Abstract
Objective
To evaluate changes in visual acuity (VA) over time in patients with Leber congenital amaurosis (LCA) and mutations in the CEP290 gene.
Patients
Forty-three patients with LCA and CEP290 mutations participated.
Methods
VA was determined at the initial and most recent visits.
Main Outcome Measures
The best-corrected VA at the initial and most recent visits, and the correlation between age and VA.
Results
At the initial visit, 14 patients had measurable chart VA in the better-seeing eye, 25 patients had non-measurable chart VA, and VA was not assessed in 4 young patients. At the most recent visit, 15 patients had measurable chart VA and 28 had non-measurable chart VA. The average duration between the two visits was 10.4 years (range: 2 to 47 years). For patients with measurable chart VA, the median logMAR value at the initial visit, 0.75 (range: 0.10 to 2.30), and most recent visit, 0.70 (range: 0.10 to 2.00), did not differ significantly (p > 0.05). There was no significant relationship between VA and age.
Conclusions
Patients with LCA and CEP290 mutations had a wide spectrum of VA that was not related to age or length of follow-up. Severe VA loss was observed in most, but not all, patients in the first decade. These data will help clinicians provide counseling on VA changes in patients with CEP290 mutations and could be of value for future treatment trials.
Introduction
Leber congenital amaurosis (LCA, MIM# 204000) is a group of hereditary infantile and childhood retinal dystrophies characterized by severe loss of visual function early in life.1 LCA is usually inherited as an autosomal recessive trait, although rare cases of dominant inheritance have been reported.2,3 The electroretinogram (ERG) can confirm the diagnosis, showing severely reduced or absent scotopic and photopic responses in these patients,.
To date, 18 LCA genes have been reported.4,5 In patients of European descent, the most commonly mutated genes are CEP290 (15% of cases) and GUCY2D (12% of cases).4 CEP290 localizes to the centromeres of dividing cells and the connecting cilia of the photoreceptors.6 Mutations in the CEP290 gene (MIM# 610142) were recently shown to be associated with the Joubert, Senior-Loken, Meckel-Gruber, and Bardet-Biedl syndromes as well as non-syndromic LCA.8–11 Mutations in this gene lead to rapid reduction in photoreceptor outer segment length and outer nuclear layer thickness. Nevertheless, a recent study has shown that LCA CEP290 patients retain substantial central cone photoreceptors and that they may be appropriate candidates for cone-directed gene therapy.12
To the best of our knowledge, there are no previous reports of VA changes over time in LCA CEP290 patients. Thus, the aim of the current multicentered retrospective study was to quantify VA changes with time in a large cohort of patients with LCA and disease-causing mutations in the CEP290 gene.
Patients and Methods
Patients
The diagnosis of LCA was based on a history of early and severe visual impairment, nystagmus (in most cases), fundus examination, and ERG recordings. For the purpose of this study, we defined LCA as a disease with onset of visual disturbance, as noted by the parents, before the age of 1 year. Patients with mutations in genes other than CEP290 and patients with non-ocular features that suggested a syndromic disorder were excluded, whereas patients with keratoconus or cataracts were included.
Because of the retrospective nature of the study, VA was recorded using different VA charts depending on the age and extent of visual impairment in individual patients. The VA charts that were used included Snellen, Early Treatment of Diabetic Retinopathy Study, Feinbloom, HOTV, and the Teller Acuity Cards. The best-corrected visual acuity (BCVA) in the better-seeing eye at the initial visit was determined and this eye was also used for analysis of VA at the follow-up visit. Some patients with hand motion or worse did not undergo refraction. All VA data were converted to the logarithm of the minimum angle of resolution (logMAR) for analysis. Patients who could only count fingers (CF), perceive hand motion (HM), had only light perception (LP), or had no light perception (NLP) were assigned the values of 2.6, 2.7, 2.8, and 2.9, respectively.13 However, these data were analyzed separately, since assigning numerical values to patients with very poor VA constitutes an ordinal scale of measurement and is somewhat arbitrary.
Data Collection
Databases from the following 7 centers were searched for patients with LCA and CEP290 mutations: the University of Illinois at Chicago and the Chicago Lighthouse for People Who Are Blind or Visually Impaired, University of Iowa, McGill University Health Center, the Cole Eye Institute of the Cleveland Clinic, Oregon Health & Science University, and University of Pennsylvania. To prevent possible duplication of patient entry into the study, patients’ dates of birth were compared across the datasets. Data identified from the records included: gender, age at appearance of first visual symptom, age at the time of visit, presence or absence of a syndromic form of LCA, and mutation(s) in CEP290. In addition, VA, chart used to measure VA, refractive error, anterior segment findings, corneal abnormalities, lens changes, macular changes, and any systemic features were recorded.
Informed consent was obtained from all participating patients or their legal guardians at the centers where they were examined. Approval was obtained from the institutional review boards at the participating centers, and the study was conducted in accordance with the Health Insurance Portability and Accountability Act and in accordance with the tenets of the Declaration of Helsinki.
Results
Table 1 provides the subject’s age at the initial visit, VA at the initial and most recent visits for each eye, the change in VA for each eye, and the duration of follow-up between the two visits for each of the 43 participants. Of the 43 patients, there were 19 females and 24 males. The mean age at the initial visit was 12.7 years with a standard deviation (SD) of 17.1 years (range: 2 months to 57 years). The mean age at the most recent follow-up visit was 23.1 years with a SD of 17.2 years (range: 2 to 61 years). The average duration between the initial and most recent visits was 10.4 years (SD = 10.7 years), with a range of 2 years to 47 years.
Table 1.
Visual acuity and follow-up data in the study cohort
| First Visit | Final Visit | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Px No | Age at initial visit (years) | VA OS | VA OD | VA OS | VA OD | VA Change OS | VA Change OD | Follow-up duration (years) |
| 1 | 0.2 | 2.90 (NLP) | 2.90 (NLP) | 2.6 (CF) | 2.6 (CF) | - | - | 2.2 |
| 2 | 0.3 | 2.90 (NLP) | 2.90 (NLP) | 2.90 (NLP) | 2.90 (NLP) | - | - | 7.8 |
| 3 | 0.3 | - | - | 2.90 (NLP) | 2.90 (NLP) | - | - | 28.7 |
| 4 | 0.3 | 2.60 (CF) | 2.60 (CF) | 2.80 (LP) | 2.80 (LP) | - | - | 18.7 |
| 5 | 0.3 | 2.90 (NLP) | 2.90 (NLP) | 2.80 (LP) | 2.80 (LP) | - | - | 6.7 |
| 6 | 0.3 | 2.90 (NLP) | 2.90 (NLP) | 2.90 (NLP) | 2.90 (NLP) | - | - | 11.7 |
| 7 | 0.4 | 2.90 (NLP) | 2.90 (NLP) | 2.80 (LP) | 2.80 (LP) | - | - | 46.6 |
| 8 | 0.4 | 2.90 (NLP) | 2.90 (NLP) | 2.80 (LP) | 2.80 (LP) | - | - | 4.6 |
| 9 | 0.7 | - | - | 0.60 | 1.90 | - | - | 17.3 |
| 10 | 0.7 | - | - | 2.80 (LP) | 2.80 (LP) | - | - | 32.3 |
| 11 | 0.7 | 2.90 (NLP) | 2.90 (NLP) | 2.80 (LP) | 2.80 (LP) | - | - | 1.7 |
| 12 | 0.7 | 2.90 (NLP) | 2.90 (NLP) | 2.80 (LP) | 2.80 (LP) | - | - | 20.3 |
| 13 | 0.8 | 2.60 (CF) | 2.60 (CF) | 2.70 (HM) | 2.80 (LP) | - | - | 28.3 |
| 14 | 1 | 2.90 (NLP) | 2.90 (NLP) | 2.80 (LP) | 2.80 (LP) | - | - | 19.1 |
| 15 | 1 | 2.90 (NLP) | 2.90 (NLP) | 2.80 (LP) | 2.80 (LP) | - | - | 6.7 |
| 16 | 2 | - | - | 2.90 (NLP) | 2.80 (LP) | - | - | 8.0 |
| 17 | 2 | 2.90 (NLP) | 2.90 (NLP) | 2.90 (NLP) | 2.90 (NLP) | - | - | 2.1 |
| 18 | 3 | 0.48 | 0.48 | 0.30 | 0.40 | 0.18 | 0.08 | 13.0 |
| 19 | 3 | 2.90 (NLP) | 2.90 (NLP) | 2.80 (LP) | 2.80 (LP) | - | - | 4.0 |
| 20 | 3 | 1.20 | 1.26 | 2.80 (LP) | 2.80 (LP) | - | - | 31.0 |
| 21 | 3 | 1.53 | 1.53 | 2.00 | 1.53 | −0.47 | 0 | 1.3 |
| 22 | 4 | 2.70 (HM) | 2.70 (HM) | 0.88 | 0.70 | - | - | 4.0 |
| 23 | 8 | 2.80 (LP) | 2.80 (LP) | 2.70 (HM) | 2.70 (HM) | - | - | 4.0 |
| 24 | 8 | 2.80 (LP) | 2.80 (LP) | 2.80 (LP) | 2.80 (LP) | - | - | 7.0 |
| 25 | 8 | 2.80 (LP) | 2.80 (LP) | 2.80 (LP) | 2.80 (LP) | - | - | 7.0 |
| 26 | 11 | 2.80 (LP) | 2.80 (LP) | 2.80 (LP) | 2.80 (LP) | - | - | 2.0 |
| 27 | 11 | 2.30 | 2.30 | 1.78 | 1.60 | 0.52 | 0.70 | 6.0 |
| 28 | 11 | 2.70 (HM) | 2.70 (HM) | 2.70 (HM) | 2.70 (HM) | - | - | 0.2 |
| 29 | 12 | 0.30 | 0.40 | 0.18 | 0.48 | 0.12 | −0.08 | 2.0 |
| 30 | 13 | 1.30 | 1.30 | - | 1.08 | - | 0.22 | 31.0 |
| 31 | 14 | 0.40 | 0.40 | 0.50 | 0.40 | −0.10 | 0.00 | 5.0 |
| 32 | 14 | 0.70 | 2.60 (CF) | 0.70 | 2.60 (CF) | 0.00 | - | 3.4 |
| 33 | 15 | 0.10 | 0.10 | 0.10 | 0.10 | 0.00 | 0.00 | 1.0 |
| 34 | 19 | 2.80 (LP) | 2.80 (LP) | 2.80 (LP) | 2.80 (LP) | - | - | 9.0 |
| 35 | 20 | 2.90 (NLP) | 2.90 (NLP) | 2.90 (NLP) | 2.90 (NLP) | - | - | 9.0 |
| 36 | 22 | 0.18 | 0.18 | 0.18 | 0.18 | 0.00 | 0.00 | 6.4 |
| 37 | 25 | 1.82 | 1.82 | 2.00 | 2.05 | −0.18 | −0.23 | 12.0 |
| 38 | 40 | 2.80 (LP) | 2.80 (LP) | 2.80 (LP) | 2.80 (LP) | - | - | 5.0 |
| 39 | 49 | 0.60 | 1.30 | 0.88 | 0.70 | −0.27 | 0.60 | 5.0 |
| 40 | 50 | 0.88 | 0.88 | 0.88 | 0.88 | 0.00 | 0.00 | 5.0 |
| 41 | 54 | 2.80 (LP) | 2.80 (LP) | 2.80 (LP) | 2.80 (LP) | - | - | 6.0 |
| 42 | 55 | 2.80 (LP) | 2.80 (LP) | 2.80 (LP) | 2.80 (LP) | - | - | 5.0 |
| 43 | 57 | 1.00 | 0.80 | 0.60 | 1.18 | 0.40 | −0.38 | 4.0 |
Px= patient, OS= left eye; OD= right eye, CF=counting fingers, HM= hand motion, LP= light perception, NLP= no light perception.
A firm diagnosis, or a highly likely diagnosis, was determined prior to obtaining the results of genotyping. The most frequently observed mutation in our sample was c.2991+1655 A > G, with approximately 77% of our patients having this mutation on at least one allele. Approximately 21% of our patients were homozygous for this mutation.
Among the study cohort, 4 patients had keratoconus at the initial and most recent visits (patient numbers: 26, 35, 41, 42) and 2 additional patients developed keratoconus during the follow-up period (patient numbers: 7 and 28). Seven patients had a cataract at the initial and most recent visits (patient numbers: 14, 16, 35, 39–42), and one patient developed a cataract while being followed (patient number 30). When cataracts were present, they were most commonly of the posterior sub-capsular type.
At the initial visit, patients had various degrees of macular changes including 32 patients with a normal or blunted foveal reflex, 9 with retinal pigment epithelium (RPE) pigmentary changes, and 2 with atrophic-appearing macular lesions. At the most recent visit, 33 patients had a normal or a blunted foveal reflex, 2 had RPE pigmentary changes, 5 had a bull’s-eye macular lesion, and 3 had an atrophic-appearing macular lesion. Only one patient, number 9, showed cystoid macular edema (CME) on spectral-domain OCT; the CME was detected only at the most recent follow-up visit.
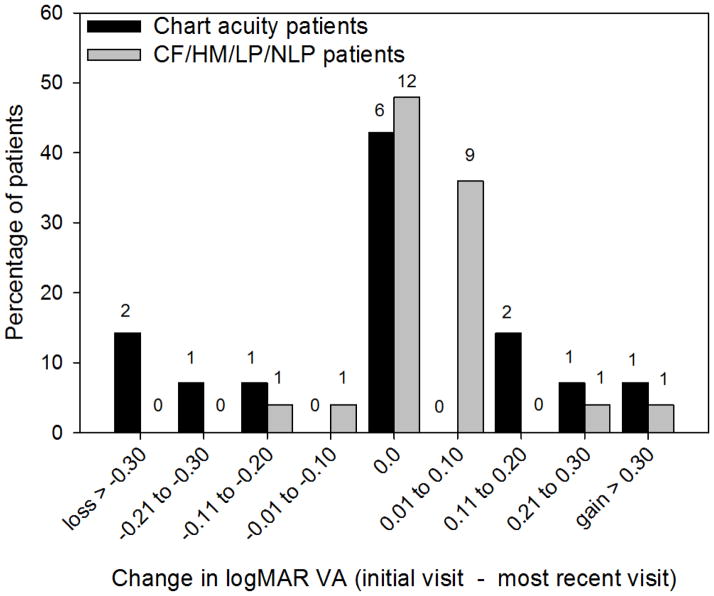
The distribution of VA among the patients is given in Figure 1. For patients with measurable chart VA, the median logMAR value at the initial visit in the better-seeing eye (black bars) was 0.75 (range: 0.10 to 2.30). While the median logMAR VA at the most recent visit (gray bars) was 0.70 (range: 0.10 to 2.00). There was no statistically significant difference in median VA between visits for the 13 patients who had measurable chart VA at both visits (Wilcoxon signed rank test, p = 0.94).
Figure 1.
Distribution of VA. Measurements made at the initial visit are represented by the dark bars and VA measurements at the most recent visit are represented by the light bars. The number above each bar represents the number of patients who had a given VA value.
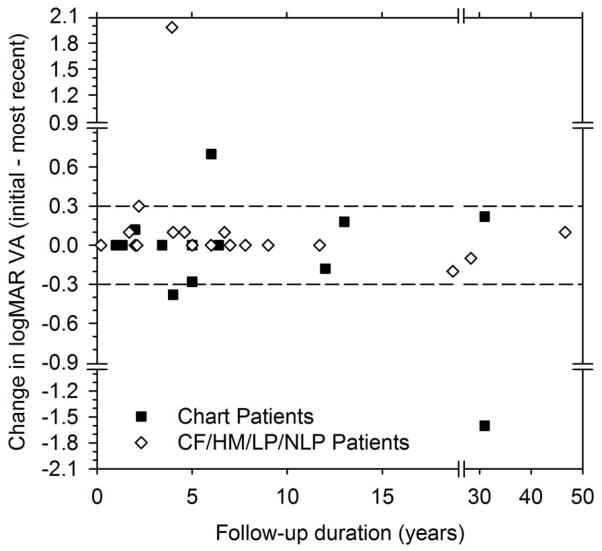
As illustrated in Figure 2, two patients with measurable chart acuity at the initial visit (black bars) had a loss in VA of more than a factor of two between the initial and most recent visits. This magnitude of VA change, a logMAR change of more than a factor of two, was used as a criterion to evaluate VA changes over the follow-up period, consistent with previous studies.14,15 One patient with measurable chart VA had an improvement of more than a factor of two. None of the patients with non-measurable chart acuity (gray bars) had more than a two level decrease in visual function (e.g. from CF to LP or HM to NLP) between the initial and most recent visits. Patient 1 (the youngest patient) improved from NLP to CF, but this change is likely attributable to the difficulty of assessing visual function in very young children. For all patients, the modal change in VA was 0 log units.
Figure 2.
Distribution of the change in VA from the initial to the most recent visit. Patients with measurable chart acuity are represented by the black bars and patients with non-measurable chart VA are represented by the gray bars. Negative values represent VA losses, whereas positive values represent gains in VA.
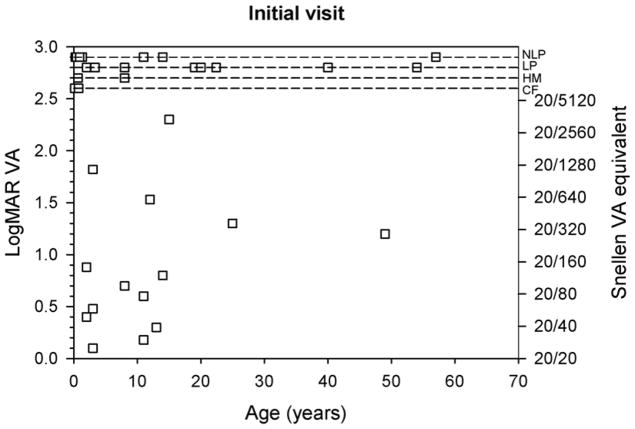
Figure 3 shows the change in logMAR VA as a function of the duration of follow-up. The dashed lines in Figure 3 define a factor of two change in VA and data points falling within the dashed lines had less than a factor of two change. Patients with measureable chart VA are represented by squares and patients for whom VA could not be measured with a chart are represented by diamonds. Nearly all of the patients had less than a factor of two change in VA. The two outliers shown in the figure are patients 20 and 22, who had changes of 1.2 logMAR to LP and HM to 0.7 logMAR, respectively. Additionally, changes in VA were not significantly related to the duration of follow-up (r = −0.32, p > 0.05).
Figure 3.
Change in VA as a function of follow-up duration. Data for patients with measurable chart acuity are represented by squares, whereas patients who had non-measurable chart VA are represented by diamonds. The dashed lines delineate a factor of two change in VA.
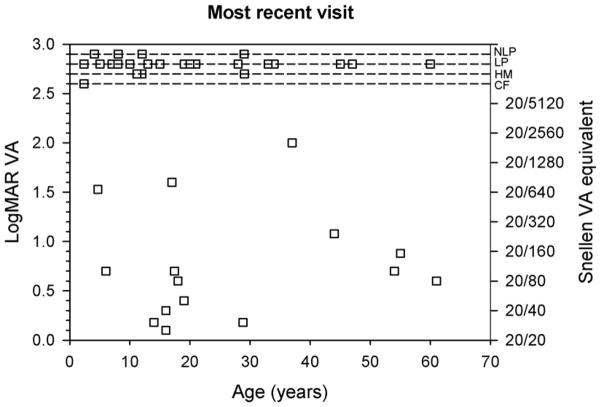
Figure 4 plots VA as a function of the patient’s age at the initial visit (top) and most recent visit (bottom). For clarity, the dashed lines at the top of each plot show the four levels of VA for patients who did not have measurable chart acuity. There was a wide range of VA values for all age ranges, with no overall tendency for VA to become better or worse with age. The correlation between logMAR VA and age was not significant at either the initial visit (r = 0.00, p > 0.05) or the most recent visit (r = −0.14, p > 0.05).
Figure 4.
LogMAR VA as a function age. VA measurements at the initial visit are shown in the top panel and data from the most recent visit are shown in the bottom panel. The Snellen equivalents of the logMAR values are shown on the right y-axes. The dashed lines, presented for clarity, indicate the four levels of non-measurable chart VA.
Comment
Severely reduced VA, beginning in the first decade of life, was observed in the majority of this cohort, which constitutes a sizeable proportion of LCA patients with CEP290 mutations from large retinal degeneration centers within the United States and Canada. At the time of presentation, 64% of the patients were classified as CF or worse in the better-seeing eye. Similar results were observed by Perrault et al,7 who examined 47 patients with CEP290 mutations and found that most patients had a severe and early reduction in VA. Den Hollander et al,6 however, found variable visual function in 4 affected siblings, ranging from LP to a VA of 20/80. Neither of these studies6,7 reported follow-up data, so an evaluation of changes in VA over time is not possible based on these studies.
The severity of the CEP290 phenotype resulted in a relatively small number of patients with measureable chart acuity, which may require some caution when generalizing to a larger population of these patients. Nevertheless, 10 of the 14 patients with measurable VA at the first visit had a change in VA measured at the most recent visit of less than a factor of two. Of the 13 patients with measurable chart acuity at both visits, the Snellen chart was used in 8 patients and the ETDRS chart was used in 2 patients. VA was measured with different charts at the initial and most recent visit in only one patient. Consequently, the use of different charts for VA measurement cannot explain the relatively constant VA over time.
Of the 25 patients with non-measurable chart acuity, only 2 lost additional visual function between the initial visit and follow-up, with patient 4 decreasing from CF to LP and patient 13 decreasing from CF to HM. Eleven patients with non-measurable chart acuity gained at least one level of visual function over the follow-up. However, these presumed changes in visual function in patients with non-measurable chart acuity are difficult to interpret.
In conclusion, the data obtained from this study showed a substantial spectrum of VA abnormalities among LCA CEP290 patients. A severe loss of VA was found in most, but not all, of the patients. Of note, there was no clinically significant additional loss of VA over the course of follow-up and we found no clinically significant relationship between age and VA, consistent with previous work.16, 17 It will be of interest to continue to follow these patients, particularly those with measurable chart VA, to verify that VA remains stable over time. The present data will aid clinicians in counseling CEP290 patients about their visual prognosis and the results may also be of value in patient selection for future treatment trials.
Acknowledgments
Supported by funds from the Grousbeck Family Foundation (EMS, RGW, SGJ, EIT, and GAF), Foundation Fighting Blindness (USA), Grant Healthcare Foundation, an unrestricted departmental grant from Research to Prevent Blindness (University of Illinois at Chicago) (GAF), NIH grant R00EY019510 (JJM), Foundation Fighting Blindness (Canada), and the Canadian Institutes for Health Research (RKK).
The authors would like to acknowledge Jeaneen L. Andorf, BA, and Tiffany Grider for their technical assistance.
Written permission has been obtained from all persons in the acknowledgment.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Cremers FP, van den hurk JA, den Hollander AI. Molecular genetics of Leber congenital amaurosis. Hum Mol Genet. 2002;11:1169–1176. doi: 10.1093/hmg/11.10.1169. [DOI] [PubMed] [Google Scholar]
- 2.Rivolta C, Berson EL, Dryja TP. Dominant Leber congenital amaurosis, cone-rod degeneration, and retinitis pigmentosa caused by mutant versions of the transcription factor CRX. Hum Mutat. 2001 Dec;18(6):488–498. doi: 10.1002/humu.1226. [DOI] [PubMed] [Google Scholar]
- 3.Sohocki MM, Sullivan LS, Mintz-Hittner HA, et al. A range of clinical phenotypes associated with mutations in CRX, a photoreceptor transcription-factor gene. Am J Hum Genet. 1998 Nov;63(5):1307–1315. doi: 10.1086/302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Koenekoop RK. Successful RPE65 gene replacement and improved visual function in humans. Ophthalmic Genet. 2008 Sep;29(3):89–91. doi: 10.1080/13816810802216480. [DOI] [PubMed] [Google Scholar]
- 6.den Hollander AI, Koenekoop RK, Yzer S, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006 Sep;79(3):556–561. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrault I, Delphin N, Hanein S, et al. Spectrum of NPHP6/CEP290 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Hum Mutat. 2007 Apr;28(4):416. doi: 10.1002/humu.9485. [DOI] [PubMed] [Google Scholar]
- 8.Cideciyan AV, Aleman TS, Jacobson SG, et al. Centrosomal-ciliary gene CEP290/NPHP6 mutations result in blindness with unexpected sparing of photoreceptors and visual brain: implications for therapy of Leber congenital amaurosis. Hum Mutat. 2007 Nov;28(11):1074–1083. doi: 10.1002/humu.20565. [DOI] [PubMed] [Google Scholar]
- 9.Papon JF, Perrault I, Coste A, et al. Abnormal respiratory cilia in non-syndromic Leber congenital amaurosis with CEP290 mutations. J Med Genet. 2010 Dec;47(12):829–834. doi: 10.1136/jmg.2010.077883. [DOI] [PubMed] [Google Scholar]
- 10.Sayer JA, Otto EA, O’Toole JF, et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006 Jun;38(6):674–681. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 11.Valente EM, Silhavy JL, Brancati F, et al. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet. 2006 Jun;38(6):623–625. doi: 10.1038/ng1805. [DOI] [PubMed] [Google Scholar]
- 12.Cideciyan AV, Rachel RA, Aleman TS, et al. Cone photoreceptors are the main targets for gene therapy of NPHP5 (IQCB1) or NPHP6 (CEP290) blindness: generation of an all-cone Nphp6 hypomorph mouse that mimics the human retinal ciliopathy. Hum Mol Genet. 2011 Apr 1;20(7):1411–1423. doi: 10.1093/hmg/ddr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grover S, Fishman GA, Anderson RJ, et al. Visual acuity impairment in patients with retinitis pigmentosa at age 45 years or older. Ophthalmology. 1999;106:1780–1785. doi: 10.1016/S0161-6420(99)90342-1. [DOI] [PubMed] [Google Scholar]
- 14.Berdeaux GH, Nordmann JP, Colin E, Arnould B. Vision-related quality of life in patients suffering from age-related macular degeneration. Am J Ophthalmol. 2005;139:271–279. doi: 10.1016/j.ajo.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Finger RP, Fleckenstein M, Holz FG, Scholl HP. Quality of life in age-related macular degeneration: a review of available vision specific psychometric tools. Qual Life Res. 2008;17:559–574. doi: 10.1007/s11136-008-9327-4. [DOI] [PubMed] [Google Scholar]
- 16.Heher KL, Traboulsi EI, Maumenee IH. The natural history of Leber’s congenital amaurosis: age-related findings in 35 patients. Ophthalmology. 1992;99:241–245. doi: 10.1016/s0161-6420(92)31985-2. [DOI] [PubMed] [Google Scholar]
- 17.Lambert SR, Kriss A, Taylor D, et al. Follow-up and diagnostic reappraisal of 75 patients with Leber’s congenital amaurosis. Am J Ophthalmol. 1989;107:624–631. doi: 10.1016/0002-9394(89)90259-6. [DOI] [PubMed] [Google Scholar]