Abstract
Background
The pathogenesis of essential tremor is poorly understood. Historically, the inferior olivary nucleus has been hypothesized to play an important role in the generation of tremor in essential tremor, yet a detailed, controlled, anatomic-pathological study of that brain region has yet to be conducted.
Methods
A detailed postmortem study was undertaken of the microscopic changes in the inferior olivary nucleus of 14 essential tremor cases vs. 15 age-matched controls at the Essential Tremor Centralized Brain Repository. A series of metrics was used to quantify microscopic neuronal and glial changes in the inferior olivary nucleus and its input and output tracts. Olivary linear neuronal density was also assessed.
Results
Cases and controls did not differ from one another with respect to any of the assessed metrics (p values ranged from 0.23 – 1.0). Olivary linear neuronal density was also similar in cases and controls (p = 0.62). Paddle-shaped neurons, a morphologic shape change in olivary neurons, which to our knowledge have not been previously recognized, occurred to an equal degree in ET cases and controls (p = 0.89), and correlated with several markers of neuronal loss and gliosis.
Discussion
A systematic postmortem study of the microscopic changes in the inferior olivary nucleus did not detect any differences between cases and controls. These data, along with positron emission tomography data, which have failed to identify any metabolic abnormality of the olive, indicate that if the olive is involved in essential tremor, there is no clearly identifiable structural or metabolic correlate.
Keywords: essential tremor, brain, pathology, inferior olivary nucleus, pathophysiology, neurodegenerative
Introduction
Although essential tremor (ET) stands out as one of the most prevalent neurological diseases,1–4 its precise pathogenesis is not understood. Recent postmortem studies point to a central role of the cerebellum, where an increasing number of structural changes have been observed in ET brains relative to age-matched control brains.5 These changes include swellings of Purkinje cell axons and dendrites,6–8 heterotopic displacement of Purkinje cells,9 structural remodeling of neighboring neuron populations (i.e., basket cells),10 increased gliosis,11 and, in some cases, more extensive structural changes within the deep cerebellar nuclei.12 Significant (approximately 30 – 40%) loss of Purkinje cells has been reported in adequately powered studies,6, 13 but not in those with smaller sample size.14, 15 Clinical neuroimaging studies also point to a central role of the cerebellum in ET.16–22
Whether other brain structures also contribute to the pathogenesis of ET has been a matter of longstanding debate. In particular, it has been hypothesized that the inferior olivary nucleus (ION), a brainstem structure with inherent oscillatory-pacemaking properties, may play the primary role in the generation of tremor in ET.23–25 Indeed, one early metabolic imaging study suggested that the ION was abnormally activated in ET,26 though no confirmatory studies have shown intrinsic ION activation in ET.27, 28
Examination of brain tissue of clinically well-characterized individuals provides investigators with a powerful and direct means to advance the understanding of disease pathogenesis, yet historically, postmortem tissue in ET has been scarce. Furthermore, although the ION is perfunctorily examined in standard postmortem surveys,6 a detailed, systematic postmortem study focused on the microscopic changes in the ION has not been undertaken in ET cases vs. age-matched controls. Capitalizing on the Essential Tremor Centralized Brain Repository (ETCBR), a tissue resource which banks the largest collection of ET brains,6, 29 we conducted an in-depth microscopic study of the ION, comparing a group of ET brains to those of age-matched controls. Our primary aim was to assess whether microscopic changes where distinctly occurring in ET ION compared to control ION. Furthermore, given the scarcity of microscopic data on the normal ION in humans, a secondary aim of the study was to establish normal reference data on the neuronal and glial changes that are observed in the ION, and their correlations with age. Finally, we observed a number of ION neurons that had an unusual “paddle-shaped” morphology. To our knowledge, these neurons have not been reported previously in the medical literature.
Methods
Cases and Controls
This study was conducted at the ETCBR, New York Brain Bank (NYBB), Columbia University Medical Center (CUMC). All ET cases were diagnosed by their treating neurologist and the ET diagnosis was confirmed using ETCBR criteria by a second neurologist specializing in movement disorders (E.D.L.).6
Age-matched control brains were normal elderly control subjects from the NYBB, derived from the Alzheimer’s Disease Research Center and the Washington Heights Inwood Columbia Aging Project; they were free of clinical diagnoses of Alzheimer’s disease (AD), ET, or Parkinson’s disease (PD) and without neuropathological diagnoses of neurodegenerative disease.6 The NYBB operates under approval of the Institutional Review Board of CUMC.
Using pilot data from five controls, we determined that a sample size of 15 cases and 15 controls would be adequate. Thus, this sample size would provide more than 80% power to detect as little as a 30% increase in each morphologic metric in ET cases versus controls (assuming a two sided test and alpha = 0.05).
Clinical Evaluation
During life, demographic and clinical data were collected using a series of semi-structured questionnaires.6 Data on lifetime exposure to medications known to cause cerebellar damage (e.g., lithium, diphenylhydantoin, chemotherapeutic agents) were collected. Heavy ethanol use was defined previously as consumption of an average of four or more standard drinks (15 ml of absolute ethanol) per day for a man, or three or more per day for a woman, at any point in their lives.6, 30 Most ET cases also underwent a standardized, videotaped neurological examination, which included an assessment of postural tremor (sustained arm extension), five tests of kinetic tremor (pouring, drinking, using spoon, finger-nose-finger maneuver, and drawing spirals), and head and voice tremors.31
Standard Neuropathological Assessment
As previously described, all brains underwent a complete neuropathological assessment by a senior neuropathologist (J.P.G.V.) at the NYBB.6 Each brain had a standardized measurement of brain weight (grams), postmortem interval (PMI, hours between death and placement of brain in a cold room or upon ice), Braak and Braak AD staging for neurofibrillary tangles,32, 33 Consortium to Establish a Registry for AD (CERAD) ratings for neuritic plaques.34 No Lewy pathology was found in any of the brains assessed using alpha-synuclein immunohistochemistry in the brain regions normally examined in our routine neuropathological assessment.
Postmortem Examination of the ION
The medulla oblongata was detached from the brainstem before performing a sagittal section of the brain through the corpus callosum. At least two transverse blocks of the medulla oblongata were harvested for microscopic examination. Each block was 3 mm thick, and included both the left and right ION. One block was obtained 3 mm caudal to the ponto-medullary junction to capture the rostral part of the hypoglossal nucleus, and one block was obtained through the caudal third of the medulla. From these blocks, 7 µm thick paraffin sections were stained with Luxol Fast Blue counterstained with Hematoxylin and Eosin (LH&E).
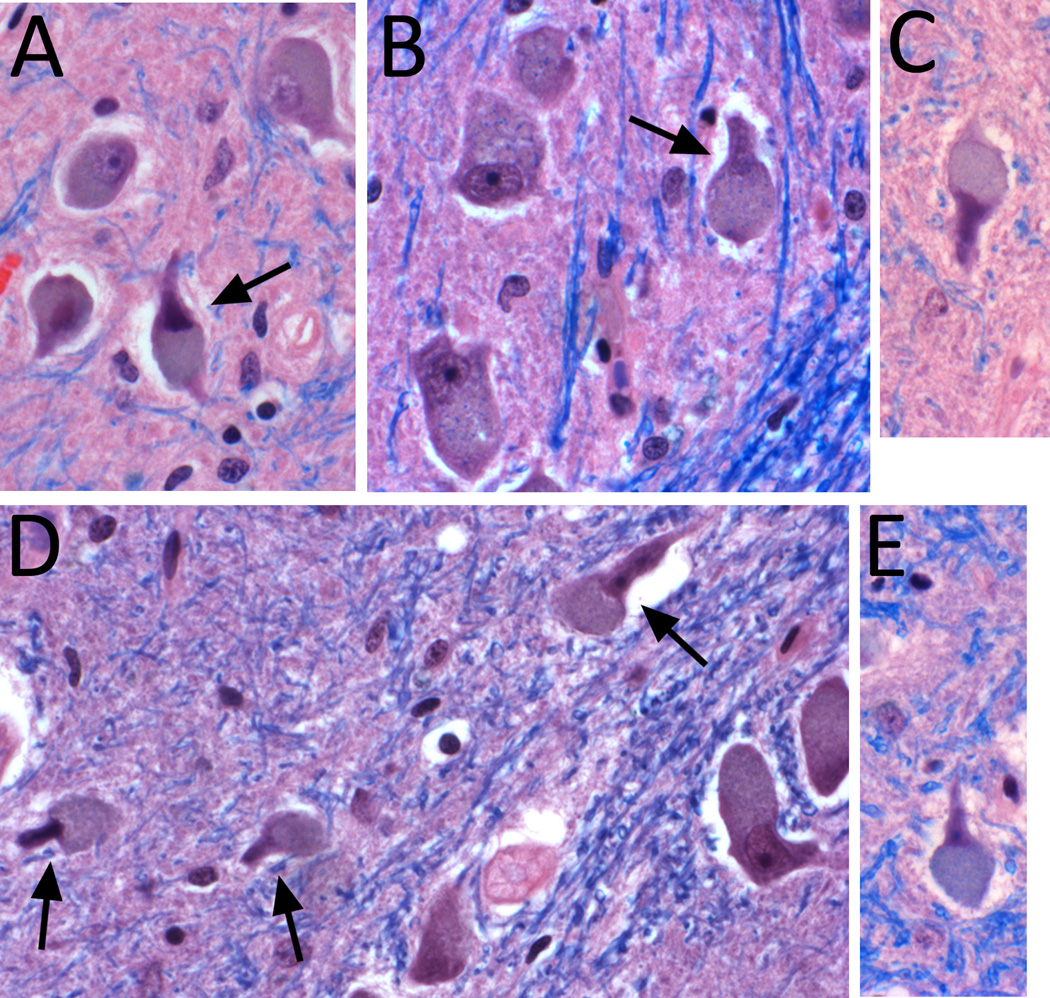
In a pilot study, two clinically-blinded senior neuropathologists (J.P.G.V. and P.L.F.) carefully examined LH&E stained medullary sections from 5 ET cases and 5 controls, and based on their survey, defined a series of metrics that would describe and quantify microscopic neuronal and glial changes in the ION and its white matter input and output tracts (Table 1). Discrete segmental areas of neuronal loss were counted, specifying the extent to which they filled a 400× field (i.e., 1/3 of the field, ½ of the field, or the entire 400× field). Neuronal density and gliosis in the ION were rated using a semi-quantitative scale. Atrophic neurons were quantified; they were defined as neurons having shrunken, densely staining nuclei and cytoplasms with typically angulated borders. Astrocytes with bizarre morphology (moderately enlarged nucleus with prominent nucleolus; Alzheimer type 2-like astrocytes) and discrete foci of neuronophagia were also quantified. Paddle-shaped neurons were identified as a shape change in ION neurons, where rounded eccentric cell body cytoplasm was seen adjacent to a sharply demarcated, elongated nuclear profile (Figure 1). Semi-quantitative metrics were defined that assessed microscopic changes in the white matter input and output tracts of the ION, including pallor of dorsal amiculum, pallor of ventral amiculum, pallor of dorsal hilum, pallor of ventral hilum, and appearance of olivo-cerebellar output fibers as they pass through the medial lemniscus (Table 1). These metrics were applied blindly to five cases and five controls in two separate rating sessions, one month apart, and there was very good agreement across sessions, indicating that the metrics could be used to produce reliable and reproducible results. For gliosis, weighted kappa = 1.0. For segmental areas of neuronal loss (1/3 of the field), weighted kappa = 0.79, for segmental areas of neuronal loss (½ of the field), weighted kappa = 0.92. For segmental areas of neuronal loss (entire 400× field), weighted kappa = 1.0. For neuronal loss, weighted kappa was 0.52, mainly due to disagreement between the 0 and 1 ratings. It was for this reason that we also created an index of ION linear density (neurons/length in microns × 1,000, see below).
Table 1.
Metrics Used to Assess Microscopic Changes in the ION
| Metric | Data or Ratings |
|---|---|
| Metrics that assess microscopic neuronal and glial changes in the ION | |
| Segmental loss of neurons # of discrete areas that fill 1/3 of 400× field # of discrete areas that fill 1/2 of 400× field # of discrete areas that fill all of 400× field |
|
| Neuronal density | 1 = nearly no neurons or no neurons 2 = marked reduction 3 = moderate reduction 4 = mild reduction 5 = normal |
| # of atrophic neurons | |
| # of paddle-shaped neurons | |
| # of foci of neuronophagia | |
| Gliosis | 0 = normal 1 = mild increase 2 = mild to moderate increase 3 = moderate increase 4 = marked increase |
| # of bizarre astrocytes | |
| Metrics that assess microscopic changes in the white matter input and output tracts of the ION | |
| Pallor of dorsal amiculum | 0 = normal 1 = mild pallor 2 = moderate pallor 3 = marked pallor 4 = no myelin |
| Pallor of ventral amiculum | 0 = normal 1 = mild pallor 2 = moderate pallor 3 = marked pallor 4 = no myelin |
| Pallor of dorsal hilum | 0 = normal 1 = mild pallor 2 = moderate pallor 3 = marked pallor 4 = no myelin |
| Pallor of ventral hilum | 0 = normal 1 = mild pallor 2 = moderate pallor 3 = marked pallor 4 = no myelin |
| Appearance of olivo-cerebellar output fibers as they pass through the medial lemniscus | 0 = normal 1 = minimal reduction 2 = mild reduction 3 = moderate reduction 4 = marked reduction |
Figure 1.
Paddle-shaped neurons in the ION in three ET cases (A – C) and two controls (D – E). Paddle-shaped neurons have an elongated, handle-like nuclear contour sharply demarcated from a highly eccentric and rounded cell soma profile (arrows). The more typical appearance of neurons in ION, with a rounded nucleus and cytoplasm within an overall rounded cell profile is also shown (A,B,D). Paraffin sections stained with Luxol-fast blue-H&E, 400× magnification.
Detailed neuronal and glial metrics were assessed within the principal olivary nucleus35 in 14 cases and 15 controls by a clinically-blinded neuropathologist (P.L.F). Several levels of medulla were available (range = 2 – 4, mean = 2.4, median = 2, mode = 2).Within-subject agreement between data generated from different levels was high (e.g., for number of atrophic neurons, r = 0.73, p = 0.005; for number of paddle=shaped neurons, r = 0.82, p = 0.001; for gliosis, Spearman’s r = 0.96, p <0.001). The median value was used in our analyses.
The number of neurons in the principal ION was quantified, counting only those neurons with an identifiable nucleolus in order to provide a point-like cell identifier. The total number of neurons with nucleoli was normalized to a traced linear length of the ION nucleus (in microns), measured with Neurolucida software (MicroBrightField Bioscience, Williston, VT). This was used to create an index of ION linear density (neurons/length in microns × 1,000). As several levels were available, and the correlation between data derived from different levels of the same brain was high (Pearson’s r = 0.997, p = 0.003), the mean value was used.
Quantification of Postmortem Changes in Cerebellum
As described, a standard 3 × 20 × 25 mm parasagittal, formalin-fixed, tissue block was harvested from the neocerebellum;6, 36 the block included the cerebellar cortex, white matter and dentate nucleus. A senior neuropathologist (P.L.F.), who was blinded to all clinical information, counted torpedoes throughout one entire LH&E section, and counted and averaged Purkinje cells in fifteen 100× fields (LH&E).36 As previously described,10 a semi-quantitative rating of the appearance of the basket cell plexus surrounding Purkinje cell bodies throughout Bielschowsky preparations was carried out by the same neuropathologist (P.L.F.) as well. The following scale was used: 0 (few, or no discernible processes); 1 (sparse number of processes); 2 (moderate number of processes); and 3 (dense tangle of processes). In some instances, the rater used intermediate values (0.5, 1.5, and 2.5).
Statistical Analyses
Analyses were performed in SPSS (version 18.0). Clinical and postmortem findings were compared in cases and controls using Student’s t tests, chi-square tests, and Fisher’s exact tests. Correlations were Pearson’s correlation coefficients (r). Non-parametric tests (Mann-Whitney test, Spearman’s r) were also used when required.
Results
Cases vs. controls
There were 15 ET cases and 15 controls, but one case was excluded because of insufficient tissue. The 14 remaining ET cases and 15 controls were similar in age and gender (Table 2). Tremor duration ranged from 17 – 81 years in ET cases (median = 55.5 years); age of onset was ≤64 years in all cases (median = 25 years). No cases or controls were heavy ethanol users or exposed to medications known to cause cerebellar damage. Cases and controls were similar in terms of PMI, brain weight, CERAD plaque score, and Braak AD stage (Table 2). Cases had more torpedoes and fewer Purkinje cells than controls (Table 2). None of the cases or controls had clinical or postmortem diagnoses of AD or PD.
Table 2.
Clinical Characteristics of ET Cases and Controls
| ET Cases N = 14 |
Controls N = 15 |
Significance | |
|---|---|---|---|
| Age (years) | 83.4 ± 7.6, 67 – 95 | 83.1 ± 7.4, 67 – 95 | p = 0.90 A |
| Female gender | 10 (71.4) | 8 (53.3) | p = 0.32 B |
| PMI (hours) | 4.1 ± 5.0 | 8.5 ± 9.9 | p = 0.15 A |
| Brain weight (grams) | 1245 ± 157 | 1224 ± 139 | p = 0.70 A |
| CERAD plaque score | p = 0.75 B | ||
| 0 | 7 (50.5) | 8 (53.3) | |
| A | 4 (28.6) | 4 (26.7) | |
| B | 2 (14.3) | 3 (20.0) | |
| C | 1 (7.1) | 0 (0.0) | |
| Braak AD stage | p = 0.45 B | ||
| 0 | 0 (0.0) | 3 (20.0) | |
| 1 | 5 (35.7) | 4 (26.7) | |
| 2 | 4 (28.6) | 3 (20.0) | |
| 3 | 4 (28.6) | 3 (20.0) | |
| 4 | 1 (7.1) | 2 (13.3) | |
| 5 | 0 (0.0) | 0 (0.0) | |
| 6 | 0 (0.0) | 0 (0.0) | |
| Torpedo count (LH&E) | Median = 13.0 | Median = 3.0 | P = 0.04 C |
| Purkinje cell count (LH&E) | 6.4 ± 2.2 | 8.8 ± 2.2 | P = 0.002 A |
| Basket cell axonal plexus density* | P = 0.15 B | ||
| 0 | 0 (0.0) | 1 (11.1) | |
| 0.5 | 0 (0.0) | 0 (0.0) | |
| 1 | 1 (7.1) | 0 (0.0) | |
| 1.5 | 4 (28.6) | 1 (11.1) | |
| 2 | 3 (21.4) | 5 (55.6) | |
| 2.5 | 0 (0.0) | 1 (11.1) | |
| 3 | 6 (42.9) | 1 (11.1) | |
AD (Alzheimer’s disease), CERAD (Consortium to Establish a Registry for AD), LH&E (Luxol Fast Blue counterstained with Hematoxylin and Eosin), PMI (Postmortem interval).
Unless specified otherwise, values are mean ± standard deviation, range or number (percentage).
Student’s t test.
Chi-square test.
Mann-Whitney test.
Some subjects did not have available data.
ION neuronal linear density was not associated with age (r = −0.20, p = 0.29), gender (p = 0.49), PMI (r = −0.06, p = 0.76), brain weight (r = −0.29, p = 0.12), or Braak AD stage (r = −0.20, p = 0.29), but was marginally associated with CERAD plaque score (r = −0.34, p = 0.07).
The measured linear length of the principal nucleus of the ION was highly similar in ET cases (2.64 ± 5.8 mm) and controls (2.59 ± 7.0 mm), indicating that a well-defined anatomic level was analyzed in this study. Cases and controls did not differ from one another with respect to microscopic changes in the ION (Table 3). This included all of the metrics that assessed microscopic neuronal and glial changes in the ION and all of the metrics that assessed microscopic changes in the input and output tracts of the ION. The ION neuronal linear density was also similar in cases and controls (9.4 ± 3.2 vs. 8.8 ± 3.1, p = 0.62, Table 3).
Table 3.
Microscopic Changes in the ION in ET Cases vs. Controls
| Metric * | Cases (N = 14) |
Controls (N = 15) |
Significance |
|---|---|---|---|
| Segmental loss of neurons | |||
| # of discrete areas that fill 1/3 of 400× field | 3.3 ± 2.0 | 3.4 ± 2.7 | p = 0.90A |
| # of discrete areas that fill 1/2 of 400× field | 1.2 ± 1.9 | 1.8 ± 1.4 | p = 0.40A |
| # of discrete areas that fill all of 400× field | 0.08 ± 0.16 | 0.03 ± 0.13 | p = 0.41A |
| Neuronal density | 0.23B | ||
| Normal | 5 (35.7) | 8 (53.3) | |
| Mild reduction | 8 (57.1) | 7 (46.7) | |
| Moderate reduction | 1 (7.1) | 0 (0.0) | |
| Nearly no neurons/no neurons | 0 (0.0) | 0 (0.0) | |
| # of atrophic neurons | 8.7 ± 3.9 | 6.9 ± 4.9 | 0.27A |
| # of paddle-shaped neurons | 9.0 ± 6.3 | 9.4 ± 7.5 | 0.89A |
| # of foci of neuronophagia | 0.19 ± 0.36 | 0.12 ± 0.35 | 0.62A |
| Gliosis | 1.00C | ||
| Normal | 5 (35.7) | 7 (46.7) | |
| Mild increase | 5 (35.7) | 7 (46.7) | |
| Mild to moderate increase | 2 (14.3) | 1 (6.7) | |
| Moderate increase | 2 (14.3) | 0 (0.0) | |
| Marked increase | 0 (0.0) | 0 (0.0) | |
| # of bizarre astrocytes | Median = 0 | Median = 0 | 0.83D |
| ION Linear Neuronal Density | 9.4 ± 3.2 | 8.8 ± 3.1 | 0.62A |
Student’s t test,
Chi-square test,
Fisher’s Exact test,
Mann-Whitney test
Data on metrics that assess microscopic changes in the white matter input and output tracts of the ION (pallor of dorsal amiculum, pallor of ventral amiculum, pallor of dorsal hilum, pallor of ventral hilum, appearance of olivo-cerebellar output fibers as they pass through the medial lemniscus) are not presented in this table as the ratings for each were 0 (normal) in all cases and all controls.
Neither the microscopic changes in the ION nor the ION neuronal linear density were correlated with torpedo counts (e.g., for linear neuronal density, r = −0.09, p = 0.65). The microscopic changes in the ION and ION neuronal linear density were not correlated with Purkinje cell counts (e.g., for neuronal linear density, r = −0.33, p = 0.14). In an analysis restricted to ET cases, ION neuronal linear density was not associated with tremor duration (r = 0.03, p = 0.92).
Neuronal and glial changes in the ION: correlations with age
In this age-matched sample of ET cases and controls, patient age ranged from 67- to 95-years-old for both groups (Table 2). We did not detect a consistent correlation between older age and segmental loss of neurons (i.e., 2 of the 3 following p values were far greater than 0.5 and one was marginal at 0.05: # of discrete areas that fill 1/3 of 400× field, r = 0.02, p = 0.94; # of discrete areas that fill 1/2 of 400× field, r = −0.07, p = 0.72; # of discrete areas that fill all of 400× field, r = −0.37, p = 0.05). We did not detect a correlation between older age and neuronal density (Spearman’s r = −0.31 p = 0.11), number of atrophic neurons (r = −0.08, p = 0.68), number of paddle-shaped neurons (r = −0.10, p = 0.61), number of foci of neuronophagia (r = −0.13, p = 0.49), gliosis (Spearman’s r = 0.15, p = 0.44), number of bizarre astrocytes (Spearman’s r = −0.19, p = 0.33), or ION neuronal linear density (r = −0.20, p = 0.29).
Correlates of paddle-shaped neurons
We defined a phenotype observed in olivary neurons as “paddle-shaped” when the nucleus appeared as an elongated, handle-like shape that was sharply demarcated from an eccentric and rounded profile of cell body cytoplasm (Figure 1). In analyses of our sample of 29 brains, higher numbers of paddle-shaped neurons were associated with other changes in the ION, including lower ION neuronal linear density (r = −0.35, marginally significant p = 0.06), more atrophic neurons (r = 0.57, p = 0.001), lower semi-quantitative rating of neuronal density (Spearman r = −0.35, marginally significant p = 0.06), larger number of regions with segmental loss of neurons in half the field (r = 0.52, p = 0.004), and more gliosis (Spearman’s r = 0.44, p = 0.016). Paddle-shaped neurons were not observed in medullary neurons outside of the ION, nor in the cerebellar dentate nucleus in the brains examined in this study.
Discussion
Examination of brain tissue provides investigators with powerful and direct means to advance the understanding of the pathogenesis of distinct neurodegenerative diseases. Recent postmortem studies in ET have indeed indicated that a hallmark of the disease is the presence of structural changes in the cerebellum.5 It has been hypothesized that the ION could play an important role in the generation of tremor in ET, yet a detailed and systematic postmortem study of the ION had not been undertaken in ET cases. Despite thorough microscopic and semiquantitative evaluations, no distinctive difference could be detected between the ION of ET cases vs. the ION of controls. Thus, if the ION is dysfunctional in ET, it occurs so far without identifiable structural correlate. Similarly, positron emission tomography studies have failed to identify any metabolic involvement of the ION in ET either.5, 27, 37
Historically, a physiological derangement in the ION, a structure which has inherent oscillatory-pacemaking properties, has been proposed as the possible prime mover in ET, although this is mainly a physiological construct. Rhythm generating networks (i.e., pacemakers) are a non-specific finding, located throughout the mammalian cerebral cortex and brainstem,38, 39 and their role in the generation of ET, although widely discussed, has not been empirically demonstrated. Based on cortico-muscular coherence studies, other investigators have suggested the existence of several rather than one central pacemaker in ET (i.e., hypothesizing the existence of a complex cortical and subcortical network that is responsible for tremor),24, 40 yet the precise location of these pacers is not clear. Furthermore, although these coherence studies posit that the structures in this network could play some role in the propagation of tremor oscillations, they do not demonstrate that these structures are involved in tremor generation per se.41
As there are surprisingly few data on the normal microscopic appearance of the ION in humans, a secondary aim of the study was to establish normal reference data on the neuronal and glial changes in the ION and their correlations with age. The few existing studies have provided cell count data rather than clinical/morphometric data.42–45 Paddle-shaped neurons, which to our knowledge have not been described previously, occurred to an equal degree in cases and controls, but did correlate with other markers of neuronal loss or gliosis, and might be a useful metric for future studies of the ION.
While the quantification of the neuronal linear density of the ION was performed without random, unbiased stereological methods, counting the neuronal nucleolus increases reliability of results, as it provides a point-like cell identifier in paraffin sections. Also, the same approach was used in cases and controls, so that it is unlikely that there was any diagnostically-selective bias.
The study also had several strengths. This is the first detailed, systematic postmortem study of the ION in ET cases vs. age-matched controls, in which a series of semi-quantitative and quantitative metrics were used to assess neuronal and glial changes. Also, the study was able to capitalize on the resources of the ETCBR, which has banked the largest, prospectively-collected number of ET brains worldwide. Finally, we provide normal reference data on the neuronal and glial changes in the ION in elderly controls, their correlations with age, and their correlations with one another.
Recent postmortem studies have revealed that structural brain changes appear to be an attribute of ET. The current study, however, failed to detect any differences between ET cases and controls in terms of morphologic changes in the ION. These data, along with positron emission tomography data, which have failed to identify any metabolic involvement of the ION in ET,5, 27, 37 demonstrate that if the ION is involved in the tremor of ET, there seems to be no clearly identifiable metabolic or structural indication of that.
Acknowledgements
Financial disclosure related to research covered in this article
This work was supported by R01 NS042859 (National Institutes of Health, Bethesda, MD) and by the Claire O’Neil Essential Tremor Research Fund (Columbia University, New York, NY).
Full financial disclosure for the previous 12 months
Elan D. Louis has received research support from the National Institutes of Health: NINDS #R01 NS042859 (principal investigator), NINDS #R01 NS39422 (principal investigator), NINDS #T32 NS07153-24 (principal investigator), NINDS #R01 NS073872 (co-principal investigator), NINDS #R21 NS077094 (co-Investigator), and NINDS #R01 NS36630 (co-Investigator), as well as the Parkinson's Disease Foundation (principal investigator), the Arlene Bronstein Essential Tremor Research Fund (Columbia University), and the Claire O'Neil Essential Tremor Research Fund (Columbia University). Jean-Paul G. Vonsattel has received research support from the National Institutes of Health: NINDS #R01 NS042859 (co-investigator). Phyllis L. Faust has received research support from the National Institutes of Health: NINDS #R21 NS077094 (principal investigator) and NINDS #R01 NS042859 (co-investigator).
Footnotes
Statistical Analyses: The statistical analyses were conducted by Dr. Louis.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest and no competing financial interests.
Author Contributions
Elan D. Louis: Research project conception, organization and execution; statistical analyses design and execution; manuscript writing (writing the first draft and making subsequent revisions).
Rachel Babij: Research project execution; manuscript writing (making subsequent revisions).
Etty Cortés: Research project execution; manuscript writing (making subsequent revisions).
Jean-Paul G. Vonsattel: Research project conception, organization and execution; manuscript writing (making subsequent revisions).
Phyllis L. Faust: Research project conception, organization and execution; manuscript writing (making subsequent revisions).
References
- 1.Benito-Leon J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord. 2003;18:389–394. doi: 10.1002/mds.10376. [DOI] [PubMed] [Google Scholar]
- 2.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;15:534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 3.Dogu O, Sevim S, Camdeviren H, et al. Prevalence of essential tremor: door-to-door neurologic exams in Mersin Province, Turkey. Neurology. 2003;61:1804–1806. doi: 10.1212/01.wnl.0000099075.19951.8c. [DOI] [PubMed] [Google Scholar]
- 4.Benito-Leon J. Essential Tremor: One of the Most Common Neurodegenerative Diseases? Neuroepidemiology. 2011;36:77–78. doi: 10.1159/000323572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis ED. Essential tremor: evolving clinicopathological concepts in an era of intensive post-mortem enquiry. Lancet Neurol. 2010;9:613–622. doi: 10.1016/S1474-4422(10)70090-9. [DOI] [PubMed] [Google Scholar]
- 6.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor, 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 7.Louis ED, Faust PL, Ma KJ, Yu M, Cortes E, Vonsattel JP. Torpedoes in the cerebellar vermis in essential tremor cases vs. controls. Cerebellum. 2011;10:812–819. doi: 10.1007/s12311-011-0291-0. [DOI] [PubMed] [Google Scholar]
- 8.Yu M, Ma K, Faust PL, et al. Increased number of Purkinje cell dendritic swellings in essential tremor. Eur J Neurol. 2012;19:625–630. doi: 10.1111/j.1468-1331.2011.03598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo SH, Erickson-Davis C, Gillman A, Faust PL, Vonsattel JP, Louis ED. Increased number of heterotopic Purkinje cells in essential tremor. J Neurol Neurosurg Psychiatry. 2011;82:1038–1040. doi: 10.1136/jnnp.2010.213330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erickson-Davis CR, Faust PL, Vonsattel JP, Gupta S, Honig LS, Louis ED. "Hairy baskets" associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol. 2010;69:262–271. doi: 10.1097/NEN.0b013e3181d1ad04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shill HA, Adler CH, Sabbagh MN, et al. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology. 2008;70:1452–1455. doi: 10.1212/01.wnl.0000310425.76205.02. [DOI] [PubMed] [Google Scholar]
- 12.Louis ED, Vonsattel JP, Honig LS, et al. Essential tremor associated with pathologic changes in the cerebellum. Arch Neurol. 2006;63:1189–1193. doi: 10.1001/archneur.63.8.1189. [DOI] [PubMed] [Google Scholar]
- 13.Axelrad JE, Louis ED, Honig LS, et al. Reduced purkinje cell number in essential tremor: a postmortem study. Arch Neurol. 2008;65:101–107. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajput AH, Robinson CA, Rajput ML, Robinson SL, Rajput A. Essential tremor is not dependent upon cerebellar Purkinje cell loss. Parkinsonism Relat Disord. 2012;18:626–628. doi: 10.1016/j.parkreldis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Louis ED, Faust PL, Vonsattel JP. Purkinje cell loss is a characteristic of essential tremor: Towards a more mature understanding of pathogenesis. Parkinsonism Relat Disord. 2012;18:1003–1004. doi: 10.1016/j.parkreldis.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Louis ED, Frucht SJ, Rios E. Intention tremor in essential tremor: Prevalence and association with disease duration. Mov Disord. 2009;15:626–627. doi: 10.1002/mds.22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louis ED, Shungu DC, Chan S, Mao X, Jurewicz EC, Watner D. Metabolic abnormality in the cerebellum in patients with essential tremor: a proton magnetic resonance spectroscopic imaging study. Neurosci Lett. 2002;333:17–20. doi: 10.1016/s0304-3940(02)00966-7. [DOI] [PubMed] [Google Scholar]
- 18.Kronenbuerger M, Konczak J, Ziegler W, et al. Balance and motor speech impairment in essential tremor. Cerebellum. 2009;8:389–398. doi: 10.1007/s12311-009-0111-y. [DOI] [PubMed] [Google Scholar]
- 19.Cerasa A, Messina D, Nicoletti G, et al. Cerebellar atrophy in essential tremor using an automated segmentation method. AJNR. 2009;30:1240–1243. doi: 10.3174/ajnr.A1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avanzino L, Bove M, Tacchino A, et al. Cerebellar involvement in timing accuracy of rhythmic finger movements in essential tremor. Eur J Neurosci. 2009;30:1971–1979. doi: 10.1111/j.1460-9568.2009.06984.x. [DOI] [PubMed] [Google Scholar]
- 21.Quattrone A, Cerasa A, Messina D, et al. Essential head tremor is associated with cerebellar vermis atrophy: a volumetric and voxel-based morphometry MR imaging study. AJNR. 2008;29:1692–1697. doi: 10.3174/ajnr.A1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicoletti G, Manners D, Novellino F, et al. Diffusion tensor MRI changes in cerebellar structures of patients with familial essential tremor. Neurology. 2010;74:988–994. doi: 10.1212/WNL.0b013e3181d5a460. [DOI] [PubMed] [Google Scholar]
- 23.Park YG, Park HY, Lee CJ, et al. Ca(V)3.1 is a tremor rhythm pacemaker in the inferior olive. Proc Natl Acad Sci U S A. 2101;107:10731–10736. doi: 10.1073/pnas.1002995107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenz D, Deuschl G. Update on pathogenesis and treatment of essential tremor. Curr Opin Neurol. 2007;20:447–452. doi: 10.1097/WCO.0b013e3281e66942. [DOI] [PubMed] [Google Scholar]
- 25.Deuschl G, Elble R. Essential tremor - Neurodegenerative or nondegenerative disease towards a working definition of ET. Mov Disord. 2009;24:2033–2041. doi: 10.1002/mds.22755. [DOI] [PubMed] [Google Scholar]
- 26.Hallett M, Dubinsky RM. Glucose metabolism in the brain of patients with essential tremor. J Neurol Sci. 1993;114:45–48. doi: 10.1016/0022-510x(93)90047-3. [DOI] [PubMed] [Google Scholar]
- 27.Wills AJ, Jenkins IH, Thompson PD, Findley LJ, Brooks DJ. Red nuclear and cerebellar but no olivary activation associated with essential tremor: a positron emission tomographic study. Ann Neurol. 1994;36:636–642. doi: 10.1002/ana.410360413. [DOI] [PubMed] [Google Scholar]
- 28.Bucher SF, Seelos KC, Dodel RC, Reiser M, Oertel WH. Activation mapping in essential tremor with functional magnetic resonance imaging. Ann Neurol. 1997;41:32–40. doi: 10.1002/ana.410410108. [DOI] [PubMed] [Google Scholar]
- 29.Louis ED, Borden S, Moskowitz CB. Essential tremor centralized brain repository: diagnostic validity and clinical characteristics of a highly selected group of essential tremor cases. Mov Disord. 2005;20:1361–1365. doi: 10.1002/mds.20583. [DOI] [PubMed] [Google Scholar]
- 30.Harasymiw JW, Bean P. Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res. 2001;25:228–235. [PubMed] [Google Scholar]
- 31.Louis ED, Zheng W, Applegate L, Shi L, Factor-Litvak P. Blood harmane concentrations and dietary protein consumption in essential tremor. Neurology. 2005;65:391–396. doi: 10.1212/01.wnl.0000172352.88359.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braak H, Braak E. Diagnostic criteria for neuropathologic assessment of Alzheimer's disease. Neurobiol Aging. 1997;18(4 Suppl):S85–S88. doi: 10.1016/s0197-4580(97)00062-6. [DOI] [PubMed] [Google Scholar]
- 33.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: a commentary. Neurobiol Aging. 1997;18(4 Suppl):S91–S94. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 35.Baizer JS, Sherwood CC, Hof PR, Witelson SF, Sultan F. Neurochemical and structural organization of the principal nucleus of the inferior olive in the human. Anat Rec (Hoboken) 294:1198–1216. doi: 10.1002/ar.21400. [DOI] [PubMed] [Google Scholar]
- 36.Louis ED, Vonsattel JP, Honig LS, Ross GW, Lyons KE, Pahwa R. Neuropathologic findings in essential tremor. Neurology. 2006;66:1756–1759. doi: 10.1212/01.wnl.0000218162.80315.b9. [DOI] [PubMed] [Google Scholar]
- 37.Wills AJ, Jenkins IH, Thompson PD, Findley LJ, Brooks DJ. A positron emission tomography study of cerebral activation associated with essential and writing tremor. Arch Neurol. 1995;52:299–305. doi: 10.1001/archneur.1995.00540270095025. [DOI] [PubMed] [Google Scholar]
- 38.Li WC, Roberts A, Soffe SR. Specific brainstem neurons switch each other into pacemaker mode to drive movement by activating NMDA receptors. J Neurosci. 30:16609–16620. doi: 10.1523/JNEUROSCI.3695-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 40.Raethjen J, Lindemann M, Schmaljohann H, Wenzelburger R, Pfister G, Deuschl G. Multiple oscillators are causing parkinsonian and essential tremor. Mov Disord. 2000;15:84–94. doi: 10.1002/1531-8257(200001)15:1<84::aid-mds1014>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 41.Raethjen J, Govindan RB, Kopper F, Muthuraman M, Deuschl G. Cortical involvement in the generation of essential tremor. J Neurophysiol. 2007;97:3219–3228. doi: 10.1152/jn.00477.2006. [DOI] [PubMed] [Google Scholar]
- 42.Porzionato A, Macchi V, Stecco C, et al. Morphometric analysis of the inferior olivary complex in infants and adults. Ital J Anat Embryol. 2008;113:65–73. [PubMed] [Google Scholar]
- 43.Lasn H, Winblad B, Bogdanovic N. Neuroglia in the inferior olivary nucleus during normal aging and Alzheimer's disease. J Cell Mol Med. 2006;10:145–156. doi: 10.1111/j.1582-4934.2006.tb00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pine SS, Landing BH, Shankle WR. Reduced inferior olivary neuron number in early Down syndrome. Pediatr Pathol Lab Med. 1997;17:537–545. [PubMed] [Google Scholar]
- 45.Wenning GK, Tison F, Elliott L, Quinn NP, Daniel SE. Olivopontocerebellar pathology in multiple system atrophy. Mov Disord. 1996;11:157–162. doi: 10.1002/mds.870110207. [DOI] [PubMed] [Google Scholar]