Abstract
The purpose of this study was to evaluate the efficacy of topical dorzolamide 2% eye drops on macular function and thickness in a case of enhanced S-cone syndrome (ESCS). A 24-year-old Asian man with enhanced S-cone syndrome treated with topical dorzolamide in the left eye participated in the study. Examinations performed before and during treatment included: visual acuity (VA), contrast sensitivity measured with briefly presented grating targets (grating CS) and the Pelli-Robson chart (P-R CS), microperimetry (MP), and spectral-domain optical coherence tomography (SD-OCT). Following four months of treatment, the mean thickness of the central 1-mm foveal subfield of the left eye, as measured by SD-OCT, decreased from 551 μm to 242 μm. Mean MP sensitivity within the central 12 degrees (28 points) increased from 9.4 dB at baseline to 11.2 dB. Although, Pelli-Robson contrast sensivity improved only minimally in the left eye, grating contrast sensivity improved by more than a factor of two. Mean log MAR VA was 0.22 OD and 1.00 OS (at baseline), which improved to 0.10 OD and 0.66 OS after four months of treatment. The results indicate that in our patient with enhanced S-cone syndrome, treatment with topical dorzolamide was effective in improving macular thickness, VA, microperimetry sensitivity, and grating contrast sensivity. These measures of retinal structure and function are sensitive tools for evaluating the effects of treatment in enhanced S-cone syndrome patients with cystoid macular edema. Further investigation is warranted to assess the relationships among visual performance for daily activities, visual sensitivity, and macular thickness.
Introduction
Hereditary retinal degenerative diseases usually affect photoreceptor cell density by reducing the number of cells through apoptosis, resulting in loss of visual function. Patients with enhanced S-cone syndrome (ESCS) are unique by manifesting enhanced sensitivity of their S-cone photoreceptors compared to visually normal subjects [1, 2]. ESCS is an autosomal recessive retinal degenerative disease often associated with NR2E3 gene mutations, which encodes a ligand-dependent transcription factor that is thought to determine photoreceptor fate during development [3]. Patients with enhanced S-cone syndrome usually complain of night blindness, variable loss of visual acuity (VA), and visual field abnormalities. The most typical fundus changes are degenerative nummular pigmentary changes along the retinal vascular arcades, and a macular disturbance, often associated with intraretinal macular cysts [3–5]. The electroretinogram (ERG) has distinctive features that include either no recordable response or a markedly reduced response to low-intensity stimuli under dark-adapted conditions, whereas large and delayed responses are recorded to high-intensity stimuli under light-adapted conditions [5]. The pattern of retinal dysfunction is a constant among enhanced S-cone syndrome patients, but the degree of clinically evident retinal degeneration can vary from minimal to severe. The latter phenotype is known as Goldmann-Favre syndrome.
The use of a carbonic anhydrase inhibitor (CAI) has been shown to be effective for the improvement of cystoid macular edema (CME) in patients with enhanced S-cone syndrome [6, 7], as well as in patients with retinitis pigmentosa and X-linked retinoschisis [8–10]. In patients with enhanced S-cone syndrome, optical coherence tomography (OCT) has been used to evaluate changes in retinal thickness following treatment with both oral [6] and topical [7] forms of a CAI. Marked improvement in retinal thickness and the resolution of CME was observed following treatment. Additionally, these studies showed that the use of a CAI improved VA in patients with enhanced S-cone syndrome, however, the extent to which other measures of visual function change remains to be determined. For example, it is not clear if visual function as measured by contrast sensitivity or fundus microperimetry (MP) would also improve following treatment, and to what extent. Fundus microperimetry has been used for direct real-time evaluation of macular sensitivity and retinal fixation, allowing for an accurate examination of visual function in different retinal disorders [11–13].
The current case report demonstrates the efficacy of topical therapy with dorzolamide hydrochloride 2% on retinal thickness and macular visual function. Retinal thickness was measured by spectral-domain OCT (SD-OCT), an imaging technique that can provide high-resolution cross-sectional images of the macular region. Retinal thickness measurements and measurements of visual function were performed before treatment was initiated and after two and four months of treatment. Measurements of visual function included: visual acuity, microperimetry sensitivity, Pelli-Robson letter contrast sensitivity (P-R CS), and contrast sensitivity for briefly presented grating targets (gating CS).
Patients and Methods
A 24-year-old Asian male with a diagnosis of enhanced S-cone syndrome was treated with dorzolamide hydrochloride 2% ophthalmic eye drops three times a day in his left eye. He was seen at the hereditary retinal diseases unit in the Ophthalmology Department at the University of Illinois at Chicago where he was enrolled in a study for the use of topical dorzolamide for the treatment of CME in hereditary retinal diseases. The study was conducted in accordance with the tenets of the Declaration of Helsinki and was approved by an institutional review board at the University of Illinois at Chicago. Informed consent was obtained after the objectives and the nature of the procedures had been fully explained.
The patient was previously seen by one of the authors (GAF). He was contacted by telephone and asked to participate in the study based on his prior diagnosis of enhanced S-cone syndrome, which was determined by his clinical fundus appearance and pathognomonic ERG changes.
The patient had a history of night blindness for as long as he could remember. Additionally, there was a 6-year history of a progressive decrease in central vision of each eye (more in the left eye than the right). He denied any photoaversion or color vision problems. A general review of systems indicated mild asthma secondary to allergies. A review of the family pedigree showed no history for hereditary eye disease.
The patient underwent a complete ocular examination, including slit-lamp biomicroscopy, and intraocular pressure measurement with Goldmann applanation tonometry. Dilated fundus examination was done by using both direct and indirect ophthalmoscopy. Blood was withdrawn from the patient for molecular genetic analysis at the baseline visit (results pending). Best-corrected visual acuity was measured with both a Snellen chart and the Early Treatment of Diabetic Retinopathy Study chart (ETDRS) (The Lighthouse Low Vision Products, Long Island City, NY). Contrast sensitivity for large letters was measured with a Pelli-Robson contrast sensitivity chart (P-R CS). Additionally, contrast sensitivity was measured using a 2 cycle-per-degree grating target that was briefly presented (100 ms) on a video display (grating CS). For the grating CS measurement, the patient’s task was to judge the orientation of the grating (horizontal or vertical), and the contrast of the grating was adjusted on each trial to determine his contrast sensitivity.
Visual field examination was performed by Goldmann kinetic perimetry 940 (Haag-Streit AG, Switzerland), using II-4-e, III-4-e, V-4-e, and II-2-e test targets. A full-field ERG (Nicolet Viking IV system, Nicolet Biomedical Inc) was performed in the left eye by using a unipolar Burian-Allen contact lens electrode (obtained at the time of presentation). The ERG responses were obtained according to the International Society for Clinical Electrophysiology of Vision (ISCEV) standards. Parameters included amplitudes and implicit times for each of the major waveform components [14].
Spectral-Domain Optical Coherence Tomography (SD-OCT) Examination
SD-OCT was performed using a spectral-domain OCT/SLO system (OPKO instrumentations, Miami, Florida, USA) to obtain both OCT and SLO images with an axial resolution of <10 microns, a transverse resolution of 20 microns (in tissue), and longitudinal and coronal (depth) scan range of 2.0 mm. The SD-OCT/SLO is a high-resolution tomographic and confocal imaging device. It is indicated for in vivo viewing of axial cross-sectional and three-dimensional images of posterior ocular structures. Both the Line scan (B-scan) and the 3D Retinal Topography scan protocols were used for image acquisition at the baseline and follow-up visits. The Line scan mode allows the capture of cross-sectional B-scan OCT images of the vitreo-retinal, retinal, and chorio-retinal structures. The 3D Retinal Topography mode covers an area of 9.0 × 9.0 mm with a 2.0 mm depth. A red scanning line on the SLO image represents the precise location of the cross sectional OCT image.
The incorporated software calculates the average retinal thickness in each of 9 ETDRS-like zones [15]. The mean ± SD macular thickness from the central 1-mm subfield and from each of the 4 sectors of the inner circle (between 1-and 3-mm in diameter) and outer circle (between 3 and 6-mm in diameter) was calculated. The macular thickness values were compared with normative data provided by the manufacturer, comprised of 225 eyes of 119 normative control subjects (mean age of 47.8 ± 16.3 years).
Fundus Autofluorescence (FAF) Examination
In vivo measurement of autofluorescence was performed with a confocal scanning laser ophthalmoscope (cSLO) (Heidelberg Retina Angiograph (HRA), Heidelberg Engineering, Heidelberg, Germany) (excitation wavelength: 488 nm, band-pass filter: 500 nm), as well as infrared reflectance (IR: 820 nm) and blue reflectance (488 nm) imaging. The size of the field of view was 30° × 30°. Image acquisition was performed in high-speed mode. Images with a 30° field of view were digitized in frames of 768 × 768 pixels with a resolution of approximately 11μm per pixel. Optical resolution was approximately 10μm.
Macular Microperimetry Testing
Microperimetry testing was performed using the spectral-domain OCT/SLO system. This instrument allows the examiner to view the fundus on a computer monitor while it is imaged in real time by a SLO fundus camera. The background luminance of the instrument is 10 cd/m2. Stimulus intensity can be varied in 2 dB (0.2 log) steps from 0 to 20, where 0 dB represents the highest luminance of 125 cd/m2. The instrument also incorporates an automated tracking system to compensate for eye movement during examination.
The examination was conducted in a darkened room. The patient underwent brief training at the beginning of the microperimetry exam that allowed for familiarization and practice with correct operation of the response trigger and the stimulus target. This was followed immediately by the formal microperimetry test. All tests were performed after dilatation of the pupil with 1% tropicamide and 2.5% phenylephrine. The non-tested eye was patched. The patient was instructed to fixate on a 0.66-degree red square as a fixation target and press a button once he identified the stimulus. Parameters that were used during the baseline and follow-up visits included: a standard grid pattern (Polar 3), Goldmann III size stimulus (area of 4 mm2, diameter of 26 min arc or 0.4 degrees), a 200 ms duration, and a 4-2 test strategy. The individual sensitivity values at 28 different loci within the central 12 degrees (Polar 3 test pattern) were compared to normative data incorporated in the microperimeter software provided by the manufacturer for the Polar 3 test pattern. Sensitivity values of 14.1 dB or higher were considered normal (unpublished data from our lab).
Results
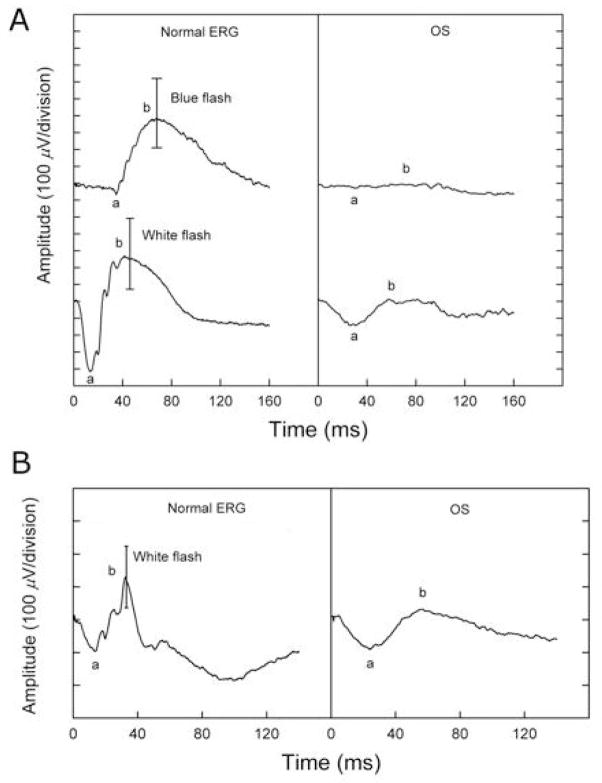
Fluorescein angiography, FAF, visual field perimetry testing, and electroretinography were performed at the time of presentation. Fluorescein angiography showed multiple punctate areas of hyperfluorescence corresponding to retinal pigment epithelium (RPE) window defects, mostly apparent along the vascular arcades, as well as areas of punctate blocked fluorescence corresponding to RPE hyperpigmentation. There was no leakage from parafoveal capillaries in late frames of the angiogram. FAF examination showed a ring of increased autofluorescence in the posterior pole of both eyes. As shown in Fig. 1, there was a faint area of enhanced autofluorescence within the fovea of the left eye. Visual field testing showed only residual temporal islands to the II-4-e target and small temporal scotomas to the V-4-e target in each eye. As shown in Fig. 2A, the dark-adapted rod ERG response to dim blue flashes was non-detectable (top panel). The lower panel of Fig. 2 shows that the amplitudes of the a- and b-waves of the dark adapted combined rod-cone response were markedly reduced. Fig. 2B shows the light adapted single flash cone response, which was also reduced. The cone waveform was atypical, consistent with an overabundance of short wavelength sensitive cones, as previously described [5].
Fig. 1.
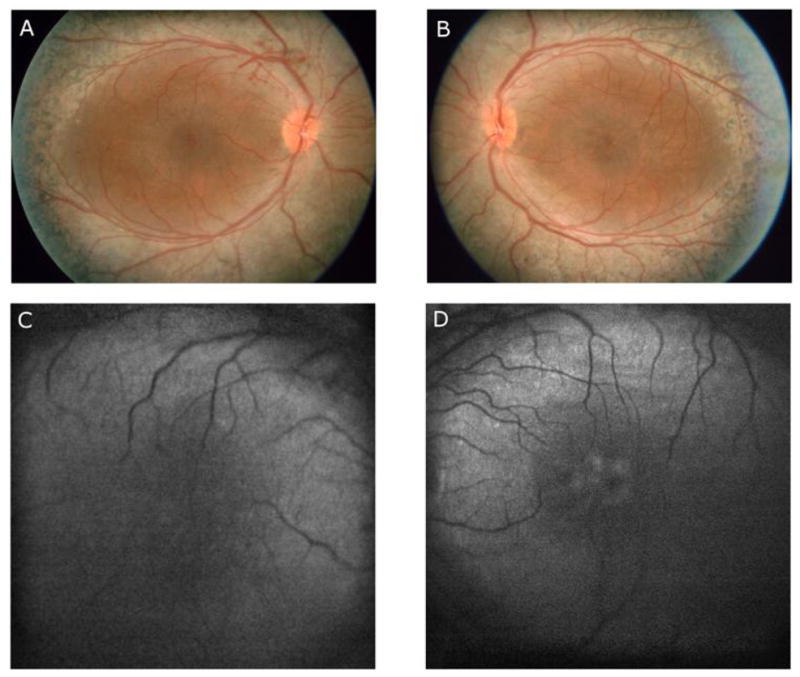
Fundus photographs obtained at the time of presentation of the right eye (A) and left eye (B), demonstrating blotchy pigment clumping along the vascular arcades and the mid-peripheral retina as well as areas of diffuse hypopigmentation for 360 degrees in the mid-peripheral retina in each eye. The left eye (B) showed cystic-appearing lesions within the macula. Autofluorescence (AF) images of the right eye (C) and left eye (D) show a ring of enhanced autofluorescence in the posterior pole in each eye. The image of the left eye (D) shows a faint ring of enhanced autofluorscece within the fovea.
Fig. 2.
The full-field ERG for the left eye (pretreatment) shows an essentially non-detectable rod response under dark-adapted conditions (right panel, A) and a mild reduction of cone responses under light-adapted conditions (right panel, B), with atypical cone waveforms (prolonged implicit time) that were consistent with an overabundance of short-wavelength-sensitive cones.
At the baseline (pretreatment) visit, the dilated fundus exam of the right eye showed a small and hyperemic-appearing optic disc, mild arteriolar attenuation, and superior telangiectatic-like vascular changes of the retinal vessels. The macula of the right eye showed a blunted foveal reflex without any evidence of macular cystic lesions. There was blotchy pigment clumping along the vascular arcades in the right eye as well as areas of diffuse hypopigmentation for 360 degrees in the mid-peripheral retina. The left eye showed similar changes with the additional finding of cystic-appearing lesions within the macula. Finding cystic-appearing lesions in only one eye is somewhat atypical. However, in other retinal degenerative diseases, such as retinitis pigmentosa, in our experience a notable number of patients can present with CME in only one eye.
Treatment with 2% topical dorzolamide hydrochloride was initiated. Following two months of treatment, the patient reported a mild subjective improvement of his central vision and also noticed that objects were brighter at distance. No changes in his peripheral, night, or color vision were subjectively reported. The fundus exams of each eye after both two and four months of treatment were similar to that of the baseline visit. However, the cystic-appearing macular lesions in the left eye were less apparent at both two and four months when compared to the baseline. After two months of treatment, the right eye showed areas of a blotchy superficial retinal hemorrhage nasal to optic disc and a dot hemorrhage temporal to the optic disc that were not seen at the baseline fundus exam. The patient reported a recent history of severe attacks of a dry cough over the week prior to his first follow-up appointment.
Ocular pressures were similar at all visits (between 8 to 10 mmHg in both eyes). The corneas showed pigment dusting on the endothelium, the anterior chambers and lenses were clear, the pupils reacted normally, and color vision screening with the Ishihara color plates was normal at each visit. The vitreous showed +1 cells in the right eye and +2 to 2 1/2 cells in the left eye at the baseline visit. There was no clinically significant change at the two follow-up visits (+1 cells in the right eye and +2 cells in the left eye at both two and four months).
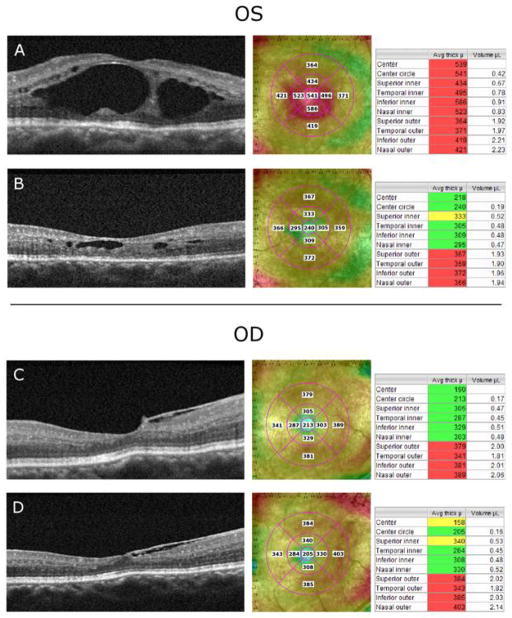
As shown in Fig. 3A, a spectral-domain OCT exam at the baseline visit indicated the presence of large intraretinal cystic lesions in the left eye. There was no evidence of cystic macular edema in the right eye, which showed only an epiretinal membrane nasal to the fovea (Fig. 3C). The retinal thicknesses of the center 1-mm foveal subfields of the left and right eyes at each visit are given in Table 1. At baseline, the retinal thickness of the left eye was much greater than normal, whereas the right eye was within the normal limits. Following two months of treatment, the retinal thickness of the left eye decreased substantially, whereas the thickness of the right eye was essentially the same as that at baseline. Comparison of Figs. 3A and 3B shows a marked decrease in the number of macular cysts in the left eye after two months of treatment. Additionally, the macular thicknesses of all 4 sectors of the inner circle of the left eye were less than the baseline thickness values (Fig. 3A versus 3B). The retinal thickness of the left and right eyes at four months of treatment was similar to that measured after two months of treatment (Table 1).
Fig. 3.
Spectral-domain OCT scans demonstrate the reduction of the macular cysts and retinal thickness while on treatment with topical dorzolamide 2% in the left (treated) eye (A and B) following two months of treatment. Also, SD-OCT scans showed that retinal thickness in the right (untreated) eye remained essentially constant.
Table 1.
Retinal thickness and visual function for each visit
| Retinal Thickness (μm) | ETDRS Log MAR visual acuity (Snellen acuity) | MP Sensitivity (dB) | Log P-R CS | Log grating CS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OS | OD | OS | OD | OS | OD | OS | OD | OS | OD | |
| Baseline | 551 | 213 | 1.00 (20/200) | 0.22 (20/30−2) | 9.4 | 12.8 | 1.5 | 1.5 | 0.71 | |
| Two-month follow-up | 249 | 205 | 0.64 (20/80−2) | 0.16 (20/30+2) | 11.1 | 12.9 | 1.5 | 1.5 | 0.25 | 1.10 |
| Four-month follow-up | 242 | 223 | 0.66 (20/80−2) | 0.20 (20/25−1) | 11.2 | 14.4 | 1.65 | 1.65 | 0.46 | 1.23 |
ETDRS= Early Treatment Diabetic Retinopathy Study; MP= Microperimetry; P-R CS= Pelli-Robson letter contrast sensitivity; CS= Contrast Sensitivity.
At the baseline visit, VA was correctable to 0.22 log MAR (20/30−2 measured with the Snellen chart) OD and to 1.00 log MAR (20/200) OS. As shown in Table 1, VA improved by more than a factor of two in the left eye following two months of treatment, whereas VA in the right eye showed a minimal change from baseline (0.12 log MAR). VA following four months of treatment did not significantly differ from that following two months of treatment. The patient’s manifest refraction at the baseline visit was +6.00+2.00×80° OD and +6.50+2.25×95° OS. There was minimal change in the refractive error at the two follow-up visits. He read J1 (20/20) for near at 12–14 inches at the baseline visit, and was essentially the same at the two follow-up visits.
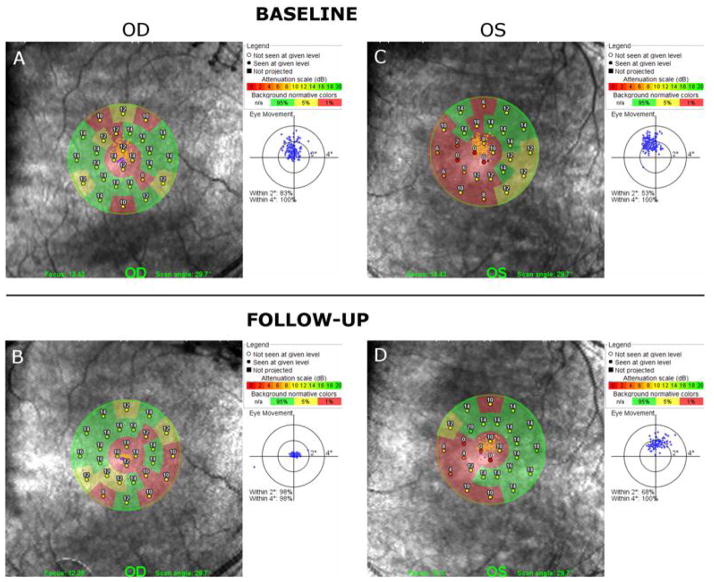
Microperimetry testing of 28 individual points within a 12 degree circle using the Polar 3 pattern showed subnormal individual point sensitivities (exceeding the 95% limits of confidence). In Fig. 4, microperimetry sensitivity is shown across the macula at baseline (Fig. 4A and 4C) and at the two-month follow-up (Fig. 4B and 4D). The overall mean microperimetry sensitivities are given in Table 1. These values were reduced below the lower limit of normal (14.1 dB). As can be seen in Figs. 4A and 4C, the patient showed relatively stable foveal fixation during the test (83% of eye movements were within 2 degrees around the projected fixation target OD and 53% OS). At the two-month follow-up, there was an increase in the overall microperimetry sensitivity in the left eye and no changes in the right eye. Fig. 4B and 4D show that patient had an improvement in fixation stability in both eyes, as 98% of eye movements were within 2 degrees around the projected fixation target OD and 68% OS. Following four months of treatment, microperimetry sensitivity in the right eye improved further, and was within the limits of normal. No change in microperimetry sensitivity was observed in the left eye.
Fig. 4.
Microperimetry (MP) testing results from the right eye (A) and left eye (C) at the baseline visit shows isolated patchy areas of subnormal retinal thresholds within the posterior pole. Improvement of microperimetry sensitivity was observed in left eye (D) as well as in the right eye (C) after treatment with topical dorzolamide 2%.
The values of Pelli-Robson contrast sensitivity and grating contrast sensitivity at each visit are given in Table 1. At the baseline visit, Pelli-Robson contrast sensitivity was equally reduced in both eyes by approximately 0.3 log units, as compared to normal [16]. Grating contrast sensitivity was markedly reduced in the left eye (at least 1.5 log units, as compared to normal). However, the full extent of the grating contrast sensitivity loss could not be determined, because the patient could not perform the task at the maximum contrast that could be produced by the display. Grating contrast sensitivity measurements could be made with the right eye at baseline, which showed an approximate 1.0 log unit loss of sensitivity, as compared to normal. Following two months of treatment, Pelli-Robson contrast sensitivity was identical to that measured at the baseline visit. Conversely, there were large improvements in grating contrast sensitivity. Measurements could be made at the two-month follow-up in the left eye, which was not possible at the baseline visit. Grating contrast sensitivity in this eye was reduced by 1.4 log units, as compared to normal. Grating contrast sensitivity improved in the right eye as well, but remained much below normal. Pelli-Robson contrast sensitivity improved slightly at the four-month follow-up visit in both eyes, but was still approximately 0.15 log units below normal [16]. Grating contrast sensitivity also continued to improve at the four-month follow-up, as shown in Table 1.
Discussion
Enhanced S-cone syndrome is a rare autosomal recessive disorder related to mutations in the NR2E3 gene. Signs include nummular pigmentary deposition at the level of the RPE around the vascular arcades and foveal schisis [17–19]. The peripheral retina (ordinarily a rod-rich region) is affected early in the disease and degenerates further because of photoreceptor cell loss [20]. Our patient showed the characteristic signs of Enhanced S-cone syndrome and his ERG results were typical of the disease. Additionally, a large macular schisis was observed on fundus clinical exam and spectral-domain OCT testing. Of note, the macular schisis was only seen in only one eye.
Our case showed that topical dorzolamide 2% eye drops are effective in the treatment of macular schisis associated with Enhanced S-cone syndrome that was, at least partially, responsible for his loss of central vision. This finding is similar to two previous studies that demonstrated the beneficial effect of CAIs in one patient with Enhanced S-cone syndrome in each study [6,7]. CAIs have been shown to be effective for the improvement of cystic macular changes in patients with other retinal diseases such as retinitis pigmentosa [8,9], uveitis [21], X-linked retinoschisis [10,22], and for patients with epiretinal membranes [23]. Improvement in our case included a marked decrease in central macular thickness on spectral-domain OCT exam and an increase in retinal sensitivity on microperimetry testing. VA, measured with both the Snellen and EDTRS charts also showed improvement in the treated eye, as did grating contrast sensitivity.
Our findings document that the foveal cystic-appearing lesions in patients with Enhanced S-cone syndrome may favorably respond to treatment with a topical CAI. A precise explanation for such a favorable response is not readily apparent. However, it may depend on the residual function of the RPE cells in individual patients, as a CAI has been shown to affect the pumping mechanism in these cells [24, 25]. It is also conceivable that continued treatment with dorzolamide could result in an even further improvement of visual function in our patient.
We observed the presence of an area of enhanced autofluorescence at the posterior pole on FAF imaging in our patient, which could suggest an increased turnover of intact photoreceptors with enhanced accumulation of lipofuscin. This finding was similar to previous studies that showed similar hyperautofluorescence in the central macula and mid-peripheral retina in patients with Enhanced S-cone syndrome [20, 26].
Although contrast sensitivity is not routinely measured in the clinic, our results show that it can be of value in evaluating visual function when monitoring changes during treatment with CAIs. Pelli-Robson contrast sensitivity was reduced in both eyes by approximately a factor of two and there was minimal improvement due to treatment. By comparison, grating contrast sensitivity showed larger losses at baseline and substantial improvement due to treatment. Additionally, grating contrast sensitivity continued to show improvements over the four months of treatment, which was not the case for VA measurements or Pelli-Robson contrast sensitivity. Differences between grating contrast sensitivity and Pelli-Robson contrast sensitivity. have been noted previously in patients with retinal disease [27], but the mechanism underlying these differences remains to be determined. A likely explanation in our case is that in the left eye there is good contrast sensitivity for low spatial frequencies, but there is a loss of contrast sensitivity for middle and high spatial frequencies. The loss of contrast sensitivity at high spatial frequencies would also account for the large acuity difference between the two eyes. Of note, grating contrast sensitivity and microperimetry sensitivity improved in the untreated (right) eye, which suggests that topical dorzolamide may produce effects in the untreated fellow eye via systemic absorption. We have also observed what appeared to be an improvement in the untreated eye in a limited number of patients with retinitis pigmentosa who were treated with dorzolamide. However, inter-test variability of these procedures and the effects of learning would need to be evaluated before meaningful conclusions can be drawn.
In conclusion, the use of dorzolamide resulted in an improvement in VA, microperimetry sensitivity, and grating contrast sensitivity in our patient, in addition to a notable reduction in cystic macular lesions on spectral-domain OCT testing. These findings support a recommendation that a trial of this topical CAI should be considered in the treatment of cystic macular lesions in patients with the Enhanced S-cone syndrome.
Acknowledgments
Supported by funds from the Foundation Fighting Blindness, Owings Mills, Maryland; Grant Healthcare Foundation, Lake Forest, Illinois; NIH core grant EYO1792; NIH research grant EY019510 (JM), and an unrestricted departmental grant from Research to Prevent Blindness.
References
- 1.Haider NB, Jacobson SG, Cideciyan AV, Swiderski R, Streb LM, Searby C, Beck G, Hockey R, Hanna DB, Gorman S, Duhl D, Carmi R, Bennett J, Weleber RG, Fishman GA, Wright AF, Stone EM, Sheffield VC. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet. 2000;24:127–131. doi: 10.1038/72777. [DOI] [PubMed] [Google Scholar]
- 2.Michaelides M, Holder GE, Moore AT. Inherited retinal dystrophies. In: Taylor D, Hoyt GS, editors. Pediatric ophthalmology and strabismus. 2. New York: Elsevier Saunders; 2005. pp. 531–557. [Google Scholar]
- 3.Fishman GA. The enhanced S-cone syndrome. In: Fishman GA, Birch DG, Holder GE, Brigell MG, editors. Ophthalmology monograph 2-Electrophysiologic testing in disorders of the retina, optic nerve, and visual pathway. 2. Singapore: The American Academy of Ophthalmology; 2001. p. 120. [Google Scholar]
- 4.Jacobson SG, Marmor MF, Kemp CM, Knighton RW. SWS (Blue) cone hypersensitivity in a newly identified retinal degeneration. Invest Ophthalmol Vis Sci. 1990;31:827–838. [PubMed] [Google Scholar]
- 5.Marmor MF, Jacobson SG, Foerster MH, Kellner U, Weleber RG. Diagnostic clinical findings of a new syndrome with night blindness, maculopathy, and enhanced S cone sensitivity. Am J Ophthalmol. 1990;110:124–134. doi: 10.1016/s0002-9394(14)76980-6. [DOI] [PubMed] [Google Scholar]
- 6.Iannaccone A, Fung KH, Eyestone ME, Stone EM. Treatment of adult-onset acute macular retinoschisis in enhanced s-cone syndrome with oral acetazolamide. Am J Ophthalmol. 2009;147(2):307–312. doi: 10.1016/j.ajo.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajali M, Fishman GA. Dorzolamide use in the management of macular cysts in a patient with enhanced S-cone syndrome. Retinal Cases & Brief Reports. 2009;3(2):121–124. doi: 10.1097/ICB.0b013e31818faa21. [DOI] [PubMed] [Google Scholar]
- 8.Fishman GA, Apushkin MA. Continued use of dorzolamide for the treatment of cystoid macular oedema in patients with retinitis pigmentosa. Br J Ophthalmol. 2007;91(6):743–745. doi: 10.1136/bjo.2006.107466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung H, Hwang JU, Kim JG, Yoon YH. Optical coherence tomography in the diagnosis and monitoring of cystoid macular edema in patients with retinitis pigmentosa. Retina. 2006;26(8):922–927. doi: 10.1097/01.iae.0000250008.83779.23. [DOI] [PubMed] [Google Scholar]
- 10.Genead MA, Fishman GA, Walia S. Efficacy of sustained topical dorzolamide therapy for cystic macular lesions in patients with X-linked retinoschisis. Arch Ophthalmol. 2010;128(2):190–197. doi: 10.1001/archophthalmol.2009.398. [DOI] [PubMed] [Google Scholar]
- 11.Vujosevic S, Midena E, Pilotto E, Radin PP, Chiesa L, Cavarzeran F. Diabetic macular edema: correlation between microperimetry and optical coherence tomography findings. Invest Ophthalmol Vis Sci. 2006;47(7):3044–3051. doi: 10.1167/iovs.05-1141. [DOI] [PubMed] [Google Scholar]
- 12.Midena E, Vujosevic S, Convento E, Manfre’ A, Cavarzeran F, Pilotto E. Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration. Br J Ophthalmol. 2007;91(11):1499–1503. doi: 10.1136/bjo.2007.119685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaike N, Kita M, Tsujikawa A, Miyamoto K, Yoshimura N. Perimetric sensitivity with the microperimeter-1 and retinal thickness in patients with branch retinal vein occlusion. Am J Ophthalmol. 2007;143(2):342–344. doi: 10.1016/j.ajo.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Marmor MF, Holder GE, Seeliger MW, Yamamoto S. (update) Standard for clinical electroretinography. Doc Ophthalmol. 2004;108:107–114. doi: 10.1023/b:doop.0000036793.44912.45. [DOI] [PubMed] [Google Scholar]
- 15.Hee MR, Puliafito CA, Duker JS, Reichel E, Coker JG, Wilkins JR, Schuman JS, Swanson EA, Fujimoto JG. Topography of diabetic macular edema with optical coherence tomography. Ophthalmology. 1998;105(2):360–370. doi: 10.1016/s0161-6420(98)93601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mäntyjärvi M, Laitinen T. Normal values for the Pelli-Robson contrast sensitivity test. J Cataract Refract Surg. 2001;27(2):261–266. doi: 10.1016/s0886-3350(00)00562-9. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi T, Kitahara K. Optical coherence tomography in enhanced S-cone syndrome: large macular retinoschisis with disorganized retinal lamination. Eur J Ophthalmol. 2005;15(5):643–646. doi: 10.1177/112067210501500517. [DOI] [PubMed] [Google Scholar]
- 18.Audo I, Michaelides M, Robson AG, Hawlina M, Vaclavik V, Sandbach JM, Neveu MM, Hogg CR, Hunt DM, Moore AT, Bird AC, Webster AR, Holder GE. Phenotypic variation in enhanced S-cone syndrome. Invest Ophthalmol Vis Sci. 2008;49(5):2082–2093. doi: 10.1167/iovs.05-1629. [DOI] [PubMed] [Google Scholar]
- 19.Marmor MF. A teenager with night blindness and cystic maculopathy: enhanced S cone syndrome (Goldmann–Favre syndrome) Doc Ophthalmol. 2006;113:213–215. doi: 10.1007/s10633-006-9031-z. [DOI] [PubMed] [Google Scholar]
- 20.Sohn EH, Chen FK, Rubin GS, Moore AT, Webster AR, MacLaren RE. Macular Function Assessed by Microperimetry in Patients with Enhanced S-Cone Syndrome. Ophthalmology. 2010;117(6):1199–1206. doi: 10.1016/j.ophtha.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 21.Farber MD, Lam S, Tessler HH, Jennings TJ, Cross A, Rusin MM. Reduction of macular oedema by acetazolamide in patients with chronic iridocyclitis: a randomized prospective crossover study. Br J Ophthalmol. 1994;78:4–7. doi: 10.1136/bjo.78.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghajarnia M, Gorin MB. Acetazolamide in the treatment of X-linked retinoschisis maculopathy. Arch Ophthalmol. 2007;125:571–573. doi: 10.1001/archopht.125.4.571. [DOI] [PubMed] [Google Scholar]
- 23.Marmor MF. Hypothesis concerning carbonic anhydrase treatment of cystoid macular edema: example with epiretinal membrane. Arch Ophthalmol. 1990;108:1524–1525. doi: 10.1001/archopht.1990.01070130026013. [DOI] [PubMed] [Google Scholar]
- 24.Marmor MF, Maack T. Enhancement of retinal adhesion and subretinal fluid resorption by acetazolamide. Invest Ophthalmol Vis Sci. 1982;23:121–124. [PubMed] [Google Scholar]
- 25.Kawasaki K, Mukoh S, Yonemura D, Fujii S, Segawa Y. Acetazolamide-induced changes of the membrane potentials of the retinal pigment epithelial cell. Doc Ophthalmol. 1986;63:375–381. doi: 10.1007/BF00220229. [DOI] [PubMed] [Google Scholar]
- 26.Vaclavik V, Chakarova C, Bhattacharya SS, Robson AG, Holder GE, Bird AC, Webster AR. Bilateral giant macular schisis in a patient with enhanced S-cone syndrome from a family showing pseudo-dominant inheritance. Br J Ophthalmol. 2008;92(2):299–300. doi: 10.1136/bjo.2007.120055. [DOI] [PubMed] [Google Scholar]
- 27.Alexander KR, Barnes CS, Fishman GA. Characteristics of contrast processing deficits in X-linked retinoschisis. Vision Res. 2005;45(16):2095–2107. doi: 10.1016/j.visres.2005.01.037. [DOI] [PubMed] [Google Scholar]