP (2007) Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods 4: 207–214. [DOI] [PubMed] [Google Scholar]
- 38. Qeli E, Ahrens CH (2010) PeptideClassifier for protein inference and targeted quantitative proteomics. Nat Biotechnol 28: 647–650. [DOI] [PubMed] [Google Scholar]
- 39. Linding R, Russell RB, Neduva V, Gibson TJ (2003) GlobPlot: Exploring protein sequences for globularity and disorder. Nucleic Acids Res 31: 3701–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8: 785–786. [DOI] [PubMed] [Google Scholar]
- 41. Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mirth CK, Shingleton AW (2012) Integrating body and organ size in Drosophila: recent advances and outstanding problems. Front Endocrinol (Lausanne) 3: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rüegg KM (1968) Untersuchungen zum Proteinstoffwechsel des Wildtyps und der Lethalmutante (ltr) von Drosophila melanogaster. Z vergl Physiol 60: 275–307. [Google Scholar]
- 44. Powell D, Sato JD, Brock HW, Roberts DB (1984) Regulation of synthesis of the larval serum proteins of Drosophila melanogaster. Dev Biol 102: 206–215. [DOI] [PubMed] [Google Scholar]
- 45. Roberts DB, Jowett T, Hughes J, Smith DF, Glover DM (1991) The major serum-protein of Drosophila larvae, larval serum protein-1, is dispensable. European Journal of Biochemistry 195: 195–201. [DOI] [PubMed] [Google Scholar]
- 46. Grobei MA, Qeli E, Brunner E, Rehrauer H, Zhang R, et al. (2009) Deterministic protein inference for shotgun proteomics data provides new insights into Arabidopsis pollen development and function. Genome Res 19: 1786–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Verleyen P, Baggerman G, D’Hertog W, Vierstraete E, Husson SJ, et al. (2006) Identification of new immune induced molecules in the haemolymph of Drosophila melanogaster by 2D-nanoLC MS/MS. J Insect Physiol 52: 379–388. [DOI] [PubMed] [Google Scholar]
- 48. Carvalho PC, Hewel J, Barbosa VC, Yates JR III (2008) Identifying differences in protein expression levels by spectral counting and feature selection. Genet Mol Res 7: 342–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bogaerts A, Baggerman G, Vierstraete E, Schoofs L, Verleyen P (2009) The hemolymph proteome of the honeybee: gel-based or gel-free? Proteomics 9: 3201–3208. [DOI] [PubMed] [Google Scholar]
- 50. Zhou ZH, Yang HJ, Chen M, Lou CF, Zhang YZ, et al. (2008) Comparative proteomic analysis between the domesticated silkworm (Bombyx mori) reared on fresh mulberry leaves and on artificial diet. J Proteome Res 7: 5103–5111. [DOI] [PubMed] [Google Scholar]
- 51. Li XH, Wu XF, Yue WF, Liu JM, Li GL, et al. (2006) Proteomic analysis of the silkworm (Bombyx mori L.) hemolymph during developmental stage. J Proteome Res 5: 2809–2814. [DOI] [PubMed] [Google Scholar]
- 52. Furusawa T, Rakwal R, Nam HW, Hirano M, Shibato J, et al. (2008) Systematic investigation of the hemolymph proteome of Manduca sexta at the fifth instar larvae stage using one- and two-dimensional proteomics platforms. J Proteome Res 7: 938–959. [DOI] [PubMed] [Google Scholar]
- 53. Perez-Hedo M, Sanchez-Lopez I, Eizaguirre M (2012) Comparative analysis of hemolymph proteome maps in diapausing and non-diapausing larvae of sesamia nonagrioides. Proteome Sci 10: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kaeslin M, Pfister-Wilhelm R, Molina D, Lanzrein B (2005) Changes in the haemolymph proteome of Spodoptera littoralis induced by the parasitoid Chelonus inanitus or its polydnavirus and physiological implications. J Insect Physiol 51: 975–988. [DOI] [PubMed] [Google Scholar]
- 55.Farrah T, Deutsch EW, Omenn GS, Campbell DS, Sun Z, et al. (2011) A high-confidence human plasma proteome reference set with estimated concentrations in PeptideAtlas. Mol Cell Proteomics 10: M110 006353. [DOI] [PMC free article] [PubMed]
- 56. Kutty RK, Kutty G, Kambadur R, Duncan T, Koonin EV, et al. (1996) Molecular characterization and developmental expression of a retinoid- and fatty acid-binding glycoprotein from Drosophila. A putative lipophorin. J Biol Chem 271: 20641–20649. [DOI] [PubMed] [Google Scholar]
- 57. Palm W, Sampaio JL, Brankatschk M, Carvalho M, Mahmoud A, et al. (2012) Lipoproteins in Drosophila melanogaster–assembly, function, and influence on tissue lipid composition. PLoS Genet 8: e1002828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Anderson NL, Anderson NG (2002) The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics 1: 845–867. [DOI] [PubMed] [Google Scholar]
- 59. Stahl-Zeng J, Lange V, Ossola R, Eckhardt K, Krek W, et al. (2007) High sensitivity detection of plasma proteins by multiple reaction monitoring of N-glycosites. Mol Cell Proteomics 6: 1809–1817. [DOI] [PubMed] [Google Scholar]
- 60. Razavi M, Frick LE, LaMarr WA, Pope ME, Miller CA, et al. (2012) High-throughput SISCAPA quantitation of peptides from human plasma digests by ultrafast, liquid chromatography-free mass spectrometry. J Proteome Res 11: 5642–5649. [DOI] [PubMed] [Google Scholar]
- 61. Liu Y, Hüttenhain R, Surinova S, Gillet LC, Mouritsen J, et al. (2013) Quantitative Measurements of N-linked Glycoproteins in Human Plasma by SWATH-MS. Proteomics 13: 1247–1256. [DOI] [PubMed] [Google Scholar]
- 62.Bryant PJ (2001) Growth factors controlling imaginal disc growth in Drosophila. Novartis Found Symp 237: 182–194; discussion 194–202. [PubMed]
- 63. Kawamura K, Shibata T, Saget O, Peel D, Bryant PJ (1999) A new family of growth factors produced by the fat body and active on Drosophila imaginal disc cells. Development 126: 211–219. [DOI] [PubMed] [Google Scholar]
- 64. Dolezal T, Dolezelova E, Zurovec M, Bryant PJ (2005) A role for adenosine deaminase in Drosophila larval development. PLoS Biol 3: e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Novakova M, Dolezal T (2011) Expression of Drosophila adenosine deaminase in immune cells during inflammatory response. PLoS One 6: e17741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mondal BC, Mukherjee T, Mandal L, Evans CJ, Sinenko SA, et al. (2012) Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell 147: 1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen J, Honeyager SM, Schleede J, Avanesov A, Laughon A, et al. (2012) Crossveinless d is a vitellogenin-like lipoprotein that binds BMPs and HSPGs, and is required for normal BMP signaling in the Drosophila wing. Development 139: 2170–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sheldon TJ, Miguel-Aliaga I, Gould AP, Taylor WR, Conklin D (2007) A novel family of single VWC-domain proteins in invertebrates. FEBS Lett 581: 5268–5274. [DOI] [PubMed] [Google Scholar]
- 69. Galko MJ, Krasnow MA (2004) Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol 2: E239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Babcock DT, Brock AR, Fish GS, Wang Y, Perrin L, et al. (2008) Circulating blood cells function as a surveillance system for damaged tissue in Drosophila larvae. Proc Natl Acad Sci U S A 105: 10017–10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bidla G, Dushay MS, Theopold U (2007) Crystal cell rupture after injury in Drosophila requires the JNK pathway, small GTPases and the TNF homolog Eiger. J Cell Sci 120: 1209–1215. [DOI] [PubMed] [Google Scholar]
- 72. Wang M, Weiss M, Simonovic M, Haertinger G, Schrimpf SP, et al. (2012) PaxDb, a database of protein abundance averages across all three domains of life. Mol Cell Proteomics 11: 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Roberts DB, Wolfe J, Akam ME (1977) The developmental profiles of two major haemolymph proteins from Drosophila melanogaster. J Insect Physiol 23: 871–878. [DOI] [PubMed] [Google Scholar]
- 74. Wyatt GR, Pan ML (1978) Insect plasma proteins. Annu Rev Biochem 47: 779–817. [DOI] [PubMed] [Google Scholar]
- 75. Burmester T, Scheller K (1999) Ligands and receptors: common theme in insect storage protein transport. Naturwissenschaften 86: 468–474. [DOI] [PubMed] [Google Scholar]
- 76. Telfer WH, Kunkel JG (1991) The function and evolution of insect storage hexamers. Annu Rev Entomol 36: 205–228. [DOI] [PubMed] [Google Scholar]
- 77. Burmester T (2002) Origin and evolution of arthropod hemocyanins and related proteins. J Comp Physiol B 172: 95–107. [DOI] [PubMed] [Google Scholar]
- 78. Farhadian SF, Suarez-Farinas M, Cho CE, Pellegrino M, Vosshall LB (2012) Post-fasting olfactory, transcriptional, and feeding responses in Drosophila. Physiol Behav 105: 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fujikawa K, Takahashi A, Nishimura A, Itoh M, Takano-Shimizu T, et al. (2009) Characteristics of genes up-regulated and down-regulated after 24 h starvation in the head of Drosophila. Gene 446: 11–17. [DOI] [PubMed] [Google Scholar]
- 80. Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, et al. (2011) The developmental transcriptome of Drosophila melanogaster. Nature 471: 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Burmester T, Antoniewski C, Lepesant JA (1999) Ecdysone-regulation of synthesis and processing of fat body protein 1, the larval serum protein receptor of Drosophila melanogaster. Eur J Biochem 262: 49–55. [DOI] [PubMed] [Google Scholar]
- 82. Bownes M (1992) Why is there sequence similarity between insect yolk proteins and vertebrate lipases? J Lipid Res 33: 777–790. [PubMed] [Google Scholar]
- 83. Reichhart JM, Gubb D, Leclerc V (2011) The Drosophila serpins: multiple functions in immunity and morphogenesis. Methods Enzymol 499: 205–225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of all identified peptides including characteristic properties and classification [38] .
(XLS)
Complete data set (including identification of predicted small secreted globular proteins and comparison with previously published proteomic analyses of larval hemolymph).
(XLS)
A listing of all identified Drosophila protein-groups (with their evidence class), ranked by differential expression (p-value calculated by DESeq). Gene symbols and FlyBase IDs (“FBgn#”) are based on the mapping table from flybase.org. The spectral counts are shown for both conditions separately and for the combined total, followed by the DESeq normalized spectral counts that were used for the MA-plot (Figure 4). The log2-fold change is calculated based on the normalized spectral counts and the significance of differential expression is indicated by the DESeq p-value.
(XLS)