Abstract
Estrogens play a salient role in the development and maintenance of both male and female nervous systems and behaviors. The plainfin midshipman (Porichthys notatus), a teleost fish, has two male reproductive morphs that follow alternative mating tactics and diverge in multiple somatic, hormonal and neural traits, including the central control of morph-specific vocal behaviors. After we identified duplicate estrogen receptors (ERβ1 and ERβ2) in midshipman, we developed antibodies to localize protein expression in the central vocal-acoustic networks and saccule, the auditory division of the inner ear. As in other teleost species, ERβ1 and ERβ2 were robustly expressed in the telencephalon and hypothalamus in vocal-acoustic and other brain regions shown previously to exhibit strong expression of ERα and aromatase (estrogen synthetase, CYP19) in midshipman. Like aromatase, ERβ1 label co-localized with glial fibrillary acidic protein (GFAP) in telencephalic radial glial cells. Quantitative PCR revealed similar patterns of transcript abundance across reproductive morphs for ERβ1, ERβ2, ERα and aromatase in the forebrain and saccule. In contrast, transcript abundance for ERs and aromatase varied significantly between morphs in and around the sexually polymorphic vocal motor nucleus (VMN). Together, the results suggest that VMN is the major estrogen target within the estrogen-sensitive hindbrain vocal network that directly determines the duration, frequency and amplitude of morph-specific vocalizations. Comparable regional differences in steroid receptor abundances likely regulate morph-specific behaviors in males and females of other species exhibiting alternative reproductive tactics.
Keywords: duplicate estrogen receptors, auditory, vocal, midshipman fish, central pattern generator
INTRODUCTION
Steroids, including estrogen, are critical in the development and function of the vertebrate nervous system in both males and females (e.g., Arnold et al., 2003; Morris et al., 2004; Remage-Healey and Bass, 2006a; Wu et al., 2009; Diotel et al., 2011; Forlano and Bass, 2011). Estrogen binds to and activates estrogen receptors (ERs) that function as transcription factors to regulate gene expression (Means and O’Malley, 1972; Nilsson et al., 2001). Hence, elucidating how estrogens influence behavioral phenotypes requires identification and localization of ERs in regions of the peripheral and central nervous systems (PNS and CNS, respectively) that are involved in producing the behavioral output. Most vertebrates possess two ER subtypes: ERα and ERβ. Teleost fish have two ERβ isoforms, ERβ1 and ERβ2 (Ma et al., 2000; Hawkins et al., 2000; Menuet et al., 2002), though only a single ERα has been maintained in most teleost species (except see Nagler et al., 2007). All of these ER subtypes are nuclear receptors, though evidence suggests that these and other receptors may also function in rapid, membrane-initiated responses to estrogen (Pedram et al., 2006; Dominguez and Micevych, 2010; Thomas et al., 2005). Here, we investigated ERβ expression in the PNS and CNS of the plainfin midshipman fish (Porichthys notatus), a vertebrate model for neural and hormonal control of alternative male reproductive tactics and vocal-acoustic communication (Bass, 1996; Bass and Remage-Healey, 2008; Forlano and Bass, 2011). After cloning midshipman ERβ1 and ERβ2, we developed antibodies to localize each ERβ isoform and used quantitative real-time PCR to measure transcript abundance in PNS and CNS regions that have been focal points for investigation of the neural basis of alternative mating tactics and acoustic communication in midshipman.
Midshipman fish show annual cycling in androgens and estrogens (Sisneros et al., 2004a) corresponding to seasonal variation in reproductive states, vocal neurophysiology (Rubow and Bass 2009), and auditory frequency sensitivity in the saccule, the main auditory division of the inner ear in midshipman and many teleost fish (Bass and McKibben, 2003; Sisneros and Bass, 2003; Sisneros, 2009; Rohmann and Bass 2011). Androgen receptor β (ARβ) and ERα transcripts were previously localized to vocal and auditory regions of the CNS and to the saccule (Forlano et al., 2005, 2010; also see Genova et al., 2012 for AR phylogeny). Additionally, aromatase (estrogen synthetase, CYP19) is abundant in these same regions, suggesting a role for local aromatase-dependent conversion of testosterone to estrogen in vocal-acoustic mechanisms (Forlano et al., 2001). Estrogen influences both auditory (Sisneros et al., 2004b) and vocal (Remage-Healey and Bass, 2004, 2007) neurophysiology in midshipman, traits shared with tetrapods and thus having broad functional and evolutionary significance among vertebrates (e.g., Yovanof and Feng, 1983; Kim et al., 2002, Kilicdag et al., 2004; Hultcrantz et al., 2006; Bass et al., 2008; Simonoska et al., 2009).
Studies of vocal mechanisms, in particular, have also been conducted in midshipman fish in the context of alternative male reproductive tactics. Midshipman have territorial type I and sneak or satellite-spawning type II male morphs distinguished by their vocal repertoires (Bass and Marchaterre, 1989a; Brantley and Bass, 1994; Bass et al., 2008). Both male morphs as well as females produce brief (msec) agonistic “grunts”, whereas only type I males generate long duration (sec - > 1h) “hums” during courtship and agonistic “growls” (msec-sec) during aggressive encounters with other males. Though intrasexual alternative tactics occur frequently among vertebrates (Mank and Avise, 2006; Oliveira et al., 2008a), little is known about the neural mechanisms that produce this form of intrasexual behavioral variation (Bass and Forlano, 2008; Oliveira et al., 2008b; Bass and Grober, 2009). Alternative tactics in midshipman and other vertebrates are linked to differences in circulating levels of steroid hormones (e.g., Brantley et al., 1993; Duckworth et al., 2004; Knapp and Neff, 2007; Schradin and Yeun, 2011), suggesting an important role of steroids in determining and maintaining morph-specific behavior, most likely through divergent actions in behaviorally relevant brain regions. It has been shown that steroids, including estrogen, have both rapid (within 5 min) and sustained (30 min to 2 h) effects on vocal neurophysiology in a male morph-specific pattern (Remage-Healey and Bass, 2004, 2007). What has been lacking in these studies, and what we now report, is that such functional differences are coupled to morph-specific patterns in the abundance of estrogen receptors (ERα, ERβ2) and aromatase in the hindbrain vocal pattern generator network. In sum, we have now identified the complete complement of known teleost estrogen receptors in the plainfin midshipman, mapped sites in the PNS and CNS where they are abundantly expressed, and identified inter-morph variation in estrogen receptor and aromatase abundances.
MATERIALS AND METHODS
Animals
Midshipman fish used in this study were collected within the tidal zone near Bodega, CA during the spring and summer reproductive period and from deep-water trawls in Monterey Bay, CA during the winter non-reproductive period. The fish were held in saltwater tanks until they were sacrificed and perfused for these experiments. All animals were sacrificed within 10 days of collection to minimize the effects of captivity. Midshipman fish held for comparable and longer periods maintain distinct seasonal/reproductive physiology in previous studies (e.g., Sisneros and Bass 2003). The animals were primarily from the breeding season when plasma levels of estrogen, and the androgens 11-ketotestosterone and testosterone peak (Brantley et al., 1993; Knapp et al., 1999; Sisneros et al., 2004a; Genova et al., 2012), nesting males produce long duration advertisement hums (Brantley and Bass, 1994), and peripheral auditory physiology shows maximum sensitivity (Sisneros and Bass 2003). Each fish was unambiguously identified as female, type I male, or type II male upon sacrifice based on body size and coloration, relative size of the gonads, and appearance of the swim bladder vocal muscle (see Bass, 1996). The mean ± SEM of the standard lengths and gonadosomatic indices (GSI), respectively, for the fish used in this study were 12.6 ± 0.25 cm and 14.9 ± 2.55 for females, 15.3 ± 0.52 cm and 1.1 ± 0.18 for type I males, and 9.61 ± 0.39 cm and 8.8 ± 1.42 for type II males. All protocols were approved by Cornell University Institutional Animal Care and Use Committee.
Estrogen Receptor Sequencing
To obtain the 3’ sequences for both ERβ receptors from midshipman we used degenerate PCR followed by 3’ RACE. Degenerate PCR was performed using primers containing restriction sites for easy cloning of the PCR products. The degenerate primer sequences are listed in Table 1, with underlined regions indicating NotI and EcoRI restriction sites, respectively. PCR with these primers produced a band of approximately 1kb, which was cloned into pBluescript vector via the NotI and EcoRI restriction sites. Individual colonies were selected and sequenced, producing partial sequences for portions of a previously reported ERα (Forlano et al., 2005; Accession # AY466470) and an ERβ (Accession #: FJ940749) as well as a previously unreported ERβ.
Table 1.
Primer sequences, 5’ to 3’, used to amplify and clone estrogen receptors; underlined regions indicate restriction enzyme cut sites.
| Degenerate Primers | |
| F | ATGCGGCCGCTAYCAYTAYGGNGTNTGG |
| R | AGAATTCTRTKNGCRTCNARCATYTC |
| 3’ RACE Primers | |
| QT | CCAGTGAGCAGAGTGACGAGGACTCGAGCTCAAGCT(17)VN |
| Q0 | CCAGTGAGCAGAGTGACG |
| QI | GAGGACTCGAGCTCAAGC |
| ERα | ACTCCGGAGCCTTTTCCTTC |
| ERα | ACATTCGCCACATGAGCAAC |
| ERβ10 | GCGGTGTGCGAAAAGAGCGTGGGAC |
| ERβ1I | CTGGGCCAAGAAGATTCCAG |
| ERβ20 | CATTCAACCATGAGCCCAGA |
| ERβ2I | ACGCATCATGGATGCTGAAC |
| GST - Fusion Primers | |
| ERβ1F | TACCATGGATGCTCACATCATGCGCAGCT |
| ERβ1R | TTCTCGAGCTACTGGGGTTCAGGGTCAC |
| ERβ2F | TTCCATGGTGTTGGATGCCAACACATCCAGC |
| ERβ2R | GTCTCGAGTTACCCCTGTAAGGTGGTTTCC |
We used 3’ RACE following the method of Scotto-Lavino et al. (2007) to obtain the 3’ends of the three ERs. Briefly, RNA was reverse transcribed using the tagged oligo-dT primer QT (Table 1). Following cDNA synthesis, two rounds of PCR were performed using nested primers designed to specifically amplify each of the ER transcripts. The reverse primers (Q0 and QI) bind within the tagged region of QT. The forward primers were all specific to one of the ERα or ERβ transcripts. Following the second nested PCR reactions, the products were cloned into the TOPO TA vector (Invitrogen) and sequenced.
A phylogeny of teleost ERs was used to assign names to the ERβs that are most consistent with previously named ERs. We produced the phylogeny of teleost ERs with MrBayes 3.1.2 (Huelsenbeck and Ronquist, 2001) at the Computational Biology Service Unit at Cornell University. The nucleotide alignment used for this analysis was generated with ClustalW based on deduced amino acid sequences. We used a general time reversible model with invariable sites and a gamma distribution for variable rate sites (GTR+I+G). Four Markov chains of 1,000,000 generations, sampled every 100 generations, with a burn-in of 25% were used. A majority rule consensus tree and posterior probabilities were generated. We assigned the names ERβ1 and ERβ2 to the midshipman receptors based on the most frequent name of receptors in the taxonomic clade in which it fell. The partial receptor sequences were uploaded to GenBank.
Antibody Production and Purification
Two antibodies were produced against GST-fusion proteins of the highly diverged C-terminal tails (F domains) of the two midshipman ERβs (Table 2). To produce the fusion proteins, the 3’ ends of ERβ1 and ERβ2 were PCR amplified using primers listed in Table 1, which contain NcoI and XhoI restriction sites. The PCR products were digested and cloned into the pGEX-KG vector. Stop codons were included in the reverse primers. The constructs were transformed into BL21(DE3) competent cells, grown in LB medium with ampicillin, and induced with IPTG to produce the GST-fusion proteins. The bacteria were lysed using EasyLyse (Epicentre Biotechnologies), the GST-fusions proteins were purified using glutathione beads, and the purified proteins dialyzed against PBS. The fusion proteins were used by Pocono Rabbit Farm and Laboratory Inc. (PRF&L, Canadensis, PA) to produce rabbit anti-ERβ1 and guinea pig anti-ERβ2 polyclonal antibodies.
Table 2.
Two antibodies were produced against GST-fusion proteins of the C-terminal tails of the two midshipman ERβs.
| Antibody | Immunogen Sequence | Manufacturer, Host Species, Type |
|---|---|---|
| pnERβ1 | AHIMRSSRLPRQPPPQESTDQREAPDRPQPSESGPLNACPAGSSDPEPQ | PRF&L, Rabbit, Polyclonal |
| pnERβ2 | ANTSSSSSQTSASPPSSDTYFDQHQYTQPSGQDQTSVPLHGPAESPILDQHLQTLSLQSTPPPHGLAGTHMGTNDYINPESWQLGTVDTDPSVEATEYILPDQVIRETTLQG | PRF&L, Guinea pig, Polyclonal |
The antibody containing sera were purified by column chromatography. GST, GST- ERβ1, and GST-ERβ2 fusion proteins were each bound to a mix of Affi-Gel 10 and Affi-Gel 15 beads (Bio-Rad) using 1.25 mg of protein with 1 ml of each Affi-Gel in PBS. Following incubation for 6 hr at 4°C with mixing, we added 2 ml of 0.2M ethanolamine for the last hour to block unbound sites on the Affi-Gel beads. The beads were then placed in columns.
To purify the sera, 2 ml of each serum were mixed with 8 ml of 10 mM Tris pH 7.4, 150mM NaCl. These mixtures were incubated for 30 min with GST Affi-Gel columns to remove some of the GST antibodies and the eluate was collected. Each eluate was then incubated with the corresponding GST- ERβ1 or GST- ERβ2 column and incubated overnight at 4°C. The columns were drained and rinsed with excess 10mM Tris pH 7.4, 150mM NaCl. The antibodies were then eluted from the columns with sequential 1 ml washes of 0.1M glycine pH 2.8 collected in tubes containing 50 Ul of 1M Tris pH 8.0. The OD280 of each fraction was examined and the most concentrated fractions were diluted 1:1 with 200mM NaCl, 10 mg/ml BSA, 20mM NaN3, 50% glycerol. The purified antibodies were stored at −20°C. The specificities of these antibodies were verified by comparison with the pre-immune serum on western blots and by preadsorption of the antibodies with the GST-fusion protein antigens. Preadsorption of the primary antibody, no primary, and no secondary controls all fully eliminated the immunohistochemical staining signals.
Antibody Characterization
To characterize the ERβ1 antibody, several approaches were utilized. Western blots performed with protein isolated from midshipman whole-brain lysate produced a single ~37 kDa band when extractions were performed with 1X Halt protease inhibitors (Thermo Scientific). When extractions were performed with 10X protease inhibitor, the ~37 kDa band was weaker and a second band of the expected ~65 kDa appeared. This suggested that the antibody recognized the ERβ1 protein and that ERβ1 was susceptible to rapid protease activity. Preadsorption of the primary antibody with an excess of the GST fusion prior to immunohistochemistry (IHC) abolished the signal.
Similar approaches were used to characterize the ERβ2 antibody. Western blots performed with protein isolated from midshipman whole-brain lysate produced a single ~42 kDa band when extractions were performed with 1X or 10X Halt protease inhibitors (Thermo Scientific). The smaller than expected (~65 kDa) size of this band is likely due in part to the high proteolytic activity evidenced by Western blotting of ERβ1, which could not be fully inhibited. The ~42 kDa band may also be the truncated product of the smaller transcript isoforms of ERβ2 previously reported (Socorro et al., 2000; Menuet et al., 2004). To further support the specificity of the antibody, we used qPCR (see below) to corroborate morph specific expression variation of ERβ2 based on antibody staining. The tissue and morph specific immunohistochemical staining was consistent with qPCR results for ERβ2, as shown for morph-specific expression in the VMN (see Results). We also performed preadsorption of the ERβ2 antibody with an excess of the GST fusion protein. This treatment eliminated staining in IHC.
Immunohistochemistry
After deep anesthesia by immersion in benzocaine (0.025%), animals were perfused transcardially with a 4% paraformaldehyde (PF) solution in phosphate buffer (PB, pH 7.2). The sensory epithelium, nearby ganglion cells and eighth nerve afferents of the saccule division of the inner ear (see Forlano et al., 2005), along with the CNS, were then removed from the skull, post-fixed for 1 hr in PF-PB and then stored in PB. The saccule was isolated from the remainder of the inner ear as it is the main auditory division of the inner ear in midshipman, as in many teleost fish (see Bass and McKibben, 2003). The tissues were cryoprotected by incubating overnight at 4°C in 30% sucrose in PB before being frozen in Cryo-M-bed (Hacker Instruments) and sectioned at 30 Um in either the transverse (CNS, saccule) or sagittal (saccule) plane. Sections were mounted on positively charged Plus slides (Erie Scientific) and stored at −80°C until used for IHC. Some whole-mount (non-sectioned) saccule staining was performed, in which case the saccule was not incubated with either sucrose or frozen, but rather taken directly from PB through the various IHC stages (see below).
Both fluorescent and enzyme based (diaminobenzidine, DAB) labeling were used to localize proteins within the CNS and saccule. For both approaches, the tissue sections or whole saccules were washed twice for 10 min at room temperature in phosphate buffered saline (PBS) followed by a 15 min incubation in PBS, 1% BSA, 0.3% Triton X-100 to permeabilize the tissue and block non-specific binding. The tissues were then incubated 4 hr to overnight at room temperature with primary antibodies diluted in PBS, 1% BSA, 0.3% Triton X-100. The primary antibodies (and dilutions) used were: guinea pig anti-ERβ1 (1:50), rabbit anti-ERβ2 (1:50), mouse anti-acetylated tubulin (1:2000; Sigma, T6793), mouse anti-glial fibrillary acidic protein (GFAP, 1:1000; Chemicon, MAB360), and the mouse anti-hair cell HCS-1 antibody (1:500, donated by J. Corwin). After incubation with the primary antibodies, tissues were washed three times with PBS, 1% BSA.
For fluorescent labeling, the tissues were then incubated 1–3 hours in Alexa Fluor- conjugated secondary antibody(ies) (Invitrogen) diluted 1:1500 in PBS, 1% BSA. The tissues were then washed three times with PBS. In some cases NeuroTrace blue fluorescent Nissl stain (Invitrogen) was used to visualize neurons and Alexa Fluor 488 or Rhodamine conjugated phalloidin (Invitrogen) used to visualize actin. The NeuroTrace staining was done by incubating 20 min in NeuroTrace diluted 1:200 in PBS, 1% BSA. The phalloidin was diluted 1:50 in PBS, 1% BSA and incubated for 20–30 min. The tissues were washed again with PBS and mounted with VectaShield (Vector Labs). Autofluorescence was checked using unlabeled tissue and, when autofluorescence occurred, unlabeled tissue was used to adjust exposure times to account for autofluorescence.
For DAB labeling, we used Vector Labs biotinylated secondary antibodies and VECTASTAIN Elite ABC kits. The tissue sections were incubated 1 hour in secondary antibody diluted 1:200 in PBS, 0.5% BSA, washed twice with PBS, and incubated 1 hour in ABC solution. After two washes in PB, the sections were incubated for approximately 2 to 5 min in a solution of 0.1% DAB, 0.01% H2O2 in PB. Tissues were counterstained with cresyl violet, dehydrated with increasing ethanol concentrations, cleared with Histo-Clear (National Diagnostics), and mounted.
Quantitative Real-Time PCR
We used quantitative real-time PCR (qPCR) to compare transcript abundance of estrogen receptors and aromatase in the saccule and CNS among the three alternative reproductive morphs of plainfin midshipman: type I males (n=7), type II males (n=6), and females (n=7). The saccular epithelia from both ears were pooled for each individual fish. All fish used for qPCR were collected during the breeding season and sacrificed five to six days after collection. All tissues were immediately removed from sacrificed fish and treated with RNAlater (Ambion, Inc.) to prevent RNA degradation. Transcript abundance was examined in the forebrain, vocal motor nucleus (VMN), hindbrain region surrounding the VMN, and the auditory saccule. As in comparable analyses (e.g., Forlano et al., 2010), the forebrain was separated from the remaining CNS by a transverse cut at the boundary between the forebrain and the midbrain roof; the sampled region includes the olfactory bulbs, telencephalon and most of the preoptic area. Also as in prior studies, the caudal hindbrain and spinal cord were separated from the remaining CNS by a transverse cut just rostral to the cerebellum followed by removal of the cerebellum from the hindbrain. We focused on the forebrain and VMN because of our studies showing abundant aromatase, ARβ, ERα and neuropeptides in these regions (Schlinger et al., 1999; Goodson et al., 2003; Forlano et al., 2001, 2005, 2010), forebrain influences on vocal motor patterning (Goodson and Bass, 2000; Kittelberger et al., 2006) and steroid modulation of VMN output (Remage- Healey and Bass, 2004, 2007). The paired, cigar-shaped VMN together contain 3,000–4,000 motoneurons embedded in a dense network of myelinated and unmyelinated fibers that together give the appearance of a single midline nucleus (Bass and Marchaterre, 1989b; Bass and Andersen, 1991). After preserving tissue in RNAlater, we found that we could remove the paired VMN in toto, separating them from the surrounding hindbrain region referred to below as hindbrain without VMN (verified in sectioned material).
We purified RNA from the three isolated CNS regions (forebrain, VMN, hindbrain without VMN) and the saccule using Trizol reagent (Invitrogen). Potential contaminating genomic DNA was digested using the DNA-free kit (Ambion, Inc.) and the RNA was reverse transcribed by random priming with SuperScript III Reverse Transcriptase (Invitrogen). We performed relative absolute qPCR with PowerSybr (Applied Biosystems) on a ViiA 7 Real-Time PCR system (Applied Biosystems) in the Cornell University Life Sciences Core Laboratories. We found no significant morph-specific variation in 18S rRNA abundance in the forebrain (F2,16=0.58, p=0.57), hindbrain (VMN removed; F2,17=0.57, p=0.57) or saccule (F2,17=3.14, p=0.07), and thus we used 18S to normalize transcript abundances in these tissues. There was, however, significant inter-morph variation in 18S abundance within the VMN (F2,17=8.66, p<0.01). We thus used the gene RING finger protein 13 (RNF13), which does not vary by morph in the VMN (F2,17=0.45, p=0.64), to normalize abundance in VMN. Normalized data were examined to ensure normality and homogeneity of variance, and were log transformed when necessary to meet the assumptions of ANOVA. Results were not normalized to the volumes of the neuroanatomical regions or the cells that may have varied among reproductive morphs (Bass et al., 1996). ANOVA was used to determine statistical significance of variation among the reproductive morphs and when appropriate Tukey HSD pairwise comparisons were used to assess differences between groups. For graphical visualization of the qPCR results on the same y-axis, data were standardized to the highest expression level for each gene and reproductive morph.
RESULTS
Sequencing of Midshipman Estrogen Receptors
Using degenerate PCR followed by 3’ RACE, we amplified the 3’ end of three ERs. Phylogenetic analyses indicated that these receptor sequences corresponded to the receptors ERα, ERβ1, and ERβ2 found in other teleost fishes. The sequences of these receptors have been cataloged in GenBank with accession numbers JF965424 (ERβ1), JF965425 (ERβ2), and JF965426 (ERα). The partial sequences of each ER consisted of substantial portions of domain C as well as full sequences of domains D, E and F. Domains C and E are conserved among different estrogen receptors and species, while domains D and F are highly variable.
Immunohistochemical Localization
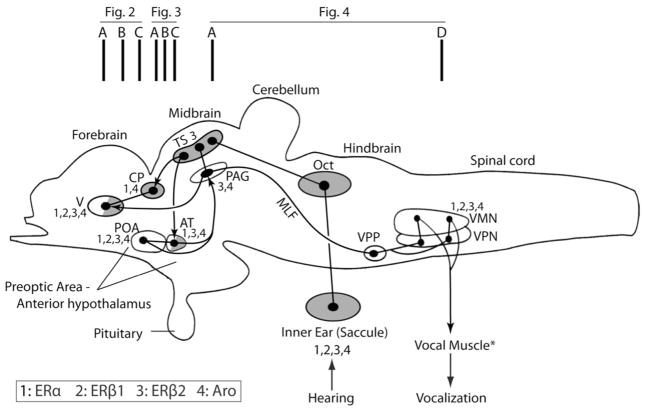
We observed some apparent low level staining above that of the negative controls for ERβ1 and/or ERβ2 in most brains sections examined. As illustrated in figures 2–4, we report only robust, unambiguous staining substantially above that of the background, suggesting particularly strong estrogen sensitivity. As noted earlier, we focused our attention on the extensively studied vocal-acoustic (VAC) network in midshipman (Goodson and Bass, 2002; Bass and McKibben, 2003; Bass and Chagnaud, 2011, 2012; Kittelberger and Bass, 2012), although we note other regions showing prominent labeling. The VAC network includes a vocal pattern generator comprised of three nuclei: a midline vocal motor nucleus (VMN, Fig. 1) that extends across the hindbrain-spinal cord transition, innervates the ipsilateral vocal muscle attached to the swim bladder and codes for vocal amplitude; a vocal pacemaker nucleus (VPN, Fig. 1) that projects to VMN and codes for call frequency/pulse repetition rate (equivalent characters in fish); a vocal pre-pacemaker nucleus (VPP, Fig. 1) that innervates VPN and codes for call duration (Bass and Baker, 1990; Bass et al., 1994, 2008; Chagnaud et al., 2011, 2012). The VPP-VPN-VMN network receives input from a vocally active midbrain site considered homologous to the periaqueductal gray (PAG) of other vertebrates (Fig. 1) that is innervated, in turn, by the forebrain preoptic area (POA)-anterior hypothalamus (Fig. 1) (Goodson and Bass, 2002; Kittelberger and Bass, 2006, 2012). The POA-PAG complex receives input from nuclei that are part of the ascending auditory system (e.g., TS, AT; Fig. 1) (Bass et al., 1994, 2000; Goodson and Bass, 2002; Kittelberger and Bass, 2006, 2012; Chagnaud et al., 2011). Figure 1 and Table 3 summarize CNS sites within the VAC network showing robust protein expression of ERβ1 and ERβ2. Additionally, Figure 1 shows VAC sites with robust ERα and aromatase (Aro) expression based on earlier studies in midshipman (Forlano et al., 2001, 2005) and the approximate location of the transverse sections shown in Figures 2–4. Except where noted, the staining patterns of ERβ1 and ERβ2 did not differ substantially between the reproductive morphs (type I males, type II males, females).
Fig. 2.
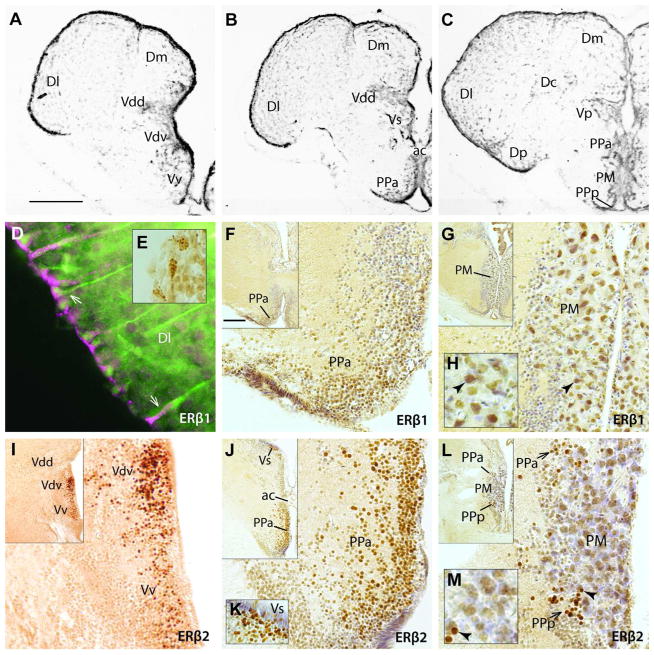
Expression of ERβ1 and ERβ2 in telencephalon and preoptic area. A–C: Atlas images provide neuroanatomical context of the ERβ1 and ERβ2 localization illustrated in D–H and I–M, respectively. Vertically aligned sections are at similar forebrain levels. D: Fluorescent co-label in the telencephalon. ERβ1 (magenta) and GFAP (green) co-localize (D, arrows) in cells along the outer ventricular surface of area dorsalis (D) of the telencephalon, consistent with radial glial expression. Inset E shows punctate-like label in periventricular region. F–M: ERβ label revealed with DAB stain (brown). Inset in the upper left corner of each panel is a lower magnification view of the region from which the larger image was taken. Cresyl violet counterstain was used, except in I. For orientation, arrowheads point to the same cell in larger image of G and H, as well as in larger image of L and M. Both receptors are expressed in the anterior parvocellular division of the preoptic area (PPa; F, J, L). ERβ1 label was robustly labeled in the magnocellular division of the preoptic area (PM, G, H), while ERβ2 label was substantially less in the PM (L,M). ERβ2 alone labeled somata in the posterior parvocellular division of the preoptic area (PPp), especially in smaller cells immediately adjacent to PM (L, arrows). ERβ2 alone was also expressed strongly in the ventral (Vv), supracommissural (Vs) and ventral division of the dorsal nucleus of area ventralis (Vdv) of the telencephalon (I – K). A–C modified from Forlano et al. (2010). Scale bar in A = 400 μm in A–C, 5 μm in D, 30 μm in E, 100 μm in magnified images in F, G, I, J, L. Scale bar in inset of F represents 200 μm in insets of F, G, I, J, L; 25 μm in H and M. Other abbreviations: Dc, central zone of D; Dl, lateral zone of D; Dm, medial zone of D; Dp, posterior zone of D; Vdd, dorsal division of the dorsal nucleus of area ventralis.
Fig. 4.
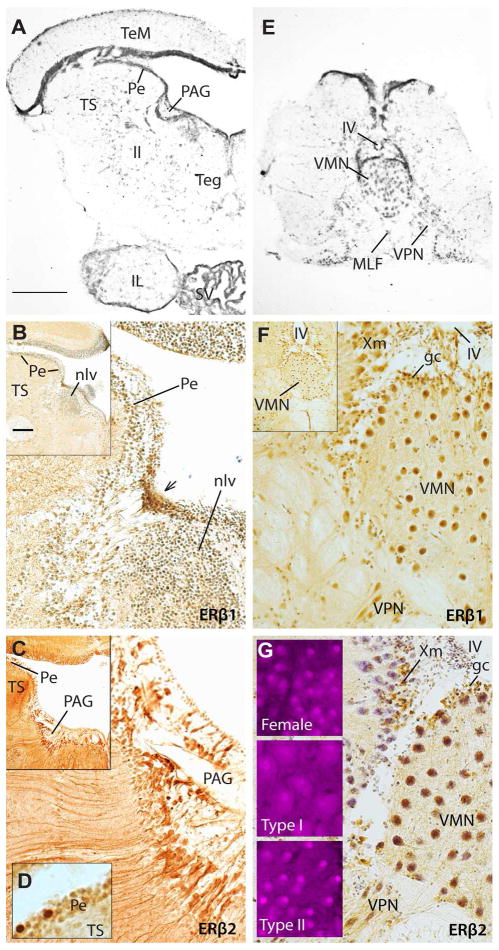
Expression of ERβ1 and ERβ2 in the brainstem. A: Atlas image of midbrain provides neuroanatomical context for ERβ1 (B) and ERβ2 (C) localization, respectively. B, C: Inset in the upper left corner is a lower magnification view of the region from which the larger image was taken. ERβ1 is found in a periventricular cluster of cells adjacent to the anterior pole of nucleus lateralis valvulae (nlv), whereas ERβ2 is broadly distributed throughout the periaqueductal gray (PAG). D: ERβ2 is also strongly expressed in a few cells in the periventricular layer (Pe) of the auditory division of the torus semicircularis (TS, see A for reference). E: Atlas image showing vocal motor nucleus (VMN) and vocal pacemaker neurons (VPN). F, G: Both ERβ1 and ERβ2 are expressed in VMN motor neurons and periventricular cells along the fourth ventricle (IV), as well as in the vagal motor nucleus (Xm). Three fluorescent insets in G highlight more robust nuclear ERβ2 staining of motor neurons in females and type II males compared to type I males (confirmed by qPCR, see Fig. 6). Scale bar in A represents 500 μm in A; 400 μm in E; 100 μm in B,C,F,G, and insets of G. Scale bar in the inset of B represents 200 μm for inset images in B,C,F. A and E modified from Forlano et al. (2010). Other abbreviations: ll, lateral lemniscus; MLF, medial longitudinal fasciculus.
Fig. 1.
Schematic sagittal view of the plainfin midshipman (Porichthys notatus) brain showing subset of vocal (open) and/or auditory (shaded) nuclei (adapted from Goodson and Bass, 2002; Bass and McKibben, 2003; Kittelberger et al., 2006; Kittelberger and Bass, 2012). Partial shading indicates involvement in both the ascending auditory and descending vocal systems. Dots and lines represent somata and axonal projections, respectively. Connected dots and arrows represent reciprocal and unidirectional connections, respectively. Vertical bars at the top indicate approximate regions of atlas sections in Figures 2–4. Nuclei that express ERα, ERβ1, ERβ2 and aromatase are labeled 1 through 4 (see key), while those without such labels do not express either ER or aromatase. *Expression data for vocal muscle is not available. Abbreviations: AT, anterior tuberal nucleus; CP, central posterior nucleus of dorsal thalamus; MLF, medial longitudinal fasciculus; Oct, hindbrain octaval (auditory nuclei); PAG, midbrain periaqueductal gray; POA, preoptic area; TS, midbrain torus semicircularis; V, area ventralis of the telencephalon; VMN, vocal motor nucleus; VPN, vocal pacemaker nucleus; VPP, vocal prepacemaker nucleus.
Table 3.
Summary of sites (see list of abbreviations) showing robust ERβ1 and ERβ2 protein expression in the midshipman fish, noting identification as part of the vocal and/or auditory pathways. A plus sign indicates that staining is unambiguously stronger than the surrounding background stain, while a minus sign indicates it was not.
| Anatomical Location | Pathway | ERβ1 | ERβ2 |
|---|---|---|---|
| Telencephalon | |||
| Ventricular surface | − | + | − |
| Vv | Auditory | − | + |
| Vd | − | − | + |
| Vs | Vocal, Auditory | − | + |
|
| |||
| Preoptic Area | |||
| PPa | Vocal, Auditory | + | + |
| PPp | Vocal, Auditory | − | + |
| PM | − | + | − |
|
| |||
| Hypothalamus | |||
| AT | Vocal, Auditory | − | + |
| Hd | − | − | + |
| Hv | − | − | + |
| LH | − | − | + |
|
| |||
| Posterior Tuberculum | |||
| TP | − | − | + |
| TPp | − | − | + |
|
| |||
| Thalamus | |||
| CP | Auditory | − | − |
| Ventricular surface | − | + | − |
|
| |||
| Brainstem | |||
| PAG | Vocal | −* | + |
| VPP | Vocal | − | − |
| VPN | Vocal | − | − |
| VMN | Vocal | + | + |
| TS-Pe | Auditory | − | + |
| Oct | Auditory | − | − |
| Xm | − | + | + |
|
| |||
| Peripheral | |||
| Saccule | Auditory | + | + |
ERβ1 is expressed in cells at the caudal end of PAG
To illustrate the cytoarchitecture of brain regions with abundant ERβ1 and ERβ2, and to facilitate comparisons with prior studies, Figures 2–4 include Nissl-stained sections from our ARβ localization study (Forlano et al., 2010). The top row in each of these figures shows sections representative of the levels at which ERβ1 and ERβ2 labeled sections are vertically aligned in the middle and/or bottom rows.
Telencephalon
ERβ1 label was observed along the entire ventricular surface that overlies areas dorsalis (Dl, Dm, Dp) and ventralis (Vv, Vdv, Vdd, Vs, Vp; see Fig. 2A–C for cytoarchitecture; a dorsal division of area dorsalis is not easily distinguished in midshipman) where it labeled cell bodies lining the surface of the telencephalon and their processes that extended centripetally into the brain (Fig. 2D). ERβ1 label resembled the previously reported expression of aromatase in radial glia cells (Forlano et al., 2001) and, like aromatase, ERβ1 was co-localized with the glial marker GFAP (Fig. 2D). ERβ1 was often observed clustered in a punctuate pattern, most notably in somata on the surface of the brain (Fig. 2E), and occasionally along tracts descending into the brain in a pattern consistent with the processes of cells co-labeled with GFAP. ERβ1 label was also found in somata of the anterior parvocellular preoptic nuclei (PPa, Fig. 2F) and the magnocellular division of the preoptic area (PM, Fig. 2G, H); GFAP did not co-label these cells or any other in the preoptic area (see below).
While ERβ1 showed substantial labeling of both non-neuronal cells and neurons, ERβ2 appeared to be expressed only in neurons based on the morphology of the cells stained and the lack of co-localization with GFAP. Also unlike ERβ1, ERβ2 label was found in area ventralis (Vv, Vdv, Vs; Fig. 2I–K) and the posterior parvocellular division of the preoptic area (PPp), although robust staining was restricted to a PPp region that lies immediately adjacent to PM (Fig. 2L, M). Label in Vv was uniformly spread throughout this region that has no obvious cytoarchitectonic subdivisions in midshipman (Fig. 2A, I). The dorsal nucleus, Vd, has more apparent ventral and dorsal divisions, Vdv and Vdd, respectively (Fig. 2A, B). Dense ERβ2 label was restricted to Vdv, with the majority of labeled cells close to the ventricle (Fig. 2I). Vdv was also distinguished by dense ARβ mRNA expression (Forlano et al., 2010; Vd is recognized as a single region in that report).
Like ERβ1, ERβ2 was present in PPa (Fig. 2J, L), an area previously identified to possess abundant ERα and ARβ mRNA (Forlano et al., 2005, 2010).
Diencephalon
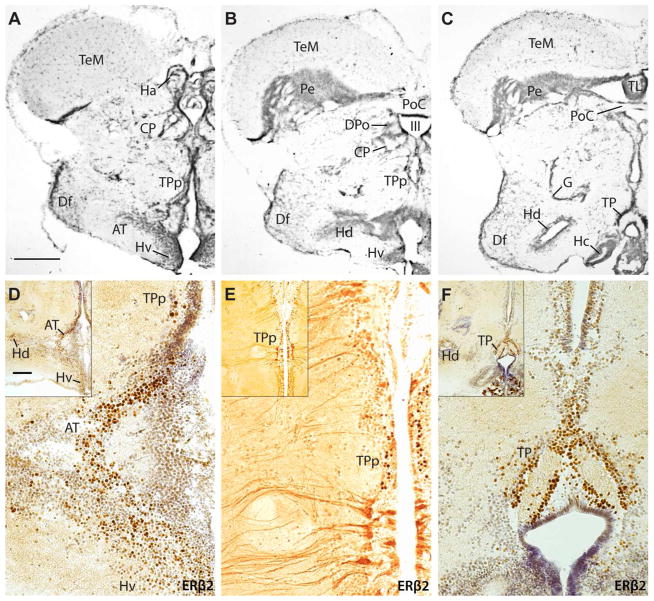
In the hypothalamus (Fig. 3A–C), there was abundant ERβ2 and little to no ERβ1 expression. More specifically, there was strong staining of ERβ2 in the anterior tuberal nucleus (AT, Fig. 3D) that receives direct input from the main auditory nucleus in the midbrain torus semicircularis (TS, Fig. 1) and provides afferent input to the vocally active midbrain PAG (Fig. 1) (Bass et al., 2000; Goodson and Bass, 2002). ERβ2 expression also occurred in the periventricular posterior tuberal nucleus (TPp, Fig. 3D, E), the posterior tuberal nucleus (TP, Fig. 3F) and periventricular hypothalamic regions (Hv, Hd; Fig. 3D, F inset). The especially robust staining in TP parallels similarly robust ARβ mRNA expression in TP, the densest site of ARβ labeling in the midshipman CNS (Forlano et al., 2010). Within TPp, there was staining of both somata and processes extending laterally from the ventricle (Fig. 3E). In the thalamus (not shown), ERβ1 expression like that observed along the telencephalic ventricle (see Fig. 2D) occurred around the third ventricle at the level of the central and dorsal posterior nuclei (CP, DPo; see Fig. 3B for general reference). GFAP staining did not show convincing co-label in these regions as it did in the telencephalon.
Fig. 3.
Expression of ERβ2 in the diencephalon. A–C: Atlas images for ERβ2 localization illustrated in D–F. Sections vertically aligned are at similar levels. Inset in the upper left corner of each panel is a lower magnification view of the region from which the larger image was taken. D–F: In the hypothalamus, robust ERβ2 label is found in the anterior tuberal nucleus (AT), with more modest expression in periventricular dorsal (Hd) and ventral (Hv) nuclei. Strong ERβ2 staining also occurs in the periventricular posterior tuberal nucleus (TPp) of the posterior tuberculum and the posterior tuberal nucleus (TP). TPp possesses both strong staining of cell bodies as well as more generally in cells projecting inward from the ventricle. A–C modified from Forlano et al. (2010). Scale bar in A represents 500 μm in A,B; 400 μm in C; 100 μm in magnified images in D–F. Scale bar in inset of D represents 200 μm in all inset images.
Midbrain
The midbrain PAG (Fig. 4A) links forebrain and hindbrain vocal regions (Kittelberger et al., 2006; Kittelberger and Bass, 2012). This area showed limited ERβ1 and broad ERβ2 staining along the ventricle (Fig. 4B, C). Rather than clearly staining the PAG itself, ERβ1 label was found in a dense cluster of small cells along the ventricle (arrow, Fig. 4B), at the rostral pole of nucleus lateralis valvulae (nlv). Although GFAP staining did not show convincing co-label, ERβ2 staining was found throughout the PAG in a pattern nearly identical to aromatase labeling of this region and may thus represent glial cells that appear to be the major, if not sole, source of aromatase in the fish CNS (Forlano et al., 2001; also see Diotel et al., 2011). Strong ERβ2 label alone occurred in the auditory division of the torus semicircularis (TS) (Bass et al., 2000) in somata scattered throughout the periventricular layer (Pe, Fig. 4D; see TS and Pe in 4A for reference).
Hindbrain
As reviewed earlier, three nuclei comprise the vocal pattern generator in midshipman: a midline motor nucleus (VMN; Figs. 1, 4E) and premotor pacemaker (VPN, Figs. 1, 4E) and pre-pacemaker (VPP, Fig. 1) nuclei. Neither ERβ1 nor ERβ2 showed staining in or around the VPP (not shown). Somata of the VPN and more lateral areas were only moderately to weakly-stained with ERβ1 (Fig. 4F), while there was no strong ERβ2 staining in this region (Fig. 4G, apparent VPN staining is primarily cresyl violet counterstain). Robust ERβ1 and ERβ2 label was found in VMN and the vagal motor nucleus (Xm) that borders the dorsolateral aspect of VMN (Fig. 4F, G). Qualitatively, the ERβ2 staining in VMN differed substantially by morph, with females and type II males showing much stronger signal than type I males (insets, Fig. 4G). This inter-morph variation was verified quantitatively with qPCR (below).
Inner ear
Both ERβ1 and ERβ2 were expressed within hair cells of the saccular hair cell epithelium, although their patterns of expression differed markedly. When viewed in sagittal sections, ERβ1 expression was observed within a very small region at the apical end (green, Fig. 5A; magenta is label with the hair cell specific, HCS-1, antibody; see Materials and Methods). When better visualized within a single focal plane in whole mounts, co-localization with actin (magenta, see Materials and Methods) demonstrated that the region of ERβ1 staining was immediately adjacent to the hair cell bundle, likely at the base of each kinocilium (Fig. 5B, green; note that magenta label is for actin here but HCS-1 in A). By contrast, ERβ2 was expressed more broadly within hair cells, although expression levels varied greatly, with some cells showing little to no ERβ2 staining (magenta, Fig. 5C, sagittal view of whole mount; green represents staining with HCS-1). ERβ2 was also expressed within the ganglion cells of the eighth nerve (VIII) proximal to the saccular epithelium (magenta, sagittal section; Fig. 5D).
Fig. 5.
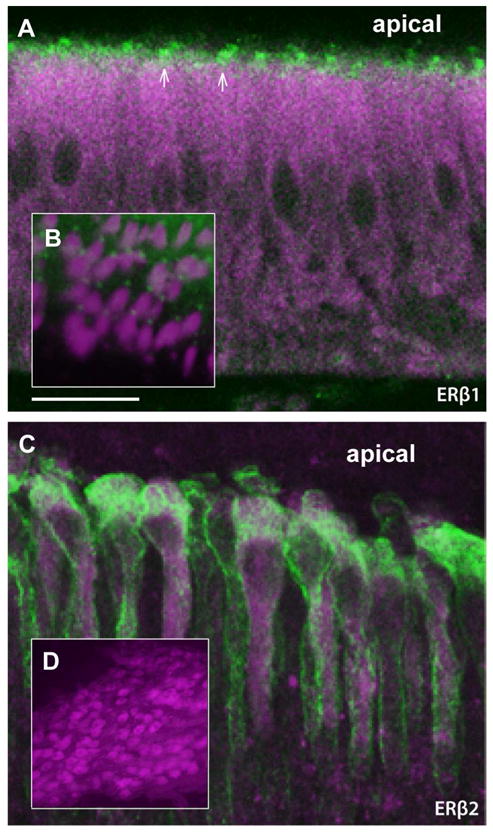
Expression of ERβ1 and ERβ2 in the saccule division of the inner ear. A: The hair cells of the sensory epithelium stain robustly throughout the cell body with the hair cell specific (HCS-1) antibody (magenta), while ERβ1 staining (green) is limited to the apical surface of the hair cells. B: Co-label of hair cells with phalloidin (magenta), which stains actin filaments of the hair cell bundles, and ERβ1 (green) demonstrates that ERβ1 protein is localized to a small region near the base of each bundle. C: Co-label of ERβ2 (magenta) and HCS-1 (green) shows that ERβ2 is distributed broadly throughout the hair cell, but with large variation in expression levels between cells across the epithelium. D: ERβ2 is also present in ganglion cells of the eighth nerve (VIII). Panels A and D are from a saccule that was frozen sectioned (30 Um), while B and C are images from non-sectioned, whole-mount saccules. Scale bar represents 20 μm in A and C; 40 μm in inset B; 200 μm in D.
Inter-morph Transcript Abundance
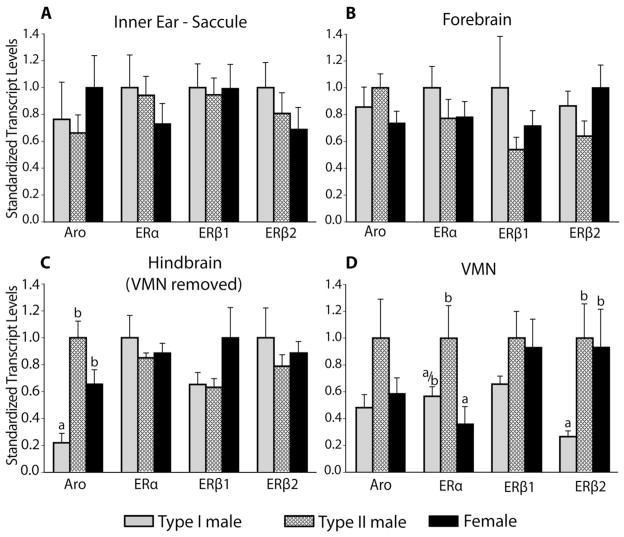
After localizing both ERβs to VMN, we wanted to measure their relative abundance given the reproductive morph-specific differences suggested by the ERβ2 staining pattern (see above). An examination of transcript abundance during the summer breeding season demonstrated distinct ER expression profiles for females, type I males, and type II males. We also assessed aromatase transcript abundance given its role in converting testosterone to estrogen and robust aromatase mRNA and protein expression throughout the forebrain and hindbrain of midshipman (Forlano et al., 2001). Similarly, we determined ERα transcript abundance given its dense and modest expression in the forebrain and VMN, respectively (Forlano et al., 2005). Notably, there were no differences between morphs in transcript abundances in the saccule (Fig. 6A), mirroring the lack of difference in seasonal frequency sensitivity between females and males (Rohmann and Bass, 2011). The forebrain was also similar among the morphs with regard to all ERs and aromatase transcript abundance (Fig. 6B).
Fig. 6.
Comparison of aromatase (Aro) and estrogen receptor (ERα, ERβ1, ERβ2) transcript abundances among the three reproductive morphs of midshipman fish (see legend at bottom). Tissue-specific transcript abundance was compared between reproductive morphs using qPCR. For visualization here, values were standardized to the morph with highest expression for each gene and tissue. Letters above bars indicate significance levels (p<0.05) among morphs for comparisons made within each gene transcript; different letters indicate significant differences in transcript abundance of that gene among morphs. A,B: No significant morph-specific differences in transcript expression were observed in the saccule or the forebrain. C,D: The hindbrain was dissected to isolate the vocal motor nucleus (VMN) from the surrounding brain tissue. Aromatase levels varied significantly among reproductive morphs in the hindbrain without VMN (C), while significant inter-morph variation in ERα and ERβ2 occurred in VMN (D). Type I males differed significantly from both type II males and females in aromatase abundance in the hindbrain and ERβ2 abundance in the VMN. For ERα in VMN, type I males were intermediate to the significant difference between females and type II males. (Sample sizes were: Type I n=7: Type II n=6; Female n=7 for all tissues and genes.)
Substantial variation in transcript abundance was found among the morphs in the hindbrain and VMN. In the hindbrain with VMN removed (see Materials and Methods), there were significant differences among morphs for aromatase (F2,17=15.28, p<0.01; Fig. 6C), with Tukey HSD pairwise comparisons indicating that type I males having significantly lower transcript abundance than either females (p<0.05) or type II males (p<0.01). The difference in aromatase between females and type II males showed a strong trend (p=0.066), suggesting females were intermediate to the male morphs.
Within the isolated VMN, ERα (F2,17=5.92, p<0.05) and ERβ2 (F2,17=7.41, p<0.01) showed significant variation among morphs (Fig. 6D). For ERα transcript abundance, females and type II males differed significantly (p<0.01) from one another, and type I males were intermediate, not differing significantly from either of the other morphs. ERβ2 abundance, on the other hand, was very similar in females and type II males, which each differed significantly from type I males (p<0.05). Neither ERβ1 nor aromatase varied significantly by morph in the VMN. However, the pattern of aromatase abundance in the VMN was similar to the surrounding hindbrain (compare Fig. 6C and D) with lowest abundance in type I males and highest abundance in type II males. This pattern was similar to that previously reported for measures based on aromatase activity levels in the VMN region and aromatase mRNA localization in VMN (Schlinger et al., 1999; Forlano and Bass 2005a; Bass and Forlano, 2008).
DISCUSSION
We identified both ERβ1 and ERβ2 in the plainfin midshipman fish and then localized these receptors within central vocal and auditory nuclei and in the saccule, the main auditory division of the inner ear. The results complement prior neuroanatomical localization in midshipman of aromatase, ERα and ARβ (Forlano et al., 2001, 2005, 2010) (also see Fig. 1). We also used qPCR to compare, for the first time, reproductive morph-specific transcript abundance for all the known ERs as well as aromatase within the CNS and saccule. The ability to remove the intact, paired VMN from the surrounding hindbrain afforded us the unique opportunity to readily examine expression within a whole brain nucleus of known function. All vocal motor neurons are found in VMN while nearby hindbrain regions contain vocal premotor nuclei (Bass and Baker, 1990; Bass et al., 1994; Chagnaud et al., 2011, 2012). We show unique estrogen receptor and aromatase transcript level profiles for the three reproductive morphs, consistent with previous findings that estrogen and androgens regulate plasticity in vocal and auditory physiology (Remage-Healey and Bass 2004, 2007, Sisneros et al., 2004b) and morph-specific patterns of aromatase expression and activity (Schlinger et al., 1999; Forlano and Bass, 2005a).
Each midshipman reproductive morph has a specific profile of plasma androgen and estrogen levels (Brantley et al., 1993; Knapp et al., 1999; Sisneros et al., 2004a; Genova et al., 2012). Type I males alone exhibit detectable plasma levels of 11-ketotestosterone, while both testosterone and estradiol are detectable in all three morphs with the highest circulating testosterone and estradiol levels in type II males and females, respectively (Brantley et al., 1993). While variation in hormone levels is undoubtedly important in regulating alternative tactics (Brantley et al., 1993; Oliveira et al., 2008b), the morph-specific steroid receptor profiles shown here for a single CNS nucleus with known function remind us that it will not be possible to determine how differences in circulating levels of steroids might affect the individual without some understanding of the identity, localization, and abundance of the specific steroid receptors.
Duplicate Estrogen Receptors
Each of the ERs may be important in mediating differential responses by neurons and glia to estrogens in both the PNS and CNS, including the auditory and vocal regions investigated here in midshipman fish. While ERs are directly activated by estrogen, testosterone may be converted to estrogen locally by the enzyme aromatase (Simpson et al., 2002; Nelson and Bulun, 2001). Thus, ERs may play a critical role in region-specific responses to circulating as well as locally synthesized estrogen (Forlano and Bass 2005b; Diotel et al., 2011). When estrogen binds the ER, this complex can either bind directly to regulatory estrogen-response-element (ERE) in the genome or can interact with other transcription factors to activate or repress transcription (Klinge 2000, 2001). However, several studies, including those in midshipman fish, have reported rapid (sec-min) responses to estrogens that are not likely mediated through transcriptional regulation (e.g., Guo et al., 2005; Remage-Healey and Bass, 2004, 2007, 2010; Wong 2010). Such rapid effects suggest a membrane bound signal transduction system. Though evidence suggests a potential G-protein coupled estrogen receptor (Thomas et al., 2005), nuclear ERs have been localized to the membrane (Pedram et al., 2006; Dominguez and Micevych, 2010) and can mediate rapid extranuclear effects of estrogen (Guo et al., 2005; Pedram et al., 2006; Wong et al., 2010). As discussed below, reproductive morph-specific profiles of transcript abundances of ER subtypes in midshipman fish imply a role of these receptors in the morph-specific patterns of steroid-dependent modulation of vocal motor excitability.
Estrogen Receptors in Vocalization
Circulating or exogenously applied estrogen has been linked to both slow and rapid modulation of vocal output across vertebrate taxa (Pfefferle et al., 2011, Simpson and Vicario 1991, Remage-Healey and Bass 2004, 2007) and estrogen receptors have been localized to vocal centers within the CNS of birds (Gahr 1994, Gahr and Metzdorf 1997) and fish (Forlano et al., 2005; this report). Furthermore, some of the effects of testosterone on the vocal system have been demonstrated to result from the local conversion of testosterone to estrogen via aromatase (Remage-Healey and Bass, 2007; Soma et al., 2003; Remage-Healey et al., 2009; Saldanha et al., 2011). Thus, it is clear that estrogen plays a critical role in the development and maintenance of vertebrate vocal networks.
With this study, we now have a more complete understanding of the localization and abundance of estrogen receptors within the physiologically defined vocal network of the plainfin midshipman. Forlano and Bass (2011) recently reviewed steroid receptor localization in fishes, including forebrain regions that show ERβ label in the current study (see Table 3) and are proposed to be homologous to mammalian nuclei as follows: Vv – septum, Vd - striatum/basal ganglia, Vs - basal amygdala, PPa/PPp – POA, PM - paraventricular nucleus, AT - ventromedial hypothalamus, Hv/Hd - arcuate nucleus, TP - ventral tegmental area/substantia nigra (see Forlano and Bass, 2011 for original references on homologies). Some of these homologies, however, are not definitively established (e.g., see Goodson, 2005 for AT). The aforementioned mammalian nuclei also express ERβ (e.g., Shughrue et al., 1997; Mitra et al., 2003; Merchenthaler et al., 2004), as does the midbrain PAG in midshipman (current study) and mammals (see above references for mammals). Several of these areas in midshipman and the closely related toadfish (Vs, PPa/PPp, AT, PAG), as in their mammalian homologues, are involved in vocal motor patterning and hence potential sites for estrogen-dependent modulation of vocal mechanisms (Fine and Perini, 1994; Goodson and Bass, 2001, 2002; Kittelberger and Bass, 2006, 2012; also see Jurgens, 2009; Bolhuis et al., 2010; Schwartz and Smotherman, 2011; Tressler and Smotherman, 2011 for mammals). Several of these sites in midshipman have an abundance of neuropeptidergic somata and/or terminals (e.g., Goodson et al., 2003), further highlighting these regions as likely neuromodulatory “hotspots” for the central control of vocalization. Recent comparative studies of birds and mammals have brought attention to the involvement of striatal dopamine in vocalization (e.g., see Bolhuis et al., 2010; Tressler and Smotherman, 2011; Leblois and Perkel, 2012). Like its proposed mammalian homologue, Vdv includes catecholaminergic neurons in midshipman, although most are immediately lateral to the dense ERβ population in Vdv (tyrosine hydroxylase immunoreactivity; Bass et al., 2001; A. Bass, unpublished observations; also see Rink and Wullimann, 2001 for other teleosts). Similarly, catecholaminergic neurons in TP overlap the region of ERβ expression. While estrogen and catecholamines may interact to modulate vocalization in midshipman, there is no evidence for the direct role of Vdv, TP or forebrain catecholamines in fish vocalization (but see Kittelberger et al., 2011 for midshipman PAG and Ball et al., 2002 for steroid-catecholamine discussion in tetrapods).
Previous work demonstrated the importance of steroid hormones for vocalization in plainfin midshipman (Remage-Healey and Bass, 2004, 2007, 2010) and closely related species (Remage-Healey and Bass, 2006b), and identified the presence of aromatase, ARβ, and ERα within vocal regions of the CNS using IHC and/or in situ hybridization histochemistry (Forlano et al., 2001, 2005, 2010). Here, we identified and localized ERβ1 and ERβ2, providing the complete complement of known teleost estrogen receptors (Hawkins et al., 2000; Menuet et al., 2002) from the plainfin midshipman.
Remage-Healey and Bass (2004, 2007) demonstrated that exogenous treatments (trunk muscle injection) of androgens and estrogen induce both rapid (within 5 min) and sustained (30 min to 2 h depending on the steroid and morph) facilitation of the duration of the vocal motor volley generated by the vocal pattern generator that determines the duration of vocal muscle contraction and, in turn, natural call duration (Chagnaud et al., 2011, 2012). However, the actions of estrogen, androgens and aromatase inhibitor on vocal excitability vary with reproductive morph. Estrogen (17β-estradiol) induces increased vocal output in all morphs, although the effect is longer lasting in females and type II males (up to 45 min) compared to type I males (up to 30 min). This pattern is consistent with greater ERβ2 transcript abundance in the VMN of type II males and females compared to type I males. The ERβ1 profile resembles that of ERβ2; although the differences were not significant, ERβ1 may yet play a role similar to ERβ2. While testosterone induces increased vocal output in only females and type II males (11-ketotestosterone is effective in only type I males), use of the aromatase inhibitor fadrozole and the AR antagonist cyproterone acetate indicate that testosterone’s effects are predominantly mediated via ER and AR mechanisms in females and type II males, respectively (Remage-Healey and Bass, 2004, 2007). Aromatase expression is highest in glial cells surrounding VMN (Forlano et al., 2001; Forlano and Bass 2005a). The moderate to high aromatase transcript abundance in the hindbrain surrounding VMN of females is consistent with the rapid effects of testosterone resulting from the conversion to estrogen. The low ERα transcript abundance in female VMN implies that this response is mediated by ERβ2 and possibly ERβ1. The high aromatase transcript abundance in type II males is not as easily related to the previous data. However, while abundant aromatase suggests that testosterone to estrogen conversion likely occurs around and in the VMN of type II males, this does not preclude a stronger direct effect of testosterone on vocal output via an AR mechanism. Androgen receptor abundance in VMN may figure prominently into testosterone’s effects. Preliminary data suggest high type II expression of both ARα and ARβ in the VMN (D. Fergus and A. Bass, unpublished observations). Hence, the direct effect of testosterone on facilitation of VMN output in type II males may depend on an abundance of AR that can bind to testosterone before it is converted to estrogen by aromatase in this morph.
It is noteworthy that only type I males produce long duration (mins to > 1h) “hums” used to attract and court females, while all adult morphs produce short duration (< 300 msec) agonistic grunts (Brantley and Bass 1994). This behavioral polymorphism parallels morph-specific differences in vocal motor excitability and the duration of sustained output from VMN (Bass and Baker, 1990; Bass and Remage-Healey, 2008; Rubow and Bass, 2009). The major distinction between the VMN output of type I males versus females and type II males suggests that the distinct receptor and aromatase expression profiles in and around the VMN of type I males relative to the other reproductive morphs likely supports the production of the courtship hums. While intramuscular injections of androgens and estrogen rapidly facilitate VMN output in all reproductive morphs, the long-term, transcriptionally-dependent effects of steroids in the VMN on courtship hums may be very different. The substantially lower abundance of ERβ2 in the type I male VMN relative to that of non-humming females and type II males suggests a potential inhibitory effect of this receptor on the neural mechanisms leading to courtship hum. ERβ activity has been shown to either facilitate or inhibit certain aggressive behaviors in apparently context, temporal, and sex dependent manners in mice (Nomura et al., 2006; Allen et al., 2010). Recently, the ability to elicit hum-like fictive calls in midshipman has been reported (Rubow and Bass, 2009), which will allow for a more thorough examination of the effects of steroid hormones on the production of humming behavior. In parallel, intracellular neurophysiology has begun to identify the intrinsic membrane and network properties of neurons in the VPP-VPN-VMN network (Chagnaud et al., 2011, 2012). These findings will allow a more targeted investigation of how estrogen (and androgens) shape vocal output via changes in VMN’s neurophysiology. This includes the dependence of synchronous VMN output, which leads to simultaneous contraction of the vocal muscles for call production, on gap junction-mediated electrical coupling within VMN, dense excitatory input from VPN (see Fig. 1) and dense inhibitory input from gamma-aminobutyric acid (GABA) neurons adjacent to VMN (Bass and Marchaterre, 1989b; Chagnaud et al., 2011, 2012), all of which are strongly influenced by estrogen in other systems. For example, estrogen-dependent events can lead to increases in the extent of neuronal synchrony mediated by gap junction coupling in the inferior olive (Smith, 1998; also see Llinas et al., 1974 and Sotelo et al., 1974). Increased excitatory and decreased GABAergic/inhibitory transmission in the hippocampus is mediated via ERβ and ERα receptors, respectively (Huang and Woolley, 2012). Comparable morph-specific events might depend upon the relative abundance of one or more of the ERs identified in midshipman VMN.
The midshipman’s three reproductive morphs differ in other vocal characters as well. For example, cortisol rapidly facilitates vocal output in type I males, but suppresses it in type II males and females (Remage-Healey and Bass, 2004, 2007). Anterior hypothalamic injections of arginine vasotocin, an arginine vasopressin homologue, suppress vocal output only in type I males, whereas injections of isotocin, an oxytocin homologue, suppress it only in type II males and females (Goodson and Bass, 2000). Neuron size in VMN (as VPP and VPN) is similar in type II males and females, but several fold smaller than in type I males (Bass et al., 1996; also see Fig. 4G insets). The development and maintenance of any one of these characters might be impacted by the divergent patterns observed here for aromatase and ERs in VMN.
Estrogen Receptors in Hearing
The role of steroids, especially estrogen, in hearing has been proposed in a number of vertebrates (e.g., Sisneros et al., 2004b; Pinaud and Tremere, 2012; Remage-Healey, 2012). In humans, both sexes exhibit high frequency hearing loss with age; however, this hearing loss extends to lower frequencies in men (2–4 kHz) than in women (4–8 kHz) (Jönsson et al., 1998). The decline in high frequency hearing in females following menopause is ameliorated by estrogen replacement therapy (Kilicdag et al., 2004; also see Hultcrantz et al., 2006). Knock-out of ERβ in mice leads to a degeneration of hair cells and spiral ganglion cells as well as a complete loss of hearing as the mice age (Simonoska et al., 2009). Seasonal cycling in peripheral auditory physiology coincident with reproductive cycles has been observed among birds (Lucas et al., 2002, 2007; Caras et al., 2010) and in midshipman fish (Sisneros and Bass, 2003). Recent work has shown that the seasonal variation in midshipman hearing occurs in both females and males, and corresponds to variation in hair cell physiology (Sisneros, 2009; Rohmann and Bass, 2011). In midshipman, the frequency sensitivity of eighth nerve afferents during the breeding season can be induced in a non-breeding individual by supplementing with either estrogen or testosterone (Sisneros et al., 2004b). Furthermore, aromatase protein has been localized to eighth nerve ganglion cells and ERα and ARβ transcripts to regions directly adjacent to hair cells (the specific cell type has not been identified) of the midshipman saccule (Forlano et al., 2005, 2010). Aromatase, ER and AR transcripts were also recently identified in the saccule of sound producing cichlids using qPCR (Maruska and Fernald, 2010), suggesting that this phenotype is likely widespread among sonic fishes (also see Maruska et al., 2012) and more generally, species that breed year round, including humans (see above). Taken together, these results strongly suggest a role for steroids, especially estrogen, in modifying auditory sensitivity.
The results presented here are the first to directly demonstrate steroid receptor expression within the hair cells of fish and complement reports of aromatase and/or ER in mammalian and avian auditory hair cells (Hultcrantz et al., 2006; Noirot et al., 2009). The presence of ERβ1 and ERβ2 in midshipman hair cells provides a direct pathway by which estrogen could regulate ion channel expression to alter auditory physiology, making these receptors strong candidates for the link between seasonal hormone cycling and auditory physiology (for extended discussion, see Rohmann and Bass, 2011). The presence of ERα protein in hair cells seems unlikely given the clustering of ERα mRNA in regions adjacent to the hair cells (Forlano et al., 2005).
The differential expression patterns of ERβ1 and ERβ2 within the saccule suggest different roles for the receptors in estrogen effects in the ear. In the hair cells both receptors show primarily non-nuclear staining, which has been previously reported for estrogen receptors α and β (reviewed by Hammes and Levin 2007). ERβ1 localization to the base of the hair cell bundle (Fig. 5A), far from the nucleus, indicates it is almost certainly involved in non-transcriptional events and may be important to the regulation of processes in the stereocillia and/or kinocillium. ERβ2, present throughout the cytoplasm of the hair cell (Fig. 5B), may regulate transcriptional (via translocation to the nucleus) or non-transcriptional events. Interestingly, ERβ2 is not abundant in all hair cells, but showed a stochastic expression within a subset of hair cells. The teleost auditory epithelium contains a heterogeneous mixture of hair cells (e.g., Furukawa and Ishii 1967; Saidel et al., 1995) and the differential expression of ERβ2 across cells of the saccule suggests a variable role of ERβ2 in different cells. This variation across the epithelium suggests differential sensitivity of hair cells to estrogen, and may be critical for the role of estrogen in frequency sensitivity in this system.
A prior study localized ARβ transcripts in the region directly adjacent to hair cells where ERα transcripts were also found (Forlano et al., 2005, 2010), and not within the hair cells themselves. The effect of testosterone on hearing may occur via local conversion by aromatase to estrogen, which then diffuses to ERs in the hair cells; however, ARα has not yet been localized and its presence in hair cells could confer an ARα-specific sensitivity to testosterone (see Ogino et al., 2009). Determining whether ARα is present in the hair cells will be important in our understanding of how steroids regulate hearing sensitivity in midshipman, and in vertebrates in general.
Estrogen receptor localization (mainly ERβ2) in the central auditory system included midbrain (TS), diencephalic (AT), preoptic (PPa, PPp) and ventral telencephalic (Vv, Vs) nuclei that form part of the ascending auditory system (see Goodson and Bass, 2002). These results suggest the potential for estrogen modulation of audition as reported in amphibians (Yovanof and Feng, 1983), but more extensively in birds (see Pinaud and Tremere, 2012; Remage-Healey, 2012). While single neuron recording studies in midshipman have investigated the response properties in the auditory torus (see review in Bass and McKibben, 2003), seasonal and steroid influences have yet to be examined in the torus and other auditory regions.
Conclusions
Steroid hormones have rapid (within 5 min) effects on vocal motor excitability, as well as slower, longer lasting effects on both vocal and auditory neurophysiology (see earlier sections). Consistent with these functions, here and in previous work (Forlano et al., 2005, 2010) we have demonstrated the presence of ERs and ARs in both the auditory and vocal pathways. Furthermore, the morph-specific differences in the vocal effects of specific steroid hormones (Remage-Healey and Bass, 2004, 2007) are paralleled by the receptor expression profiles observed within the vocal network in this study. In and around the VMN, which provides the final neural code to the vocal muscles (Bass and Baker, 1990; Chagnaud et al., 2012), type I males have ERβ2 and aromatase profiles that are distinct from those of type II males and females, which are similar to one another. In contrast, the VMN of type II males is distinctly different from that of females in ERα abundance, with type I males intermediate to the other morphs. Together with testosterone-dependent facilitation of vocal output via either androgen or estrogen receptor-dependent mechanisms in both male morphs and females, respectively, the ER profiles presented here suggest that the vocal neuron phenotype of type II males is unique compared to other morphs, although it generally is more similar to that of females than to type I males (also see Bass and Baker, 1990; Bass et al., 1996; Goodson and Bass, 2000; Forlano and Bass, 2005a; Remage-Healey and Bass, 2007). These findings support earlier proposals that regional increases in estrogen levels in the vocal motor system of type II males feminize its morphology and physiology (Schlinger et al., 1999). Our results also underscore the important role of steroid hormones in the differentiation and maintenance of alternative reproductive tactics and indicate that hormone levels likely interact dynamically with divergent patterns of steroid receptor abundances to modulate specific neural circuits controlling behaviors leading to the production of alternative reproductive tactics by males and females.
Acknowledgments
Grant Sponsor: National Institutes of Health (DC00092) and National Science Foundation (IOS 1120925).
We thank David Deitcher, Ni Y. Feng, Jad Husseini, Margaret Marchaterre and Kevin Rohmann for technical advice and support, and Ni Y. Feng for comments on an earlier version of the manuscript.
ABBREVIATIONS
- ac
anterior commissure
- Aro
aromatase
- AT
anterior tuberal nucleus
- CP
central posterior nucleus of the thalamus
- D
area dorsalis of the telencephalon
- Dc
central zone of D
- Df
diffuse nucleus of the hypothalamus
- Dl
lateral zone of D
- Dm
medial zone of D
- Dp
posterior zone of D
- DPo
dorsal posterior nucleus of the thalamus
- ERα
estrogen receptor α
- ERβ1
estrogen receptor β1
- ERβ2
estrogen receptor β2
- G
nucleus glomerulosus
- Ha
habenula
- Hc
caudal periventricular hypothalamus
- Hd
dorsal periventricular hypothalamus
- Hv
ventrolateral nucleus of the hypothalamus
- III
third ventricle
- IL
inferior lobe of the hypothalamus
- IP
isthmal paraventricular nucleus
- IV
fourth ventricle
- ll
lateral lemniscus
- MLF
medial longitudinal fasciculus
- nlv
nucleus lateralis valvulae
- Oct
hindbrain octaval nuclei
- PAG
periaqueductal gray
- Pe
periventricular cell layer of the torus semicircularis
- PM
magnocellular preoptic nucleus
- POA
preoptic area
- PPa
anterior parvocellular preoptic nucleus
- PPp
posterior parvocellular preoptic nucleus
- PoC
posterior commissure
- SV
saccus vasculosus
- Teg
midbrain tegmentum
- TeM
midbrain tectum
- TL
torus longitudinalis
- TP
posterior tuberal nucleus
- TPp
periventricular posterior tuberal nucleus
- TS
torus semicircularis
- V
area ventralis of the telencephalon
- Vd
dorsal division of V
- Vdd
dorsal division of Vd
- Vdv
ventral division of Vd
- VMN
vocal motor nucleus
- VPN
vocal pacemaker neurons
- VPP
vocal prepacemaker nucleus
- Vs
supracommissural nucleus of V
- vT
ventral tuberal hypothalamus
- Vv
ventral nucleus of V
- VIII
eighth nerve
- Xm
vagal motor nucleus
Footnotes
Conflict of Interest Statement:
We identify no known or potential conflicts of interest.
Role of Authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: DJF and AHB. Acquisition of data: DJF. Analysis and interpretation of data: DJF and AHB. Drafting of the manuscript: DJF. Critical revision of the manuscript for important intellectual content: DJF and AHB. Obtained funding: AHB.
LITERATURE CITED
- Allen AEC, Cragg CL, Wood AJ, Pfaff DW, Choleris E. Agonistic behavior in males and females: Effects of an estrogen receptor beta agonist in gonadectomized and gonadally intact mice. Psychoneuroendocrinol. 2010;35(7):1008–1022. doi: 10.1016/j.psyneuen.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Rissman EF, De Vries GJ. Two perspectives on the origin of sex differences in the brain. Ann N Y Acad Sci. 2003;1007(1):176–188. doi: 10.1196/annals.1286.018. [DOI] [PubMed] [Google Scholar]
- Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: multiple sites of action of sex steroid hormones. Front Neuroendocrinol. 2002;23(2):137–178. doi: 10.1006/frne.2002.0230. [DOI] [PubMed] [Google Scholar]
- Bass AH. Shaping brain sexuality. Am Sci. 1996;84(4):352–363. [Google Scholar]
- Bass AH, Andersen K. Inter- and intrasexual dimorphisms in the vocal control system of a teleost fish: Motor axon number and size. Brain Behav Evol. 1991;37(4):204–214. doi: 10.1159/000114359. [DOI] [PubMed] [Google Scholar]
- Bass AH, Baker R. Sexual dimorphisms in the vocal control-system of a teleost fish - morphology of physiologically identified neurons. J Neurobiol. 1990;21(8):1155–1168. doi: 10.1002/neu.480210802. [DOI] [PubMed] [Google Scholar]
- Bass AH, Bodnar DA, Marchaterre MA. Midbrain acoustic circuitry in a vocalizing fish. J Comp Neurol. 2000;419(4):505–531. doi: 10.1002/(sici)1096-9861(20000417)419:4<505::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Bass AH, Chagnaud BP. Shared developmental and evolutionary origins for neural basis of vocal-acoustic and pectoral-gestural signaling. Proc Natl Acad Sci USA. 2012;109(Suppl 1):10677–10684. doi: 10.1073/pnas.1201886109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Forlano PM. Neuroendocrine mechanisms of alternative reproductive tactics: The chemical language of social plasticity. In: Oliveira RF, Taborsky M, Brockmann J, editors. Alternative Reproductive Tactics – an Integrative Approach. Cambridge: Cambridge Univ. Press; 2008. pp. 109–131. [Google Scholar]
- Bass AH, Gilland EH, Baker R. Evolutionary origins for social vocalization in a vertebrate hindbrain-spinal compartment. Science. 2008;321(5887):417–421. doi: 10.1126/science.1157632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Grober MS. Reproductive plasticity in fish: Evolutionary lability in the patterning of neuroendocrine and behavioral traits underlying divergent sexual phenotypes. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Rubin R, editors. Hormones, Brain and Behavior. 2. San Diego: Elsevier; 2009. pp. 579–610. [Google Scholar]
- Bass AH, Horvath BJ, Brothers EB. Nonsequential developmental trajectories lead to dimorphic vocal circuitry for males with alternative reproductive tactics. J Neurobiol. 1996;30(4):493–504. doi: 10.1002/(SICI)1097-4695(199608)30:4<493::AID-NEU5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Bass AH, Marchaterre MA. Sound-generating (sonic) motor system in a teleost fish (Porichthys notatus): sexual polymorphism in the ultrastructure of myofibrils. J Comp Neurol. 1989a;286(2):141–153. doi: 10.1002/cne.902860202. [DOI] [PubMed] [Google Scholar]
- Bass AH, Marchaterre MA. Sound-generating (sonic) motor system in a teleost fish (Porichthys notatus): sexual polymorphisms and general synaptology of sonic motor nucleus. J Comp Neurol. 1989b;286(2):154–169. doi: 10.1002/cne.902860203. [DOI] [PubMed] [Google Scholar]
- Bass AH, Marchaterre MA, Baker H. Catecholaminergic innervation of central auditory system in a vocal teleost. Soc Neurosci Abstr. 2001;27:88.15. [Google Scholar]
- Bass AH, Marchaterre MA, Baker R. Vocal-acoustic pathways in a teleost fish. J Neurosci. 1994;14(7):4025–4039. doi: 10.1523/JNEUROSCI.14-07-04025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, McKibben JR. Neural mechanisms and behaviors for acoustic communication in teleost fish. Prog Neurobiol. 2003;69(1):1–26. doi: 10.1016/s0301-0082(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Bass AH, Remage-Healey L. Central pattern generators for social vocalization: Androgen-dependent neurophysiological mechanisms. Horm Behav. 2008;53(5):659–672. doi: 10.1016/j.yhbeh.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis JJ, Okanoya K, Scharff C. Twitter evolution: Converging mechanisms in birdsong and human speech. Nat Rev Neurosci. 2010;11(11):747–759. doi: 10.1038/nrn2931. [DOI] [PubMed] [Google Scholar]
- Brantley RK, Bass AH. Alternative male spawning tactics and acoustic-signals in the plainfin midshipman fish Porichthys notatus girard (Teleostei, Batrachoididae) Ethology. 1994;96(3):213–232. [Google Scholar]
- Brantley RK, Wingfield JC, Bass AH. Sex steroid levels in Porichthys notatus, a fish with alternative reproductive tactics, and a review of the hormonal bases for male dimorphism among teleost fishes. Horm Behav. 1993;27(3):332–347. doi: 10.1006/hbeh.1993.1025. [DOI] [PubMed] [Google Scholar]
- Campbell RE, Ducret E, Porteous R, Liu X, Herde MK, Wellerhaus K, Sonntag S, Willecke K, Herbison AE. Gap junctions between neuronal inputs but not gonadotropin-releasing hormone neurons control estrous cycles in the mouse. Endocrinol. 2011;152:2290–2301. doi: 10.1210/en.2010-1311. [DOI] [PubMed] [Google Scholar]
- Caras ML, Brenowitz E, Rubel EW. Peripheral auditory processing changes seasonally in Gambel's white-crowned sparrow. J Comp Physiol A. 2010;196(8):581–599. doi: 10.1007/s00359-010-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnaud BP, Baker R, Bass AH. Vocalization frequency and duration are coded in separate hindbrain nuclei. Nat Commun. 2011;2:342. doi: 10.1038/ncomms1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnaud BP, Zee MC, Baker R, Bass AH. Innovations in motoneuron synchrony drive rapid temporal modulations in vertebrate acoustic signaling. J Neurophysiol. 2012;107(12):3528–3542. doi: 10.1152/jn.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diotel N, Do Rego JL, Anglade I, Vaillant C, Pellegrini E, Vaudry H, Kah O. The brain of teleost fish, a source, and a target of sexual steroids. Front Neurosci. 2011;5:137. doi: 10.3389/fnins.2011.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez R, Micevych P. Estradiol rapidly regulates membrane estrogen receptor alpha levels in hypothalamic neurons. J Neurosci. 2010;30(38):12589–12596. doi: 10.1523/JNEUROSCI.1038-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth RA, Mendonca MT, Hill GE. Condition-dependent sexual traits and social dominance in the house finch. Behav Ecol. 2004;15(5):779–784. [Google Scholar]
- Fine ML, Perini MA. Sound production evoked by electrical stimulation of the forebrain in the oyster toadfish. J Comp Physiol A. 1994;174:173–185. doi: 10.1007/BF00193784. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Bass AH. Seasonal plasticity of brain aromatase mRNA expression in glia: divergence across reproductive and vocal phenotypes. J Neurobiol. 2005a;65(1):37–49. doi: 10.1002/neu.20179. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Bass AH. Steroid regulation of brain aromatase expression in glia: female preoptic and vocal motor nuclei. J Neurobiol. 2005b;65(1):50–58. doi: 10.1002/neu.20178. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Bass AH. Neural and hormonal mechanisms of reproductive-related arousal in fishes. Horm Behav. 2011;59(5):616–629. doi: 10.1016/j.yhbeh.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Bass AH. Distribution of estrogen receptor α mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J Comp Neurol. 2005;483(1):91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Myers DA, Bass AH. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: aromatase enzyme and mRNA expression identify glia as source. J Neurosci. 2001;21(22):8943–8955. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Marchaterre M, Deitcher DL, Bass AH. Distribution of androgen receptor mRNA expression in vocal, auditory, and neuroendocrine circuits in a teleost fish. J Comp Neurol. 2010;518(4):493–512. doi: 10.1002/cne.22233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Ishii Y. Neurophysiology studies on hearing in goldfish. J Neurophysiol. 1967;30(6):1377–1403. doi: 10.1152/jn.1967.30.6.1377. [DOI] [PubMed] [Google Scholar]
- Gahr M. The role of estrogen in the differentiation of the vocal control system of songbirds. In: Davey KG, Peter RE, Tobe SS, editors. Perspectives in Comparative Endocrinology. Ottawa: National Research Council of Canada; 1994. pp. 455–463. [Google Scholar]
- Gahr M, Metzdorf R. Distribution and dynamics in the expression of androgen and estrogen receptors in vocal control systems of songbirds. Brain Res Bull. 1997;44(4):509–517. doi: 10.1016/s0361-9230(97)00233-5. [DOI] [PubMed] [Google Scholar]
- Genova RM, Marchaterre MA, Knapp R, Fergus D, Bass AH. Glucocorticoid and androgen signaling pathways diverge between advertisement calling and non-calling fish. Horm Behav. 2012 doi: 10.1016/j.yhbeh.2012.07.010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variation. Horm Behav. 2005;48(1):11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature. 2000;403(6771):769–772. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: convergence with terrestrial vertebrates reveals conserved traits. J Comp Neurol. 2002;448(3):298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Bass AH. Putative isotocin distributions in sonic fish: relation to vasotocin and vocal-acoustic circuitry. J Comp Neurol. 2003;462(1):1–14. doi: 10.1002/cne.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Razandi M, Pedram A, Kassab G, Levin ER. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors α and β. J Biol Chem. 2005;280(20):19704–19710. doi: 10.1074/jbc.M501244200. [DOI] [PubMed] [Google Scholar]
- Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28(7):726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- Hawkins MB, Thornton JW, Crews D, Skipper JK, Dotte A, Thomas P. Identification of a third distinct estrogen receptor and reclassification of estrogen receptors in teleosts. Proc Natl Acad Sci USA. 2000;97(20):10751–10756. doi: 10.1073/pnas.97.20.10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74(5):801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Hultcrantz M, Simonoska R, Stenberg AE. Estrogen and hearing: a summary of recent investigations. Acta Otolaryngol. 2006;126(1):10–14. doi: 10.1080/00016480510038617. [DOI] [PubMed] [Google Scholar]
- Jerger J, Hall J. Effects of age and sex on auditory brainstem response. Arch Otolaryngol. 1980;106(7):387–391. doi: 10.1001/archotol.1980.00790310011003. [DOI] [PubMed] [Google Scholar]
- Jönsson R, Rosenhall U, Gause-Nilsson I, Steen B. Auditory function in 70- and 75-year- olds of four age cohorts. A cross-sectional and time-lag study of presbyacusis. Scand Audiol. 1998;27:81–93. doi: 10.1080/010503998420324. [DOI] [PubMed] [Google Scholar]
- Jurgens U. The neural control of vocalization in mammals: a review. J Voice. 2009;23:1–10. doi: 10.1016/j.jvoice.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Kilicdag EB, Yavuz H, Bagis T, Tarim E, Erkan AN, Kazanci F. Effects of estrogen therapy on hearing in postmenopausal women. Am J Obstet Gynecol. 2004;190(1):77–82. doi: 10.1016/j.ajog.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kang BM, Chae HD, Kim CH. The association between serum estradiol level and hearing sensitivity in postmenopausal women. Obstet Gynecol. 2002;99(5 Part 1):726–730. doi: 10.1016/s0029-7844(02)01963-4. [DOI] [PubMed] [Google Scholar]
- Kittelberger JM, Bass AH. Vocal-Motor and auditory connectivity of the midbrain periaqueductal gray in a teleost fish. J Comp Neurol. 2012 doi: 10.1002/cne.23202. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittelberger JM, Heisler EK, Allen AK. Dopamine injections to the midbrain periaqueductal gray rapidly and reversibly inhibit vocal production in a teleost fish. Soc Neurosci Abstr. 2011:413.11. doi: 10.1016/j.physbeh.2023.114131. [DOI] [PubMed] [Google Scholar]
- Kittelberger JM, Land BR, Bass AH. Midbrain periaqueductal gray and vocal patterning in a teleost fish. J Neurophysiol. 2006;96(1):71–85. doi: 10.1152/jn.00067.2006. [DOI] [PubMed] [Google Scholar]
- Klinge CM. Estrogen receptor interaction with co-activators and co-repressors. Steroids. 2000;65(5):227–251. doi: 10.1016/s0039-128x(99)00107-5. [DOI] [PubMed] [Google Scholar]
- Klinge CM. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001;29(14):2905–2919. doi: 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp R, Neff BD. Steroid hormones in bluegill, a species with male alternative reproductive tactics including female mimicry. Biol Lett. 2007;3(6):628–631. doi: 10.1098/rsbl.2007.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp R, Wingfield JC, Bass AH. Steroid hormones and paternal care in the plainfin midshipman fish (Porichthys notatus) Horm Behav. 1999;35(1):81–89. doi: 10.1006/hbeh.1998.1499. [DOI] [PubMed] [Google Scholar]
- Leblois A, Perkel DJ. Striatal dopamine modulates song spectral but not temporal features through D1 receptors. Eur J Neurosci. 2012;35(11):1771–1781. doi: 10.1111/j.1460-9568.2012.08095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Baker R, Sotelo C. Electronic coupling between neurons in cat inferior olive. J Neurophysiol. 1974;37(3):560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- Lucas JR, Freeberg TM, Krishnan A, Long GR. A comparative study of avian auditory brainstem responses: correlations with phylogeny and vocal complexity, and seasonal effects. J Comp Physiol A. 2002;188(11–12):981–992. doi: 10.1007/s00359-002-0359-x. [DOI] [PubMed] [Google Scholar]
- Lucas JR, Freeberg TM, Long GR, Krishnan A. Seasonal variation in avian auditory evoked responses to tones: a comparative analysis of Carolina chickadees, tufted titmice, and white-breasted nuthatches. J Comp Physiol A. 2007;193(2):201–215. doi: 10.1007/s00359-006-0180-z. [DOI] [PubMed] [Google Scholar]
- Ma CH, Dong KW, Yu KL. cDNA cloning and expression of a novel estrogen receptor β-subtype in goldfish (Carassius auratus) Biochimica et Biophysica Acta. 2000;1490(1–2):145–152. doi: 10.1016/s0167-4781(99)00235-3. [DOI] [PubMed] [Google Scholar]
- Mank JE, Avise JC. Comparative phylogenetic analysis of male alternative reproductive tactics in ray-finned fishes. Evolution. 2006;60(6):1311–1316. [PubMed] [Google Scholar]
- Maruska KP, Fernald RD. Steroid receptor expression in the fish inner ear varies with sex, social status, and reproductive state. BMC Neurosci. 2010;11:58. doi: 10.1186/1471-2202-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruska KP, Ung US, Fernald RD. The African cichlid fish Astatotilapia burtoni uses acoustic communication for reproduction: sound production, hearing, and behavioral significance. PLoS One. 2012;7(5):e37612. doi: 10.1371/journal.pone.0037612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means AR, O'Malley BW. Mechanism of estrogen action: Early transcriptional and translational events. Metabolism. 1972;21(4):357–370. doi: 10.1016/0026-0495(72)90081-9. [DOI] [PubMed] [Google Scholar]
- Menuet A, Le Page Y, Torres O, Kern L, Kah O, Pakdel F. Analysis of the estrogen regulation of the zebrafish estrogen receptor (ER) reveals distinct effects of ERα, ERβ1 and ERβ2. J Mol Endocrinol. 2004;32(3):975–986. doi: 10.1677/jme.0.0320975. [DOI] [PubMed] [Google Scholar]
- Menuet A, Pellegrini E, Anglade I, Blaise O, Laudet V, Kah O, Pakdel F. Molecular characterization of three estrogen receptor forms in zebrafish: binding characteristics, transactivation properties, and tissue distributions. Biol Reprod. 2002;66(6):1881–1892. doi: 10.1095/biolreprod66.6.1881. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor α and β in the mouse central nervous system: In vivo autoradiographic and immunocytochemical analyses. 2004;473(2):270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor β in the mouse brain: Comparison with estrogen receptor α. Endocrinol. 2003;144(5):2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7(10):1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Nagler JJ, Cavileer T, Sullivan J, Cyr DG, Rexroad C., III The complete nuclear estrogen receptor family in the rainbow trout: discovery of the novel ERα2 and both ERβ isoforms. Gene. 2007;392(1–2):164–173. doi: 10.1016/j.gene.2006.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45(3):S116–S124. doi: 10.1067/mjd.2001.117432. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson J-Å. Mechanisms of estrogen action. Physiol Rev. 2001;81(4):1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- Noirot IC, Adler HJ, Cornil CA, Harada N, Dooling RJ, Balthazart J, Ball GF. Presence of aromatase and estrogen receptor alpha in the inner ear of zebra finches. Hear Res. 2009;252(1–2):49–55. doi: 10.1016/j.heares.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Nomura M, Andersson S, Korach KS, Gustafsson J-A, Pfaff DW, Ogawa S. Estrogen receptor-β gene disruption potentiates estrogen-inducible aggression but not sexual behavior in male mice. Eur J Neurosci. 23(7):1860–1868. doi: 10.1111/j.1460-9568.2006.04703.x. [DOI] [PubMed] [Google Scholar]
- Ogino Y, Katoh H, Kuraku S, Yamada G. Evolutionary history and functional characterization of androgen receptor genes in jawed vertebrates. Endocrinol. 2009;150(12):5415–5427. doi: 10.1210/en.2009-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RF, Canario AVM, Ros AFH. Hormones and alternative reproductive tactics in vertebrates. In: Oliveira RF, Taborsky M, Brockmann HJ, editors. Alternative Reproductive Tactics - an Integrative Approach. Cambridge: Cambridge University Press; 2008b. pp. 132–174. [Google Scholar]
- Oliveira RF, Taborsky M, Brockmann HJ, editors. Alternative Reproductive Tactics - an Integrative Approach. Cambridge: Cambridge University Press; 2008a. [Google Scholar]
- Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20(9):1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- Pfefferle D, Heistermann M, Pirow R, Hodges JK, Fischer J. Estrogen and progestogen correlates of the structure of female copulation calls in semi-free-ranging barbary macaques (Macaca sylvanus) Int J Primatol. 2011;32(4):992–1006. doi: 10.1007/s10764-011-9517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Tremere LA. Control of central auditory processing by a brain-generated oestrogen. Nat Rev Neurosci. 2012;13(8):521–527. doi: 10.1038/nrn3291. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J Neurosci. 2004;24(26):5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. A rapid neuromodulatory role for steroid hormones in the control of reproductive behavior. Brain Res. 2006a;1126(1):27–35. doi: 10.1016/j.brainres.2006.06.049. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. From social behavior to neural circuitry: steroid hormones rapidly modulate advertisement calling via a vocal pattern generator. Horm Behav. 2006b;50(3):432–441. doi: 10.1016/j.yhbeh.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J Neurosci. 2007;27(5):1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Estradiol interacts with an opioidergic network to achieve rapid modulation of a vocal pattern generator. J Comp Physiol A. 2010;196(2):137–146. doi: 10.1007/s00359-009-0500-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L. Brain estrogen signaling effects acute modulation of acoustic communication behaviors: A working hypothesis. Bioessays. 2012;34(12):1009–1016. doi: 10.1002/bies.201200081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink E, Wullimann MF. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum) Brain Res. 2001;889:316–330. doi: 10.1016/s0006-8993(00)03174-7. [DOI] [PubMed] [Google Scholar]
- Rohmann KN, Bass AH. Seasonal plasticity of auditory hair cell frequency sensitivity correlates with plasma steroid levels in vocal fish. J Exp Biol. 2011;214(11):1931–1942. doi: 10.1242/jeb.054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubow TK, Bass AH. Reproductive and diurnal rhythms regulate vocal motor plasticity in a teleost fish. J Exp Biol. 2009;212(20):3252–3262. doi: 10.1242/jeb.032748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidel WM, Lanford PJ, Yan HY, Popper AN. Hair cell heterogeneity in the goldfish saccule. Brain Behav Evol. 1995;46(6):362–370. doi: 10.1159/000113286. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev. 2011;32(4):532–549. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Creco C, Bass AH. Aromatase activity in the hindbrain vocal control region of a teleost fish: divergence among males with alternative reproductive tactics. Proc R Soc Lond, Ser B: Biol Sci. 1999;266(1415):131–136. [Google Scholar]
- Schradin C, Yuen C-H. Hormone levels of male African striped mice change as they switch between alternative reproductive tactics. Horm Behav. 2011;60(5):676–680. doi: 10.1016/j.yhbeh.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Schwartz CP, Smotherman MS. Mapping vocalization-related immediate early gene expression in echolocating bats. Behav Brain Res. 2011;244(2):358–368. doi: 10.1016/j.bbr.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotto-Lavino E, Du G, Frohman MA. Amplification of 5' end cDNA with 'new RACE'. Nat Protocols. 2007;1(6):3056–3061. doi: 10.1038/nprot.2006.479. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and –β mRNA in the rat central nervous system. J Comp Neurol. 1998;63(10):498–504. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Simonoska R, Stenberg AE, Duan M, Yakimchuk K, Fridberger A, Sahlin L, Gustafsson J-A, Hultcrantz M. Inner ear pathology and loss of hearing in estrogen receptor-β deficient mice. J Endocrinol. 2009;201(3):397–406. doi: 10.1677/JOE-09-0060. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M. Aromatase-A brief overview. Annu Rev Physiol. 2002;64(1):93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- Simpson HB, Vicario DS. Early estrogen treatment alone causes female zebra finches to produce learned, male-like vocalizations. J Neurobiol. 1991;22(7):755–776. doi: 10.1002/neu.480220710. [DOI] [PubMed] [Google Scholar]
- Sisneros JA. Seasonal plasticity of auditory saccular sensitivity in the vocal plainfin midshipman fish, Porichthys notatus. J Neurophysiol. 2009;102(2):1121–1131. doi: 10.1152/jn.00236.2009. [DOI] [PubMed] [Google Scholar]
- Sisneros JA, Bass AH. Seasonal plasticity of peripheral auditory frequency sensitivity. J Neurosci. 2003;23(3):1049–1058. doi: 10.1523/JNEUROSCI.23-03-01049.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisneros JA, Forlano PM, Deitcher DL, Bass AH. Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science. 2004b;305(5682):404–407. doi: 10.1126/science.1097218. [DOI] [PubMed] [Google Scholar]
- Sisneros JA, Forlano PM, Knapp R, Bass AH. Seasonal variation of steroid hormone levels in an intertidal-nesting fish, the vocal plainfin midshipman. Gen Comp Endocrinol. 2004a;136(1):101–116. doi: 10.1016/j.ygcen.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Smith SS. Estrous hormones enhance coupled, rhythmic olivary discharge in correlation with facilitated limb stepping. Neurosci. 1998;82:83–95. doi: 10.1016/s0306-4522(97)00211-x. [DOI] [PubMed] [Google Scholar]
- Socorro S, Power DM, Olsson PE, Canario AV. Two estrogen receptors expressed in the teleost fish, Sparus aurata: cDNA cloning, characterization and tissue distribution. J Endocrinol. 2000;166(2):293–306. doi: 10.1677/joe.0.1660293. [DOI] [PubMed] [Google Scholar]
- Soma KK, Tramontin AD, Featherstone J, Brenowitz EA. Estrogen contributes to seasonal plasticity of the adult avian song control system. J Neurobiol. 2004;58(3):413–422. doi: 10.1002/neu.10288. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Llinas R, Baker R. Structural study of inferior olinary nucleus of the cat: morphological correlates of electronic coupling. J Neurophysiol. 1974;37(3):541–559. doi: 10.1152/jn.1974.37.3.541. [DOI] [PubMed] [Google Scholar]
- Tressler J, Schwartz C, Wellman P, Hughes S, Smotherman M. Regulation of bat echolocation pulse acoustics by striatal dopamine. J Exp Biol. 2011;214(19):3238–3247. doi: 10.1242/jeb.058149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinol. 2005;146(2):624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Wong WPS, Tiano JP, Liu SH, Hewitt SC, May C, Dalle S, Katzenellenbogen JA, Katzenellenbogen BS, Korach KS, Mauvais-Jarvis F. Extranuclear estrogen receptor-α stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proc Natl Acad Sci USA. 2010;107(29):13057–13062. doi: 10.1073/pnas.0914501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, Harada N, Shah NM. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139(1):61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yovanof S, Feng AS. Effects of estradiol on auditory evoked responses from the frog’s auditory midbrain. Neurosci Lett. 1983;36(3):291–297. doi: 10.1016/0304-3940(83)90015-0. [DOI] [PubMed] [Google Scholar]