Abstract
Background
Delineating the natural history of metabolic syndrome (MetS) is prerequisite to prevention. This study aimed to build Markov models to simulate each component’s progress and to test the effect of different initial states on the development of MetS.
Methods
MetS was defined with revised AHA/NHLBI criteria. Each reversible multistate Markov chain consisted of 8 states (no component, five isolated component states, 2-component state, and MetS state). Yearly transition probabilities were calculated from a five-year population-based follow up studywhich enrolled 2,247 individuals with mean aged 32.4 years at study entry.
Results
In men, high BP or a 2-component state was most likely to initiate the progress of MetS. In women, abdominal obesity or low HDL were the most likely initiators. Metabolic components were likely to occur together. The development of MetS was an increasing monotonic function of time. MetS was estimated to develop within 15 years in 12.7% of young men with no component, and 2 components developed in 16.3%. MetS was estimated to develop in 10.6% of women with at the age of 47, and 2 components developed in 14.3%. MetS was estimated to develop in 24.6% of men and 27.6% of women with abdominal obesity, a rate higher than in individuals initiating with no component.
Conclusions
This modeling study allows estimation of the natural history of MetS. Men tended to develop this syndrome sooner than women did, i.e., before their fifth decade of life. Individuals with 1 or 2 components showed increased development of MetS.
Introduction
Metabolic syndrome (MetS) is a precursor of cardiovascular disease and type 2 diabetes mellitus [1]. The burden of Mets and its component risk profile appears to be large and increases worldwide. Estimates suggest that MetS is present in at least a third of the population in most countries [2]. Several studies of incidence of MetS have reported. Those previous studies investigated the incidence of MetS and/or each component of MetS according to risk factors, such as older and less educated participants [3], smoking habit [4], alcohol consumption [5], high waist-to-hip ratio and fasting insulin levels [6]. Delineating the natural history of MetS is prerequisite to detecting high-risk groups and preventing its development. MetS is defined as the presence of three or more out of five components; therefore, there are 32 different states and1,024 transitions between states. It is difficult to establish which metabolic component leads to the cascade of disorders that characterize the syndrome. Haring et al. used the map generator to generate a network-based approach to model the progression of MetS components and their change [7]. However, many obstacles hinder the assessment of the natural history of MetS.The Markov model enables the collection, calculation, and summarization of data from groups with a wide variety of characteristics [8]. In the Markov model, the clinical states that individuals pass through are defined individually and are integrated in a system of transition probability from one state to another within a cycle. Markov models are well-recognized methods for simulating the natural history of chronic diseases [9]–[13]. Silverstein et al. applied a Markov model analysis of a population-based cohort to describe the clinical course of Crohn’s disease [13].
The Taiwanese Survey on the Prevalence of Hyperglycemia, Hyperlipidemia, and Hypertension (TwSHHH) was a valuable, large-sized, population-based study in Taiwan [14].The aim of this analysis of the application of Markov simulation was to describe the longitudinal course of the development of MetS and to determine gender differences in the natural history of MetS components and to test the effect of different initial states on the development of MetS.
Materials and Methods
The basic data for this study came from the TwSHHH, a nation-wide cross-sectional survey that was conducted in 2002. A total of 6600 individuals had completed questionnaire interviews, blood pressure (BP) and other biomarker measurements. The follow-up program, named TwSHHH-II, was conducted in 2007 to estimate the incidences of cardiometabolic diseases for people in Taiwan. There were 4,682 individuals including in the TwSHHH-II, resulting in a response rate of 70.9%. The mean follow-up was 5.4 years. Details of the TwSHHH-II cohort were described previously [15]. The protocols for the TwSHHH were approved by the Institutional Review Board at the Bureau of Health Promotion, Department of Health, Executive Yuan, in Taiwan. All individuals gave written informed consent for participation.
Because the rates of cardiometabolic risk factors were significantly different in individuals aged over 50 years [14], data were limited from individuals aged 18–45 years for taking focus on young adult group. Therefore, individuals who were younger than 18 years (n = 293) or older than 45 years (n = 1,972), had a history of stroke or coronary heart disease (n = 79), or did not provide a blood specimen (n = 91) were excluded from this analysis, leaving data from 2247 participants (1,032 men and 1,215 women).
Data Collection
Procedures and measurements were done during home visits. Demographic data were collected and the medical history was assessed. During the visit, sitting BP and anthropometric measurements were made. Waist circumference was measured with a inelastic tape measure placed parallel to the floor at the end of a relaxed expiration. Two BP measurements were taken using a random-zero mercury column sphygmomanometer in 2002 and an electric sphygmomanometer (BP3AC1-1, Microlife Cooperation, Berneck, Switzerland) in 2007. A third BP measurement was made if the first two BP readings differed by more than 10 mmHg. The average of the two closest readings was calculated and used in the analysis. Venous blood (20 ml) was taken for blood biochemistry and lipid profile. Biochemical variables, fasting total cholesterol (Lieberman-Burchard method), triglyceride (TG) (Bucolo method) and fasting plasma glucose (FPG) (glucose oxidase method) were measured by an automated system (Vitros 550/750, Johnson and Johnson, USA) in 2002. Total cholesterol (CV 1.03%), TG (CV 0.93%), and FPG (CV 1.32%) were measured with an automatic analyzer (TBA-200 FR, Toshiba Cooperation, Tokyo, Japan) in 2007. In addition, electrophoresis was performed to measure high density lipoprotein cholesterol (HDL, CV 2.52%) and low density lipoprotein cholesterol (CV 2.85%). Smoking status was categorized as current, past and never. Regular alcohol drinking was defined as consuming alcohol beverage at least once per week for at least 6 months.
Metabolic Syndrome Criteria
There are five components in MetS. The criteria for the MetS were fulfilled if 3 or more of the following components were present [16]: (i) high BP: BP of more than 130/85 mmHg or use of antihypertensive agents; (ii) high FPG: FPG of more than 100 mg/dl or use of antidiabetic agents; (iii) high TG: fasting TG levels of more than 150 mg/dl; (iv) low HDL: HDL level of less than 40 mg/dl in men and less than 50 mg/dl in women; (v) abdominal obesity: waist circumference greater than 90 cm in Asian men or greater than 80 cm in Asian women.
Markov Chain Models
Each Markov chain consisted of 8 states: no component state, isolated abdominal obesity state, isolated high TG state, isolated low HDL state, isolated high BP state, isolated high FPG state, 2-component state, and MetS state. The 2-component state indicated any two combinations of components. A graphical presentation of the Markov model is presented in Figure 1. These states are mutually exclusive and collectively exhaustive [8].
Figure 1. An 8-state Markov model to describe the progression of metabolic components.
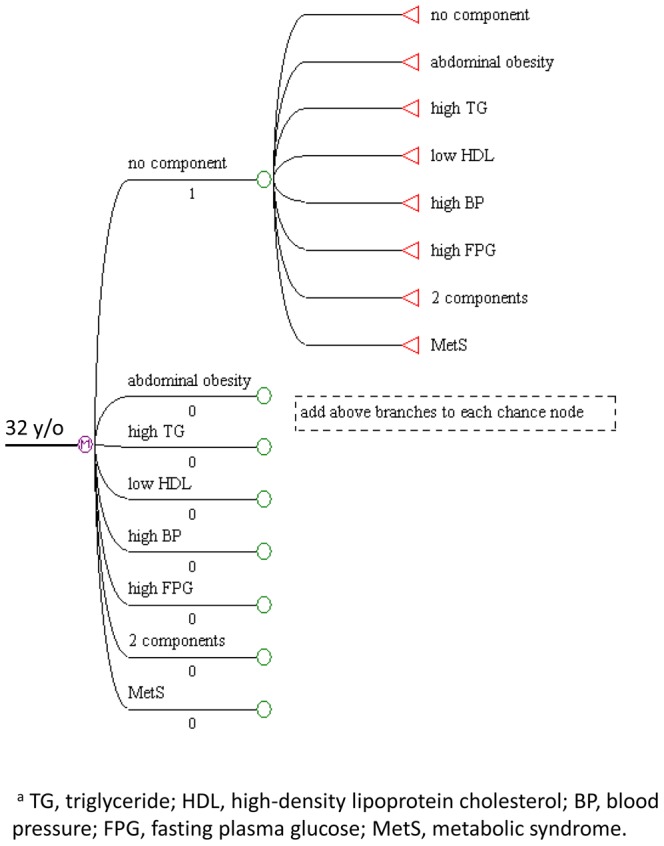
It was expected that individuals would experience a steady risk in the development of MetS over time. It was also expected that others would experience either steady improvement (resolution of MetS) or a highly variable course. Therefore, to capture all of these possible changes over time, we adopted a reversible multistate Markov model that allows for transitions to and from any state [9].
No component could transit into any “isolated component”, “2-component”, “MetS”, or maintain its steady state. Any isolated component could transit into “no component”, other “isolated component”, “2-component”, original “isolated component” or “MetS”. Two components state could transit into “no component”, “2-component”, any “isolated component” or “MetS”. MetS could reverse into other seven states or maintain its steady state.
During each Markov cycle, an individual in each gender group was assigned a probability of transitioning from one state to another. We analyzed our cohort survey data for annualized transitional probabilities. Evolution of MetS during the follow-up period was included in the simulation; therefore, there was no absorbing state in our Markov chains. At the start of the model simulation, the individual was assumed to have no component. Individuals were allowed to change their state every year, their outcome being determined by the probabilities defined at each chance node in the model. In addition, we constructed other Markov chains of different initial states, such as starting with isolated abdominal obesity, or starting with isolated high BP, and so on.
To simplify the model, a number of assumptions were necessary [8]. 1) The future progression depended only on a individual’s current state without any memory of prior states. 2) We assumed that each baseline transition rate remained constant throughout the follow-up period. 3) We also assumed that individuals with MetS could experience evolution in every cycle.
Validation of the Model
To validate the MetS development of the model, we compared these outcomes with the empiric data. For the comparison between estimated prevalence of model and the empiric data, the time horizons of the model were set to 5 years to match the follow-up duration of the original cohort data.
Statistical Analysis
SAS software (SAS Institute Inc, Cary, NC) was used for basic data analyses and calculation of annualized transition probabilities. Transition probabilities were calculated using a formula: P = 1−EXP∧(−rt); where P is the transition probability, r is the annual incidence rate and t is time. Differences in cross-tabulations were analyzed by using the Chi-square test. The Markov models were constructed using TreeAge pro 2008 software from Tree Age Software, Inc. (Williamstown, MA). The criterion for statistical significance was p<0.05.
Results
Characteristics of the study individuals, stratified by gender are displayed in Table 1. Of 2,247 participants aged 18–45 years at study entry (in 2002), the mean age of the study cohort was 32.4 (SD: 8.0) years, and 54.1% were women. MetS developed in 164 participants who were without MetS at baseline examination, with incidence rates of 19.5/1000 person-years in men and 11.4/1000 person-years in women. MetS had reversed in 96 participants, with resolution rates of 79.8/1000 person-years in men and 72.0/1000 person-years in women during the 5.4-year follow-up.
Table 1. Basic characteristics of the study sample.
| Men | Women | ||||
| Basic surveya,b | N = 1032 | N = 1215 | p | ||
| Age (years) | 32.1 | ±8.1 | 32.7 | ±7.9 | 0.0629 |
| Body massindex (kg/m2) | 24.0 | ±3.8 | 22.2 | ±3.6 | <0.0001 |
| Abdominalobesity | 234 | (22.7) | 202 | (16.6) | 0.0003 |
| High TG | 317 | (30.7) | 144 | (11.9) | <0.0001 |
| Low HDL | 222 | (21.5) | 314 | (25.8) | 0.0163 |
| High BP | 214 | (20.7) | 83 | (6.8) | <0.0001 |
| High FPG | 97 | (9.4) | 92 | (7.6) | 0.1199 |
| MetS in 2002 | 145 | (14.1) | 84 | (6.9) | <0.0001 |
| Currentsmoker (%) | 541 | (52.4) | 77 | (6.3) | <0.0001 |
| Regular alcoholdrinker (%) | 254 | (24.6) | 58 | (4.8) | <0.0001 |
Values for continuous characteristics are expressed as mean ± SD; values for categorical data were expressed as n (%).
TG, triglyceride; HDL, high-density lipoprotein cholesterol; BP, blood pressure; FPG, fasting plasma glucose; MetS, metabolic syndrome.
The Markov Chain Model and Transition Probabilities
The Markov chain model for the natural history of MetS depended on probabilities for transition between any 2 states at 1-year intervals. The results are shown as transition probabilities in Table 2 and Table 3. The probabilities for transition from the “no component” state, 2-component state, isolated high BP state, high FPG state, or high TG state to MetS were higher in men than in women. However, the probabilities for transition from the “no component” state to abdominal obesity or isolated low HDL were higher in women. MetS components always occurred simultaneously. In men, the progress of MetS components was likely to be initiated by high BP or the 2-component state, but in women, it was initiated by abdominal obesity or the low HDL state.
Table 2. Annualized transition probabilities (%) in Markov chain models for men.
| Event state | |||||||||
| Starting state | No component | abdominal obesity | high TG | low HDL | high BP | high FPG | 2 components | MetS | |
| No component | 92.89 | 0.91 | 1.08 | 0.74 | 1.99 | 0.20 | 1.73 | 0.45 | |
| Isolated abdominal obesity | 2.16 | 86.77 | 0.35 | 0.70 | 0.35 | 0.01 | 6.76 | 2.93 | |
| Isolated high TGa | 4.65 | 0.25 | 84.35 | 0.25 | 0.75 | 0.25 | 6.89 | 2.62 | |
| Isolated low HDL | 12.89 | 0.01 | 0.32 | 79.64 | 2.31 | 0.01 | 3.02 | 1.63 | |
| Isolated high BP | 6.10 | 0.32 | 0.64 | 0.96 | 85.66 | 0.64 | 3.38 | 2.31 | |
| Isolated high FPG | 11.29 | 0.90 | 1.84 | 0.00 | 1.84 | 79.41 | 3.84 | 0.90 | |
| 2 components | 2.27 | 1.29 | 1.17 | 0.69 | 0.81 | 0.58 | 86.33 | 6.87 | |
| MetS | 0.52 | 0.65 | 0.26 | 0.13 | 0.91 | 0.26 | 6.14 | 91.14 | |
TG, triglyceride; HDL, high-density lipoprotein cholesterol; BP, blood pressure; FPG, fasting plasma glucose; MetS, metabolic syndrome.
Table 3. Annualized transition probabilities (%) in Markov chain models for women.
| Event state | |||||||||
| Starting state | No component | abdominal obesity | high TG | low HDL | high BP | high FPG | 2 components | MetS | |
| No component | 92.82 | 1.67 | 0.32 | 2.73 | 0.41 | 0.16 | 1.49 | 0.41 | |
| Isolated abdominal obesity | 2.22 | 85.42 | 0.00 | 0.27 | 0.01 | 0.01 | 6.39 | 5.70 | |
| Isolated high TGa | 5.58 | 1.27 | 80.28 | 5.58 | 0.63 | 0.01 | 4.05 | 2.62 | |
| Isolated low HDL | 10.24 | 0.66 | 0.11 | 85.23 | 0.54 | 0.01 | 3.11 | 0.54 | |
| Isolated high BP | 4.23 | 0.01 | 0.79 | 2.44 | 83.91 | 0.01 | 6.19 | 2.44 | |
| Isolated high FPG | 6.70 | 5.19 | 0.59 | 2.44 | 0.59 | 80.81 | 3.10 | 0.59 | |
| 2 components | 4.82 | 1.55 | 0.17 | 1.73 | 0.85 | 0.01 | 86.67 | 4.21 | |
| MetS | 0.45 | 0.90 | 0.22 | 1.13 | 0.45 | 0.45 | 4.10 | 92.31 | |
TG, triglyceride; HDL, high-density lipoprotein cholesterol; BP, blood pressure; FPG, fasting plasma glucose; MetS, metabolic syndrome.
Initiating with No Component
A representative component progression in this cohort of the “no component” state, divided by gender, is presented in Table 4. The development of MetS was an increasing monotonic function of time. After 15 years, MetS was estimated to develop in 12.7% (CI, 10.7–14.9) of young men with “no component” state which had started at the age of 32 years, and 2-component was estimated to develop in 16.3% (CI, 14.1–18.8). Only 46.7% of men remained steady in the “no component” state (Table 4). MetS was estimated to develop in 10.6% (CI, 8.8–12.7) of young women, and 2-component developed in 14.3% (CI, 12.2–16.7). Of note, 49.8% of women were steady in the no component state.
Table 4. The predictive development of metabolic syndrome and its components (%) in individuals starting with no component according to the Markov chain models.
| Young men | Young women | ||||||||
| Year 1 | Year 5 | Year 10 | Year 15 | Year 1 | Year 5 | Year 10 | Year 15 | ||
| No component | 100 | 92.89 | 71.88 | 56.15 | 46.71 | 92.82 | 72.30 | 57.89 | 49.81 |
| Isolated abdominal obesity | 0 | 0.91 | 3.30 | 4.68 | 5.28 | 1.67 | 5.77 | 7.78 | 8.41 |
| Isolated high TGa | 0 | 1.08 | 3.71 | 4.94 | 5.26 | 0.32 | 1.00 | 1.23 | 1.26 |
| Isolated low HDL | 0 | 0.74 | 2.40 | 3.02 | 3.09 | 2.73 | 9.13 | 11.85 | 12.41 |
| Isolated high BP | 0 | 1.99 | 6.80 | 8.91 | 9.32 | 0.41 | 1.50 | 2.19 | 2.52 |
| Isolated high FPG | 0 | 0.20 | 0.76 | 1.13 | 1.31 | 0.16 | 0.49 | 0.60 | 0.64 |
| 2 components | 0 | 1.73 | 7.64 | 12.88 | 16.34 | 1.49 | 6.77 | 11.43 | 14.32 |
| MetS | 0 | 0.45 | 3.52 | 8.30 | 12.69 | 0.41 | 3.04 | 7.04 | 10.63 |
TG, triglyceride; HDL, high-density lipoprotein cholesterol; BP, blood pressure; FPG, fasting plasma glucose; MetS, metabolic syndrome.
Initiating with any Isolated Component or 2-component State
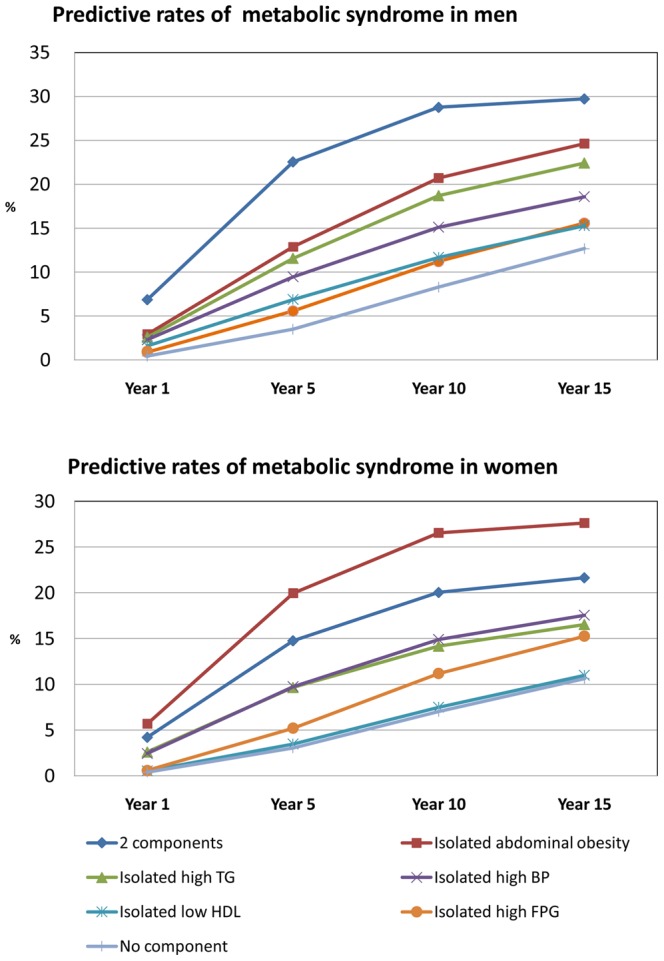
Individuals starting with any isolated component or 2-component state were more likely to develop MetS (Figure 2). MetS was estimated to develop after 15 years (at age 47 years) in 24.6% (CI, 22.0–27.4) of men and 27.6% (CI, 24.9–30.5) of women in whom the disease was initiated with the isolated abdominal obesity state. MetS was estimated to develop after 15 years in 29.7% (CI, 26.9–32.7) of men and 21.7% (CI, 19.1–24.3) of women in whom the disease was initiated with the 2-component state, a rate higher than in individuals in whom the disease initiated with the “no component” state. The results of this study suggest that MetS develops in more individuals who have 1 or 2 components.
Figure 2. The predictive development of metabolic syndrome according to various starting component.
Estimated prevalence of MetS after 5 years in men and women who had no component at study entry was 3.5% and 3.0%, respectively. We compared these outcomes with the empiric data for validation of model and found that estimated prevalence was similar with empiric data from original cohort in 2007 that showed 3.1% (CI, 1.7–5.1) in men and 2.5% (CI, 1.4–3.9) in women.
Discussion
We developed a reversible multi-state Markov model and showed through simulation how these MetS components progress over 15 years in a young population. These results demonstrate that the main initiating components in men were high BP and a combination of 2 components. However, in women, the main isolated components were low HDL or abdominal obesity. Metabolic components were likely to occur together, which implies a close relation between these metabolic components.
Some researchers believe that high BP is not a main metabolic component. Macchia et al. showed that a clinically diagnostic score for MetS improves its predictivity of diabetes mellitus [17]. Their score system does not include a high BP component. Haring et al. generate a map of risk factor flow to visualize clustered MetS components and their change over a five-year follow-up period and identified hypertension and central obesity as the predominant risk factor cluster [7]. Our data revealed that in most young men MetS initiated with high BP. People with MetS are at increased risk of diabetes mellitus as well as at increased risk of cardiovascular events [18]. Hypertension is a well-defined risk factor for cardiovascular diseases. In our results, high BP components showed its important role in MetS, especially among young men.
The model initiated with any isolated MetS component or combination of 2 components accelerated the development of MetS in both genders; contrarily, the model also initiated with the “no component” state. Many researchers agree that obesity, dysglycemia, dyslipidemia, and hypertension coexist frequentlys [19], [20]. These MetS components have partially overlapping mechanisms of pathogenic actions mediated through common metabolic pathways. It should be specifically pointed out that individuals with 1 MetS component need to be examined for other components and have to be regularly followed up to identify the risk of development of MetS.
We found that women with abdominal obesity had an increased likelihood for incidences of a MetS event when compared with women with 2 components. Conceptually, MetS is a consequence of obesity and insulin resistance [21]. People with MetS usually pass through phases of excessive adipogenesis (obesity) [19]–[22]. MetS has been more prevalent in men than in women in recent years but has risen particularly in young women, where it is mainly driven by obesity [23].
In the absence of studies comprising metabolic components and longitudinal follow-up of large numbers of population, this Markov simulation modeling study allows estimation of the natural history of MetS. A Markov process is a frequently used mathematical model to describe disease progression over time [11], [24]–[27]. Sonnenberg and Naugler showed Markov models of influence in chronic liver disease [25]. Veenstra et al. constructed Markov processes that have been used to analyze hepatitis B virus infections [26]. Their results demonstrated that modeling the progression of a disease becomes more accurate than simple incidence rate presentation. Denton et al. constructed a Markov model with health states composed of cardiovascular events and metabolic factors to evaluate the optimal start time of statin treatment for different combinations of cardiovascular risk models and patient attributes [28]. Grassi et al. [29] analyzed data from a longitudinal study on a group of women with a history of gestational diabetes and constructed a three-state Markov chain model to represent the dynamics of changes between metabolic states. Our model with reversible chances (including resolution of MetS and its components) had the advantage of conceptualizing an intricate disease process.
This study had limitations. First, we estimated the natural history of MetS development, but it was not necessarily “natural” due to interventions of health services that were not taken into account in these analyses. Secondly, we assumed constant transition probability for following years, but probability might also increase with age [30]. On the contrary, increasing age was found to decrease the likelihood of resolution among individuals with MetS. Thirdly, we did not include medication use in high TG and low HDL components. The term “clean-blood agents” was represented as “lipid-lowering agents.” This concept is vague in Taiwan and would underestimate the high TG and low HDL components. Fourthly, mortality was not taken into account in this model. Slight overestimation of the cumulative incidence of MetS was anticipated. Decreased survival in persons with MetS is associated with the presence of other life-threatening diseases, such as diabetes mellitus and cardiovascular disease. Mortality rates for the general Taiwanese population were not included in these analyses and led to overestimation. This is especially true when gender differences are considered. Mortality rates in 2002 were 0.822% for middle-aged men and 0.385% for women. In this case, we assumed that the influence of mortality on the incidence of MetS is very small.
In conclusion, these results of this study suggest that men in whom MetS was initiated with no component tended to develop this syndrome sooner than women (i.e., before their fifth decade of life), but women with abdominal obesity develop this syndrome sooner than men. MetS develops in more individuals who have 1 or 2 components. The conclusions that can be drawn from our model are valid, in spite of some limitations, and the probability for bias is small. These findings confirm the importance of incorporating BP monitoring programs and weight control interventions into health services for assessing high-risk groups and preventing the development of MetS.
Acknowledgments
The authors thank the nurses at the study areas for data collection.
Funding Statement
This study was supported by a grant from the Bureau of Health Promotion, Department of Health (DOH95-HP-2103), Executive Yuan, Taiwan, Republic of China. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB (2005) Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 112: 3066–3072. [DOI] [PubMed] [Google Scholar]
- 2. Prabhakaran D, Anand SS (2004) The metabolic syndrome: an emerging risk state for cardiovascular disease. Vasc Med 9: 55–68. [DOI] [PubMed] [Google Scholar]
- 3. Santos AC, Severo M, Barros H (2010) Incidence and risk factors for the metabolic syndrome in an urban South European population. Prev Med 50: 99–105. [DOI] [PubMed] [Google Scholar]
- 4. Wilsgaard T, Jacobsen BK (2007) Lifestyle factors and incident metabolic syndrome. The Tromso Study 1979–2001. Diabetes Res Clin Pract 78: 217–224. [DOI] [PubMed] [Google Scholar]
- 5. Kim BJ, Kim BS, Kang JH (2012) Alcohol consumption and incidence of metabolic syndrome in korean men. A 3-year follow-up study. Circ J 76: 2363–2371. [DOI] [PubMed] [Google Scholar]
- 6. Han TS, Williams K, Sattar N, Hunt KJ, Lean ME, et al. (2002) Analysis of obesity and hyperinsulinemia in the development of metabolic syndrome: San Antonio Heart Study. Obes Res 10: 923–931. [DOI] [PubMed] [Google Scholar]
- 7. Haring R, Rosvall M, Volker U, Volzke H, Kroemer H, et al. (2012) A network-based approach to visualize prevalence and progression of metabolic syndrome components. PLoS One 7: e39461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silverstein MD, Albert DA, Hadler NM, Ropes MW (1988) Prognosis in SLE: comparison of Markov model to life table analysis. J Clin Epidemiol 41: 623–633. [DOI] [PubMed] [Google Scholar]
- 9. Andersen PK, Keiding N (2002) Multi-state models for event history analysis. Stat Methods Med Res 11: 91–115. [DOI] [PubMed] [Google Scholar]
- 10. Beck JR (1988) Markov models of natural history. J Clin Epidemiol 41: 619–621. [DOI] [PubMed] [Google Scholar]
- 11. Sonnenberg FA, Beck JR (1993) Markov models in medical decision making: a practical guide. Med Decis Making 13: 322–338. [DOI] [PubMed] [Google Scholar]
- 12. Craig BA, Fryback DG, Klein R, Klein BE (1999) A Bayesian approach to modelling the natural history of a chronic condition from observations with intervention. Stat Med 18: 1355–1371. [DOI] [PubMed] [Google Scholar]
- 13. Silverstein MD, Loftus EV, Sandborn WJ, Tremaine WJ, Feagan BG, et al. (1999) Clinical course and costs of care for Crohn’s disease: Markov model analysis of a population-based cohort. Gastroenterology 117: 49–57. [DOI] [PubMed] [Google Scholar]
- 14. Hwang LC, Bai CH, Chen CJ (2006) Prevalence of obesity and metabolic syndrome in Taiwan. J Formos Med Assoc 105: 626–635. [DOI] [PubMed] [Google Scholar]
- 15. Yang T, Chu CH, Bai CH, You SL, Chou YC, et al. (2012) Uric acid level as a risk marker for metabolic syndrome: a Chinese cohort study. Atherosclerosis 220: 525–531. [DOI] [PubMed] [Google Scholar]
- 16. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, et al. (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 17. Macchia A, Levantesi G, Borrelli G, Franzosi MG, Maggioni AP, et al. (2006) A clinically practicable diagnostic score for metabolic syndrome improves its predictivity of diabetes mellitus: the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico (GISSI)-Prevenzione scoring. Am Heart J 151: 754.e7–754.e17. [DOI] [PubMed] [Google Scholar]
- 18. Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, et al. (2010) The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 56: 1113–1132. [DOI] [PubMed] [Google Scholar]
- 19. Tenenbaum A, Fisman EZ (2011) “The metabolic syndrome… is dead”: these reports are an exaggeration. Cardiovasc Diabetol 10: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Despres JP, Lemieux I (2006) Abdominal obesity and metabolic syndrome. Nature 444: 881–887. [DOI] [PubMed] [Google Scholar]
- 21. Eckel RH, Alberti KG, Grundy SM, Zimmet PZ (2010) The metabolic syndrome. Lancet 375: 181–183. [DOI] [PubMed] [Google Scholar]
- 22. Cameron AJ, Boyko EJ, Sicree RA, Zimmet PZ, Soderberg S, et al. (2008) Central obesity as a precursor to the metabolic syndrome in the AusDiab study and Mauritius. Obesity (Silver Spring) 16: 2707–2716. [DOI] [PubMed] [Google Scholar]
- 23.Regitz-Zagrosek V, Lehmkuhl E, Mahmoodzadeh S (2007) Gender aspects of the role of the metabolic syndrome as a risk factor for cardiovascular disease. Gend Med 4 Suppl B: S162–177. [DOI] [PubMed]
- 24. Spiegel BM, Targownik L, Dulai GS, Karsan HA, Gralnek IM (2003) Endoscopic screening for esophageal varices in cirrhosis: Is it ever cost effective? Hepatology 37: 366–377. [DOI] [PubMed] [Google Scholar]
- 25. Sonnenberg A, Naugler WE (2010) Models of influence in chronic liver disease. Liver Int 30: 718–724. [DOI] [PubMed] [Google Scholar]
- 26. Veenstra DL, Spackman DE, Di Bisceglie A, Kowdley KV, Gish RG (2008) Evaluating anti-viral drug selection and treatment duration in HBeAg-negative chronic hepatitis B: a cost-effectiveness analysis. Aliment Pharmacol Ther 27: 1240–1252. [DOI] [PubMed] [Google Scholar]
- 27. Sweeting MJ, Farewell VT, De Angelis D (2010) Multi-state Markov models for disease progression in the presence of informative examination times: an application to hepatitis C. Stat Med. 29: 1161–1174. [DOI] [PubMed] [Google Scholar]
- 28. Denton BT, Kurt M, Shah ND, Bryant SC, Smith SA (2009) Optimizing the start time of statin therapy for patients with diabetes. Med Decis Making 29: 351–367. [DOI] [PubMed] [Google Scholar]
- 29. Grassi A, Gaetano L, Pacini G, Kautzky-Willer A, Tura A (2010) A Markov chain probability model of glucose tolerance in post gestational diabetes follow up study. Stud Health Technol Inform 160: 1155–1159. [PubMed] [Google Scholar]
- 30. Rigopoulou EI, Dalekos GN (2010) Can mathematical models be useful in clinical hepatology? Liver Int 30: 637–638. [DOI] [PubMed] [Google Scholar]


