Abstract
Introduction
Percutaneous coronary intervention (PCI) in patients with congenital coagulation factor deficiencies presents a unique challenge. They are not only at increased risk of perioperative bleeding but can also suffer thrombosis of the stent since preventive anticoagulation and antiplatelet therapy is difficult. Several cases of successful PCI have been described in patients with hemophilias A and B, but there are no reports in patients with combined coagulation factor deficiencies.
Aim
We used PCI to treat the coronary artery disease in a patient with the combined deficiency of factor V and factor VIII (F5F8D) and analyzed the molecular basis of the disorder for this patient.
Methods
A 68 year old patient was admitted for urgent PCI with bare metal stent placement after the diagnosis of the combined deficiency of F5F8D. Peripheral blood DNA was extracted for the sequence analysis of LMAN1 and MCFD2 genes. Mutation in LMAN1 was confirmed by molecular cloning of the PCR product and resequencing of the resulting clones.
Results
The patient underwent successful PCI with good long-term outcome. He tolerated well anticoagulation therapy with unfractionated heparin and double antiplatelet therapy while he was initially supported with fresh frozen plama and recombinant FVIII. Molecular analysis revealed that the patient carries unusual compound heterozygous frameshift mutations on the same microsatellite repeat region in exon 8 of LMAN1, one of which is a novel mutation (c.912delA).
Conclusion
Our results suggest that patients with F5F8D can safely undergo PCI for coronary artery disease, with the treatment individualized to the specific patient.
Introduction
Several cases of successful PCI have been described in patients with hemophilia A and B (1–7) but there are no reports in patients with combined coagulation factor deficiencies. Deficiencies in more than one coagulation factor can present additional challenges in the management of bleeding before and after the procedure and the prevention of stent thrombosis in the long term.
Combined deficiency of factor V and factor VIII (F5F8D) is a rare autosomal recessive disorder associated with both factor V (FV) and factor VIII (FVIII) levels in the 5–30% range. It is characterized by spontaneous bleeding including easy bruising, epistaxis and menorrhagia and excessive bleeding after trauma or surgery (8;9). Despite a deficiency of two key factors in coagulation, patients with F5F8D generally present bleeding symptoms similar to those of mild to moderate single factor deficiencies (10). F5F8D is caused by mutations in either LMAN1 or MCFD2 (11), resulting in the lack of either protein expression in cells. LMAN1 and MCFD2 bind to each other to form a complex that functions as a cargo receptor required for the efficient trafficking of FV and FVIII from the endoplasmic reticulum to the Golgi complex (12;13). The LMAN1-MCFD2 cargo receptor function is conserved in mammals as mice with LMAN1 deficiency also have decreased FV and FVIII levels in plasma (14). We report the first successful PCI with bare metal stent placement in a patient with F5F8D with good long term outcome.
Materials and Methods
Patient
A 68 year old white male (hereby designated as B29) presented with a three month history of dyspnea on exertion and palpitations lasting less than 5 minutes. A Thallium Stress Test showed anteroseptal ischemia with a Left Ventricular Ejection Fraction (LVEF) of 64%. He was then admitted for urgent PCI and stenting. His cardiac biomarkers were normal with a CK-Mb of 1.2, Troponin I of 0.3. Electrocardiogram (EKG) showed no evidence of acute myocardial infarction. Medical history revealed that he was diagnosed with FV deficiency at 18 years of age, after he had a large contusion on his upper extremity provoked by a motor vehicle accident. Earlier in life he reports minor episodes of mucosal bleeds with tooth brushing, but no episodes of hemarthroses, hematuria, gastroinstestinal bleeding or epistaxis. He remained asymptomatic until the age of 25 when he had prolonged bleeding after a tooth extraction, which required treatment with fresh frozen plasma (FFP) and hospitalization. Screening colonoscopy was successfully performed with prophylactic treatment with FFP at the age of 50, with the removal of a benign adenoma without excessive bleeding. Coagulation workup on admission revealed a FV level of 11% and a FVIII level of 7%, thus a correct diagnosis of F5F8D was made.
Mutation analysis
Peripheral blood sample was obtained in acid citrate dextran venous blood vacuum collection tubes from the proband. Study protocol was approved by the Cleveland Clinic Institutional Review Board on Human Subject Research. Informed consent was obtained from the proband in accordance with the Declaration of Helsinki. Genomic DNA was extracted from peripheral blood leukocytes. All exons and intron-exon junctions of the LMAN1 and MCFD2 genes were amplified by polymerase chain reaction (PCR) and sequenced using primers reported previously (15;16). The PCR product of exon 8 was cloned using the TA cloning kit (Invitrogen). Plasmid DNA was isolated from 10 individual colonies and subject to Sanger sequencing.
Results and Discussion
During stent placement, local hemostasis at the site of artery incision is of great concern, usually managed by repletion of the deficient factor to 60–80% of normal and by using local vascular closure systems (17;18). Because of the patient’s underlying coronary artery disease (CAD) we had to balance the risk of fluid overload versus hemorrhage. Our goal of FV and FVIII was >30%. Therefore, before cardiac stent placement, the patient was given a loading dose of 30 ml/kg of FFP. The levels of FV and FVIII were 37% and 31% respectively after the procedure. Our major postoperative concerns were local bleeding at the femoral artery site, acute thrombosis of the implanted stent and hypervolemia. During the next 24 hours, the patient was given 25 U/kg of recombinant FVIII (helixate FS c) at 12 and 24 hours post PCI with a resultant FVIII level of 71% at 36 hours post PCI. Given the 24 hour half life of FV and the uncomplicated asymptomatic course of our patient, no further FFP was given to support the FV levels. There were no signs of local bleeding at the femoral access site through the postoperative period.
In our patient a bare metal stent was chosen because it requires a shorter duration of dual antiplatelet therapy compared to drug eluting stents (one to three months vs 12 months) (19). During the procedure anticoagulation was achieved using 45u/kg unfractionated heparin (UFH). The PCI was performed using a 6 French GL3.5 Viking/Abbott® guiding catheter, the lesion was crossed with a 0.014″ Luge™ wire. Intravascular ultrasound was performed using an Atlantis SR Pro Catheter®. Pre-dilation was performed using a 2.75 × 10mm Angiosculpt® balloon at 10 atm, stenting was then performed using a 2.75 × 28 mm Vision® bare metal stent at 14 atm, the length was chosen to not only cover the critical stenosis but also a slightly distal stenotic region in the LAD. Post-dilation was performed using a 3.25 × 6 mm NC Stormer® balloon in the proximal and distal portion of the diseased vessel segment. The final result was excellent, with restored flow confirmed by both angiography (Figure 1) and intravascular ultrasound. Groin hemostasis was achieved with a 6 French Perclose® closure device without complication. The patient was treated with clopidogrel 75 mg PO daily for 30 days after the procedure, and he is to take aspirin 81 mg PO daily indefinitely. He was discharged home with a FVIII level of 49% and a FV level of 24% and did not receive any further product support as an outpatient. He underwent a nuclear stress tests at 3, 12 and 24 months post procedure with normal results; at 36 months post procedure the patient reports to us no symptoms and a full activity level. There is a shortage of data on the use of antiplatelet agents in hemophiliacs and in combined coagulation disorders. Their use during and after PCI is essential given that thrombosis of stents is a significant source of mortality, especially in the first few months following the procedure. The incidence of bare metal stent thrombosis has been estimated in the range of 0.5–2%. It has been postulated that in patients with classic hemophilia, platelet adhesion and aggregation is generally intact and therefore these patients suffer similar risks of rethrombosis (19;20). Bovenzi F. et al. reports a case of a patient with hemophilia B, pretreated with heparin but not with aspirin, who was found to have acute coronary occlusion during stent implantation (7). Our patient has been thrombosis free and no cardiac problems since undergoing PCI with bare metal stent in October 2008 with dual antiplatelet therapy for 30 days and aspirin therapy long term.
Figure 1.
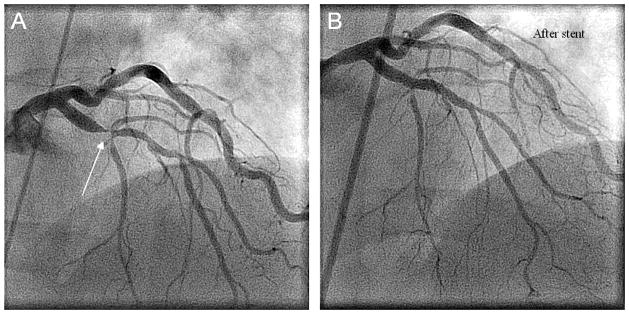
Angiographic images taken during percutaneous coronary intervention. A) Pre-stenting imaging depicting critical stenosis (white arrow) of the left anterior descending coronary artery (LAD). B) Post-stenting imaging showing excellent flow through the LAD.
Sequencing revealed overlapping peaks following a string of adenines in exon 8 of LMAN1, suggesting heterozygosity due to the length variations of this mononucleotide repeat, which normally contains a repeat of 9 adenines [(A)9] from nucleotide 904 to 912. No other mutations/polymorphisms were identified in LMAN1 or MCFD2. To confirm mutations, we cloned PCR products of exon 8 from the patient and identified two populations: one with mononucleotide repeat of (A)8 and the other with (A)10. No wild-type clones were identified in the 10 clones analyzed. These results indicate that the patient is compound heterozygous with (A)10 on one chromosome (c.912-913insA), and (A)8 on the other chromosome (c.912delA). The c.912delA mutation is a novel mutation, while the c.912-913insA mutation has been reported previously in patients of Iranian origin (21). Both mutations cause frameshift of the open reading frame of LMAN1 and premature stop of translation. In the case of c.912delA, a stop codon occurs 22 amino acid residues after the mononucleotide repeat. Our patient had FV and FVIII around 10% with mild spontaneous bleeding and excessive bleeding upon trauma, which are typical clinical presentations of F5F8D (10). As reported previously, the levels of FV and FVIII correlate poorly with bleeding symptoms (22), and are therefore not useful in predicting bleeding severity.
Compound heterozygosity usually results from inheritance of two different mutations from both parents. However, it is unusual to find two different mutations at the same location of the gene in the same patient. Another possibility is that one of the mutations was a de novo mutation, considering that the (A)9 microsatellite repeat is probably a mutation hot spot, with three mutations already identified at this location in patients of distinct origins (c.912-913insA, c.912delA and c.904A>T) (21;23). Unfortunately DNA samples from his parents were not available for analysis. Interestingly, this same (A)9 repeat was recently reported to be frequently mutated in microsatellite unstable colon cancer cell lines and tissues (24).
Conclusion
We report on successful PCI with a bare metal stent for CAD in a patient with a moderate bleeding diathesis due to F5F8D caused by novel compound heterozygous LMAN1 mutations. The patient has a history of spontaneous bleeding and excessive bleeding upon trauma and tooth extraction. After initially supported with FFP and recombinant FVIII, he tolerated well anticoagulation therapy with UFH and double antiplatelet therapy after surgery. Our experience suggests that patients with F5F8D can safely undergo PCI for CAD, with treatment individualized to the specific patient.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (HL094505) to BZ. The authors wish to thank Xiaolan Zhao for her help with DNA sequencing.
Footnotes
Author contributions
VM created the coagulation factor therapy protocol and RAL performed the PCI and angiographic images. AJP assisted with patient care and HHL performed molecular analysis; AJP and BZ wrote the paper.
Disclosures
The authors have no competing interests.
References
- 1.Arora UK, Dhir M, Cintron G, Strom JA. Successful multi-vessel percutaneous coronary intervention with bivalirudin in a patient with severe hemophilia A: a case report and review of literature. J Invasive Cardiol. 2004;16:330–332. [PubMed] [Google Scholar]
- 2.Krolick MA. Successful percutaneous coronary intervention in a patient with severe haemophilia A using bivalirudin as the sole procedural anticoagulant. Haemophilia. 2005;11:415–417. doi: 10.1111/j.1365-2516.2005.01103.x. [DOI] [PubMed] [Google Scholar]
- 3.Petrillo G, Cirillo P, Leosco D, Maresca F, Piscione F, Chiariello M. Percutaneous coronary intervention in a patient with acute non-ST-elevation myocardial infarction and haemophilia A: a ‘genous’ experience. Haemophilia. 2011;17:e245–e246. doi: 10.1111/j.1365-2516.2010.02355.x. [DOI] [PubMed] [Google Scholar]
- 4.Quintero D, Biria M, Meyers DG. Percutaneous coronary intervention in a patient with acute ST-elevation myocardial infarction and hemophilia A. J Invasive Cardiol. 2008;20:240–241. [PubMed] [Google Scholar]
- 5.Smolka G, Kulach A, Dabek J, Szulc A, Gasior Z. Percutaneous coronary intervention with stent implantation in haemophilic A patient with unstable angina. Haemophilia. 2007;13:428–431. doi: 10.1111/j.1365-2516.2007.01436.x. [DOI] [PubMed] [Google Scholar]
- 6.Virtanen R, Kauppila M, Itala M. Percutaneous coronary intervention with stenting in a patient with haemophilia A and an acute myocardial infarction following a single dose of desmopressin. Thromb Haemost. 2004;92:1154–1156. [PubMed] [Google Scholar]
- 7.Bovenzi F, De LL, Signore N, Fusco F, de LI. Abciximab for the treatment of an acute thrombotic coronary occlusion during stent implantation in a patient with severe hemophilia B. Ital Heart J. 2003;4:728–730. [PubMed] [Google Scholar]
- 8.Spreafico M, Peyvandi F. Combined FV and FVIII deficiency. Haemophilia. 2008;14:1201–1208. doi: 10.1111/j.1365-2516.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B. Recent developments in the understanding of the combined deficiency of FV and FVIII. Br J Haematol. 2009;145:15–23. doi: 10.1111/j.1365-2141.2008.07559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B, Ginsburg D. Familial multiple coagulation factor deficiencies. In: Colman RW, Marder VJ, Clowes AW, George JN, Goldhaber SZ, editors. Hemostasis and Thrombosis, Basic Principles and Clinical Practice. Lippincott Williams & Wilkins; Philadelphia: 2006. pp. 953–960. [Google Scholar]
- 11.Zhang B, McGee B, Yamaoka JS, Guglielmone H, Downes KA, Minoldo S, Jarchum G, Peyvandi F, de Bosch NB, Ruiz-Saez A, et al. Combined deficiency of factor V and factor VIII is due to mutations in either LMAN1 or MCFD2. Blood. 2006;107:1903–1907. doi: 10.1182/blood-2005-09-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng C, Liu HH, Zhou J, Zhang B. EF hand domains of MCFD2 mediate interactions with both LMAN1 and coagulation factor V or VIII. Blood. 2010;115:1081–1087. doi: 10.1182/blood-2009-09-241877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng C, Liu HH, Yuan S, Zhou J, Zhang B. Molecular basis of LMAN1 in coordinating LMAN1-MCFD2 cargo receptor formation and ER-to-Golgi transport of FV/FVIII. Blood. 2010;116:5698–6706. doi: 10.1182/blood-2010-04-278325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, Zheng C, Zhu M, Tao J, Vasievich MP, Baines A, Kim J, Schekman R, Kaufman RJ, Ginsburg D. Mice deficient in LMAN1 exhibit FV and FVIII deficiencies and liver accumulation of {alpha}1-antitrypsin. Blood. 2011;118:3384–3391. doi: 10.1182/blood-2011-05-352815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols WC, Seligsohn U, Zivelin A, Terry VH, Hertel CE, Wheatley MA, Moussalli MJ, Hauri HP, Ciavarella N, Kaufman RJ, et al. Mutations in the ER-Golgi intermediate compartment protein ERGIC-53 cause combined deficiency of coagulation factors V and VIII. Cell. 1998;93:61–70. doi: 10.1016/s0092-8674(00)81146-0. [DOI] [PubMed] [Google Scholar]
- 16.Zhang B, Cunningham MA, Nichols WC, Bernat JA, Seligsohn U, Pipe SW, Mcvey JH, Schulte-Overberg U, de Bosch NB, Ruiz-Saez A, et al. Bleeding due to disruption of a cargo-specific ER-to-Golgi transport complex. Nat Genet. 2003;34:220–225. doi: 10.1038/ng1153. [DOI] [PubMed] [Google Scholar]
- 17.Lim MY, Pruthi RK. Outcomes of management of acute coronary syndrome in patients with congenital bleeding disorders: A single center experience and review of the literature. Thromb Res. 2012;130:316–322. doi: 10.1016/j.thromres.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 18.Schutgens RE, Tuinenburg A, Roosendaal G, Guyomi SH, Mauser-Bunschoten EP. Treatment of ischaemic heart disease in haemophilia patients: an institutional guideline. Haemophilia. 2009;15:952–958. doi: 10.1111/j.1365-2516.2009.02020.x. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura M, Yamashita T, Yajima J, Oikawa Y, Ogasawara K, Kirigaya H, Nagashima K, Sawada H, Aizawa T. Long-term safety and efficacy of sirolimus-eluting stents in Japanese patients: a single-center cohort study. J Invasive Cardiol. 2009;21:526–531. [PubMed] [Google Scholar]
- 20.Wenaweser P, Dorffler-Melly J, Imboden K, Windecker S, Togni M, Meier B, Haeberli A, Hess OM. Stent thrombosis is associated with an impaired response to antiplatelet therapy. J Am Coll Cardiol. 2005;45:1748–1752. doi: 10.1016/j.jacc.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 21.Neerman-Arbez M, Johnson KM, Morris MA, Mcvey JH, Peyvandi F, Nichols WC, Ginsburg D, Rossier C, Antonarakis SE, Tuddenham EGD. Molecular analysis of the ERGIC-53 gene in 35 families with combined factor V factor VIII deficiency. Blood. 1999;93:2253–2260. [PubMed] [Google Scholar]
- 22.Seligsohn U, Zivelin A, Zwang E. Combined factor V and factor VIII deficiency among non-Ashkenazi Jews. N Engl J Med. 1982;307:1191–1195. doi: 10.1056/NEJM198211043071907. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B, Spreafico M, Yang A, Platzer P, Callaghan MU, Avci Z, Ozbek N, Mahlangu J, Haw T, Kaufman RJ, et al. Genotype-phenotype correlation in combined deficiency of factor V and factor VIII. Blood. 2008;111:5592–5600. doi: 10.1182/blood-2007-10-113951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roeckel N, Woerner SM, Kloor M, Yuan YP, Patsos G, Gromes R, Kopitz J, Gebert J. High frequency of LMAN1 abnormalities in colorectal tumors with microsatellite instability. Cancer Res. 2009;69:292–299. doi: 10.1158/0008-5472.CAN-08-3314. [DOI] [PubMed] [Google Scholar]


