Abstract
Metal contamination was investigated in soils of the Vallecamonica, an area in the northern part of the Brescia province (Italy) where ferroalloy industries were active for a century until 2001. The extent in which emissions from ferroalloy plants affected metal concentration in soils is not known in this area. In this study the geogenic and/or anthropogenic origin of metals in soils were estimated. A modified Community Bureau of Reference sequential chemical extraction method followed by inductive coupled plasma optical emission spectroscopy analyses were employed to evaluate the potential bioavailability of Al, Cd, Mn, Fe, Cr, Zn, and Pb in soils. Principal components analysis was used to assess the relationships among metal sources in soil samples from different locations. This approach allowed to distinguish different loadings and mobilities of metals in soils collected in different areas. Results showed high concentrations and readily extractability of Mn in the Vallecamonica soils, which may suggest potential bioavailability for organisms and may create an environmental risk and potential health risk of human exposure.
Keywords: soils fractionation, heavy metals, Principal Component Analysis (PCA)
Introduction
Manganese (Mn), an essential element for humans, is also known to be a potent neurotoxicant at elevated exposures (Lucchini et al. 2007). Manganese contained in dust and soils can be absorbed into the human body through different routes including ingestion and inhalation: in dusty environments, it has been estimated that adults could ingest up to 100 mg of dust per day (ATSDR 2005). Children are more susceptible than adults to metal exposure because of the hand-to-mouth activities and greater gastrointestinal absorptive rates for ingested metals (Centers for Disease Control, 2005).
Studies on children suggest adverse effects of elevated Mn exposure on their neurobehavioral functioning (Gail et al. 2011).
In a recent study by Lucchini et al. (2012) elevated environmental Mn was associated with sub-clinical deficits in olfactory and motor function among adolescents in the Vallecamonica, a valley in the pre-Alps in the Northern part of Brescia province where ferroalloy production ceased in 2001 after about a century. This study showed an association between Mn exposure reflected by Mn levels in surface soil, hair and blood and impairment of motor coordination (hand dexterity), tremor intensity and odor identification. The results showed that these effects were associated with past or cumulative environmental exposures to Mn, but not current exposure.
Ecological, environmental and health effects of metal exposure from soil depend on the mobility and availability of the elements (Violante et al. 2010). Total metal levels in soil reflect the soil’s geological origin and weathering, and in some cases anthropogenic inputs of metals from industrial processes (Kersten and Förstner 1989). In addition to the total concentration of metals in soil it may also be important to assess the speciation, i.e. chemical forms, of the metals that may influence their environmental mobility and availability to organisms. To address this, selective chemical extraction methods can be used to estimate the relative mobility of metals in soils including the extent that metals may be bound to particular soil phases (Rao et al. 2008). For example, several different reagents can be used to estimate metal mobility, including the use of reagents to release metals on exchange sites, or metals bound or associated with soil organic matter (Keller and Hammer 2004; Rauret et al. 2001). It has also been shown that a single chemical extraction procedure does not provide an accurate estimate of the amounts of metals released from soil (Madrid et al. 2007). Rather, it is now recognized that more detailed and potentially valuable information may be gained by using sequential chemical extraction methods that combine a series of reagents in a sequential extraction procedure, in which the residue from one extraction is used as the material for the next extraction through a pre-determined number of stages.
During the last decade, a ‘BCR’ three-step protocol, proposed by the Standards, Measurements and Testing Programme of the European Commission has been extensively used, either in its original form (Ure et al. 1993) or with modifications (Rauret et al. 1999).
Here, our main objectives were: (a) To determine the chemically labile levels of various metals (Cd, Cr, Al, Fe, Zn, Pb, and Mn) in the soil of Vallecamonica compared to the reference area of Garda Lake, using the revised BCR sequential extraction protocol, and (b) to assess the contribution of geogenic and anthropogenic sources of these metals in soil, especially of Mn. The previous studies on metals in environmental media conducted in Vallecamonica by our group have focused on the total levels of metals, and the information on their prevailing chemical forms is scarcely available (Lucchini et al. 2012).
The previous environmental measurements were also mainly focused on airborne particulate matter (Bontempi et al. 2009; Borgese et al. 2011; Borgese et al. 2012). The information obtained with the analysis of soil after sequential chemical extraction may be useful to better understand the origin of metal contamination in soil of the Vallecamonica and potential risk for human metal exposure.
Experimental design
Study site, sample collection, and preparation
The province of Brescia is located within the Lombardia Region in Northern Italy and covers an area of 4748 km2. The Vallecamonica is an area in the Northern part of the Brescia province where three previously operating Mn-ferroalloy plants were located. The three plants sites are located in different municipalities about 12 km from each other. A more detailed description of the study site is reported in previous publications (Lucchini et al., 2007; Zacco et al., 2009). A total number of 85 soil samples were collected for this work, 69 of them were collected in the Vallecamonica and 16 samples in the Garda Lake reference area. Results of a previously reported study (Borgese et al. 2011) showed that the content of Mn in the Vallecamonica air particulates, are on average two to three times higher than those in the reference area of Garda Lake. However, those studies reported a limited number of sampling sites, which showed some degree of variation in metal levels, suggesting the need for a larger amount of sampling particularly around sites of presumed metal contamination. For this reason the number of Vallecamonica samples is three times larger than the Garda Lake samples.
The samples in the Vallecamonica area were collected at different linear distances between 0.44 and 24.4 Km from the ferroalloy sites, whereas the soil samples from the Garda Lake reference area, which is totally outside the Vallecamonica area, were collected at a linear distance of more than 17 km from the nearest ferroalloy plant.
Surface soil samples were collected within the top 10 cm of the soil surface. All samples were dried (65 °C), ground in a centrifugal ball mill to homogenize, sieved to <150 μm, stored in polyethylene containers unit analysis.
Extraction procedure
A modification of the three step BCR sequential extraction method described by Tokalioglu et al. (2010) was applied to 1 g of soil in duplicate. The main modification with respect to Tokalioglu et al. (2010) was that the final extraction step used 7.5 N HNO3, rather than aqua regia. This is an important difference if one cares about the chemically immobile silicate-bound fractions which may not be extracted with 7.5 N HNO3, but it is unimportant for exposure risk assessment, since silicate-bound metals are biologically unavailable. We used 7.5N HNO3, rather than aqua regia largely because our primary interests in soil metal levels was in the fraction of metals that might be chemically mobile in the environment. Clearly, differences in soil metal extractions via 7.5N HNO3 versus aqua regia are not important in this regard, since both extraction methods, when preceded by weaker extraction steps as performed here, would mobilize metals that would not be expected to be mobilized under environmental conditions.
The four steps of the adopted sequential extraction procedure identify four fractions (f1, f2, f3 and f4) reflecting different degrees of elemental solubility, with f1 containing the readily extractable metals. The sum of elemental concentration of each fraction represents the “near total concentration” of one element in the sample.
Validation of the analytical method
Limits of detection (LOD) of inductively coupled plasma optical emission spectroscopy (ICP-OES) measurements for the elements of interest were calculated as three times the standard deviation (SD) for blank measurements. One half the detection limit was used to estimate the concentration of metals in samples reported as not detected.
Quality assurance (QA) and quality control (QC), were performed according to US EPA Method 200.7. A selection of seven different soil samples analyzed triplicate was included to evaluate intra-sample and intra-measurement variability for the elements of interest. The higher values of relative standard deviation occur when the metal concentration is very near to the LOD. Analysis of QA/QC data revealed an acceptable degree of accuracy and precision.
The certified reference material CRM483 from the Community Bureau of Reference was treated according to the modified BCR sequential extraction scheme followed by ICP-OES analysis. This standard reference materials was chosen for the extractable contents of Cd, Cr, Cu, Ni, Pb and Zn with EDTA and acetic acid extraction method by Quevauviller et al. (1997), and it was also adopted for the three step modified BCR-sequential extraction by Rauret et al. (2000). Low values of SD with some exception, maybe due to the closeness with the LOD, have been found. There are high discrepancies among data reported from many authors about CRM483 (Rauret et al., Mossop et al., Sutherland et al., Kubova et al.). In the work of Kubova et al. (2004) the modified BCR three-step sequential procedure (Whalley and Grant 1994) was applied to CRM483. The basis for the modified BCR, as detailed by Mossop et al (2003), is that significant inter-laboratory variability was apparent in the original BCR, in particular in step 2 of the extraction. This limited the number of elements for which certified values could be agreed and, eventually, led to a thorough reevaluation of this step of the protocol. Using CRM 601 as the test substrate, comparison studies led to the development of the modified BCR sequential extraction procedure. The revised protocol involves use of an increased concentration of NH2OH.HCl and lower pH. It improves reproducibility due, it is thought, to a more efficient dissolution of the reducible fraction of the soil matrix. Our results are in agreement with that reported by Kubova et al. (2004). The main differences occur in the last fraction (f4) probably because of the use of nitric acid instead of aqua regia.
Data analysis
Principal component analysis (PCA) is a suitable tool for resolution and modeling of large environmental multivariate datasets generated in fractionation studies (Dong et al. 2010). PCA is a data reduction procedure (Singh et al. 2005) that provides an easy visualization of latent relationships among objects or variables in large and complex data sets. In this work, PCA was applied to the data matrix composed of 85 soil samples and seven metals to elucidate common factors affecting the chemical availability of the metals relative to their near-total concentration in soil. Metals may be complexed differently in soil according to their natural or anthropogenic origin.
Results from the fractionation methods are used for univariate analysis (i.e. element by element and/or sample by sample), but this approach can hardly identify interrelations between different elements in large datasets. Multivariate statistical analysis is a more useful technique to show common patterns in data distributions, as for example, a common origin or source of values, leading to a reduction of the initial dimension of data sets and facilitating its interpretation. Therefore, PCA is a useful approach for the analysis of very large sets of experimental data, as in the present case, for interpretation and to separate the variables in subgroups in a data matrix.
Results
The concentration of metals in each of the four fractions of each sample was measured. Table I shows the concentration range and median of Al, Cr, Mn, Fe, Zn, Cd and Pb in soil samples of Vallecamonica and Garda Lake. Near total metal concentrations were calculated as the sum of values from all four fractions (f1, f2 f3 and f4). The median value for each metal in each fraction was considered to discuss the overall data set.
Table I.
Median metal content in different fractions of extracted soils (mg/kg).
| Element | Al | Cr | Mn | Fe | Zn | Cd | Pb | ||
|---|---|---|---|---|---|---|---|---|---|
| Exchange able fraction (f1) | Vallecamonica | Range | 2.3 – 49.6 | 0.02 – 2.07 | 11 – 258 | 6.6 – 13 | 1.2 – 110 | 0.05 – 1.40 | 0.4 – 9.8 |
| Median | 5.2 | 0.2 | 79 | 9 | 24 | 0.4 | 3.1 | ||
|
| |||||||||
| Lake Garda | Range | 2.3 – 4.6 | 0.02 – 1 | 11 – 182 | 1.7 – 12.8 | 0.6 – 93 | 0.05 – 1.25 | 0.4 – 11 | |
| Median | 3.4 | 0.4 | 92 | 9.3 | 12 | 0.8 | 7.0 | ||
|
| |||||||||
| Reducible fraction (f2 ) | Vallecamonica | Range | 5.9 – 5063 | 0.17 – 6.56 | 245 – 1779 | 62.7 – 3980 | 3.3 – 286 | 0.32 – 1.60 | 6.1 – 107 |
| Median | 1782 | 1.8 | 649 | 1375 | 71 | 0.6 | 32 | ||
|
| |||||||||
| Lake Garda | Range | 2.9 – 1373 | 0.18 – 7.94 | 105 – 407 | 23 – 1077 | 1.5 – 139 | 0.27 – 1.05 | 2.9 – 37 | |
| Median | 179 | 1.1 | 225 | 316 | 33 | 1 | 10 | ||
|
| |||||||||
| Oxidizable fraction (f3) | Vallecamonica | Range | 195 – 2667 | 1.31 – 121 | 6.8 – 297 | 31.6 – 4026 | 2 – 184 | 0.03 – 0.79 | 1.4 – 221 |
| Median | 1079 | 4.4 | 30 | 1135 | 15 | 0.2 | 9.4 | ||
|
| |||||||||
| Lake Garda | Range | 194 – 1359 | 1.31 – 193 | 6.9 – 121 | 32 – 1758 | 2.0 – 78 | 0.03 – 0.65 | 1.4 – 49 | |
| Median | 928 | 7.5 | 22 | 579 | 26 | 0.3 | 18 | ||
|
| |||||||||
| Insoluble compounds fraction (f4) | Vallecamonica | Range | 627 – 8887 | 1.25 – 14.5 | 12.7 – 309 | 878 – 10184 | 6.5 – 77 | 0.11 – 1.39 | 0.4 – 31 |
| Median | 3153 | 4.7 | 72 | 4958 | 29 | 0.7 | 3.1 | ||
|
| |||||||||
| Lake Garda | Range | 627 – 4036 | 1.25 – 16.2 | 13 – 57 | 879 – 5927 | 6.5 – 79 | 0.11 – 0.75 | 0.4 – 54 | |
| Median | 1967 | 4.6 | 28 | 2909 | 19 | 0.4 | 4.9 | ||
|
| |||||||||
| Total Metal Level | Vallecamonica | Range | 1801 – 15619 | 3.8 – 139 | 380 – 2384 | 2518 – 14035 | 28 – 456 | 0.84 – 4.39 | 14 – 358 |
| Median | 6019 | 11 | 830 | 7476 | 140 | 1.9 | 48 | ||
|
| |||||||||
| Lake Garda | Range | 1801 – 5153 | 3.8 – 216 | 239 – 601 | 2464 – 6973 | 17 – 363 | 0.66 – 2.9 | 14 – 103 | |
| Median | 3077 | 14 | 367 | 3814 | 90 | 2.1 | 39 | ||
The sum total content of metals in a soil sample are well represented by the near-total metal concentrations in soil measured here. Here the near total concentrations of all elements are considerably lower in the Garda Lake soil samples compared to Vallecamonica area, with the exception of Cr and Zn in f4 and Pb in f1 for which the values are comparable between the two regions. The median values of near-total concentration for Mn, Zn, Pb, Fe and Al are higher in the Vallecamonica, while Cd and Cr concentrations are similar.
The assessment of soil metal contamination may be accomplished comparing the total metal concentrations to protective exposure standard values or to background values. Comparative results for metal concentrations in contaminated soil samples are reported in Table II. These values represent critical concentrations of metals for microbiota (Vanmechelen et al. 1997).
Table II.
Metal concentrations in contaminated soil samples (mg/kg) Kabata-Pendias and Pendias (1984) (Vanmechelen et al. 1997).
| Element | Concentration (mg/kg) |
|---|---|
| Cr | 75 |
| Mn | 1500 |
| Zn | 170 |
| Cd | 3 |
| Pb | 100 |
The median value of near-total concentrations of Mn in the Vallecamonica soil samples (see Table I) is 830 mg/kg (with a maximum value of about 2384 mg/kg). This value agrees with results found by XRF analyses (Lucchini et al. 2012) and it is very close to the Mn contaminated soil concentration of about 1000 mg/kg reported by other authors (Tokalioglu et al. 2004). The majority of soils in Europe contain a Mn concentration lower than 1000 mg/kg (Vanmechelen et al. 1997). Kabata-Pendias and Pendias (1984) proposed 1500 mg/kg as the threshold value at which manganese toxicity may occur. For some of the Vallecamonica soils Mn concentrations exceeded this suggested threshold value.
Discussion
Evaluation of experimental data sets
Results suggest that many of the soils in the Vallecamonica municipal districts located in the investigated area may be contaminated with metals. In the Vallecamonica region the primary anthropogenic sources of Mn in the environment are emissions from industrial plants, such as ferroalloy production plants and iron and steel foundries (Santamaria 2008). However the crustal origin of Mn in soil must also be considered as an underlying important source. To estimate the geogenic contribution of Mn in the Vallecamonica soil samples, it is necessary to distinguish the different extractable fractions.
In a typical soil metal extraction procedure, metal compounds present in the first fraction are those that are weakly bound at cation-exchange sites in the matrix and hence chemically very labile. Subsequent processing steps typically extract metals from the carbonate phase, organic matter, etc. Metals in the water/acid soluble and exchangeable fractions are considered the most mobile and potentially bioavailable forms present in soils, and may best capture the anthropogenic contribution of greatest possible concern for human exposure, followed by the carbonate phase. Iron and Mn oxides fractions are relatively stable under typical environmental conditions, and can be mobilized (typically in the laboratory) under acidic conditions. Metals associated with the organic phase are relatively stable in nature, but can be mobilized under strong oxidizing conditions that degrade the organic matter, leading to a release of metals.
Finally, more refractory soil components, related to the geogenic origins of metals in soil (for example including the primary silicates) can be dissolved to obtain the so-called “residual” or chemically inert fraction of metals in soil. This fraction typically contained within the silicate crystal matrix of the minerals and represents the relatively immobile fraction of metals in soil (Tessier et al. 1979).
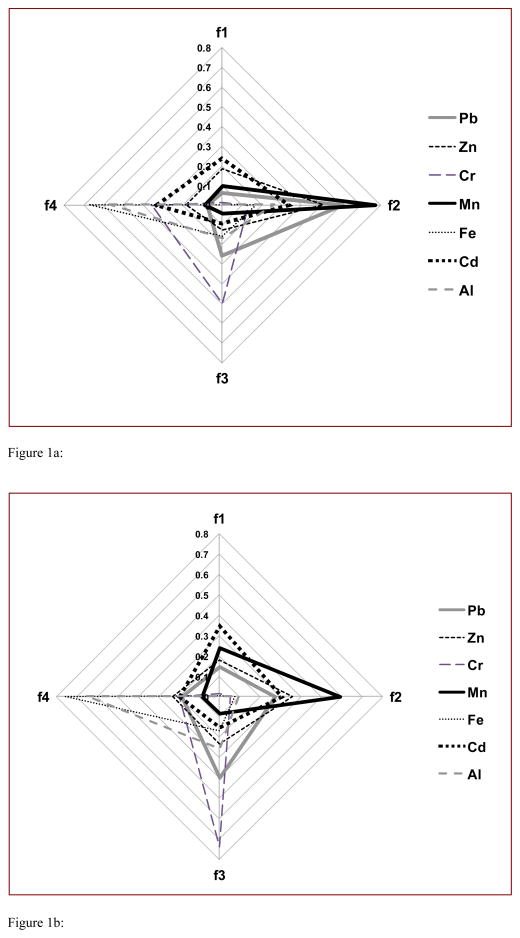
Because of the large set of data, and to discuss the results globally, median values are represented in percent fractions in Figure 1.
Figure 1.
Figure 1a. Percent of the f1, f2, f3, and f4 fractions median values, obtained for Vallecamonica soils.
Figure 1b. Percent of the f1, f2, f3, and f4 fractions median values, obtained for Garda Lake soils.
Aluminum and Fe show the highest percentage in the residual fraction (f4) (respectively 52% and 66% for Vallecamonica and 64% and 76% for Garda Lake). Chromium shows the highest percentage in the oxidizable (f3) and residual fractions (respectively 40% and 43% for Vallecamonica and 55% and 34% for Garda Lake). Relatively high metal levels in the residual fraction indicates that a relatively large fraction of metals in the soil sample are strongly bounded to clay minerals. As a consequence, the metals present in the residual, strong acid extractable fractions may be considered biologically unavailable. Cadmium is the only element that exist at comparable levels in both the Vallecamonica and the Garda Lake soils, and shows comparable levels in the exchangeable (f1), reducible (f2) and residual fractions for the two areas (only the oxidizable fraction is slightly lower). Also Pb and Zn show comparable levels in the exchangeable, reducible and residual fractions of soils from the Garda Lake region, but not the Vallecamonica. Lead and Zn show the highest concentrations in the reducible fraction (respectively 67% and 51%) for the Vallecamonica samples. It was reported that Zn in clay soil, very diffused in the studied area (Sesana et al. 2005), occurs mostly in the residual fraction (73%), while Zn is more mobile in sandy soils where it is relatively abundant in the reducible fraction (Brazauskiene et al. 2008). The relatively high percent of Pb in the reducible fraction (24%) of the Garda Lake samples may be due to its tendency to adsorb onto clay minerals, organic matter and Fe, Mn, and Al oxides, and co-precipitate with metal oxides (Favas et al. 2001). Lead is mainly contained in the oxidizable fraction of the Garda Lake samples (about 45%), probably because of its complexation with sediments being released after degradation of organic matter or oxidation from sulfides to sulfates (Diaz-de Alba et al. 2011). Lead, Zn, Mn, Cr and Fe concentrations associated with the exchangeable and reducible fractions are higher in the Vallecamonica samples, compared to the Garda Lake samples, so their potential bioavailability is greater in that area (Cd shows comparable values). Manganese levels in the Vallecamonica soils shows the following order of association: reducible (78%) > exchangeable (10%) > residual (8%) > oxidizable (4%). The reducible fraction of extracted Mn accounts for Mn that is primarily bound to Fe-Mn oxides in soil. While it was reported that in slightly contaminated soils Mn was mainly associated with the reducible fraction, such as Mn oxides (Pueyo et al. 2003), the large difference in the absolute Mn concentration and in the reducible fraction from samples from the Vallecamonica (78%) and the Garda Lake (59%), strongly suggests its anthropogenic origin in the former. This interpretation is supported by the observation that the percentage of Mn in the residual fraction (8%), is lower compared to other published data (Tokalioglu et al. 2010).
We next performed analysis of the data by considering the readily extractable fractions (f1+f2) were considered separately from the less extractable ones (f3+f4). Soils metals of anthropogenic origin are most likely selectively enriched in the two first fractions that include the adsorptive, exchangeable and bound to carbonates metals, which are considered to be weakly bound and may be relatively labile with the aqueous phase thus possibly becoming more readily bioavailable. In contrast, metals in f3+f4 represent the less soluble fraction of metals in soils and reflect the geogenic origin of the soil material.
Figure 2 shows Cr, Cd, Fe and Mn concentrations in f3+f4 fractions, normalized to the Al concentration in the f3+f4 fractions for all the analyzed samples. It is evident that these concentrations are correlated, suggesting the same natural origin for these metals. As already pointed out the soil in the whole Brescia province is calcareous with the presence of silicates and clay (Sesana et al. 2005). This is supported by the fact that the soil levels of all metals, except Mn and Fe, are very comparable throughout soils of the Vallecamonica. In contrast the median values of f3+f4 for Mn and Fe is about twice as high in Vallecamonica compared to the
Figure 2.
f3+f4 fractions metal concentration as a function of Al f3+f4 concentration
Garda Lake region, indicating that from a geological standpoint Mn and Fe are relatively enriched in the Vallecamonica area. Indeed the Vallecamonica is well known from antiquity because its habitants worked with metals, largely because of the presence of natural siderite (Fe carbonate minerals containing Mn) deposits in this region (Brigo and Venerandi 2005). In light of this, it is important to understand if the higher levels of Mn in soils of the Vallecamonica area are due only to natural sources or also to anthropogenic contributions.
The radar charts in Figure 3a and b show the median proportions of each element associated with f1+f2 and f3+f4 fractions respectively in Vallecamonica and Garda Lake samples. As expected metals that show lower concentration in the readily extractable fractions also show higher contributions in less extractable fractions and vice-versa. It is very interesting to notice that the behavior of metals in the Vallecamonica soils seems to be very similar to the Garda Lake samples and no substantial differences can be highlighted from these data. This strongly supports the interpretation that Al, Fe and Cr in soils from these two areas are mainly of geogenic origin.
Figure 3.
Figure 3a. Radar chart showing the median proportions distribution of each element associated with f1+f2 and f3+f4 fractions for Vallecamonica soils
Figure 3b. Radar chart showing the median proportions distribution of each element associated with f1+f2 and f3+f4 fractions for Garda Lake soils
Principal component analysis
PCA analysis was performed on data of f3+f4 and f1+f2 fractions from all the 85 sampling sites. The results are shown in Figure 4. Figure 4a shows the PCA of the less extractable fractions (f3 and f4). It is evident that, despite higher scattering in the Vallecamonica data, the Garda lake samples do not show evident segregation from the Vallecamonica samples. Therefore it is possible to conclude that PCA of f3+f4 cannot distinguish between the two investigated areas. This is probably due to the primarily crustal origin of metals in these fractions (f3+f4) and the similar geology of the two investigated areas, in agreement with the results reported in Figure 3.
Figure 4.
Figure 4a. PCA results performed on f3+f4 fractions for all the 85 sampling sites.
Figure 4b. PCA results performed on f1+f2 fractions for all the 85 sampling sites.
Figure 4b shows the PCA of the readily extractable fraction of metals (f1+f2). It is evident that the Garda Lake samples form a cluster (as highlighted with the circle), suggesting similarities in the data set. This result suggests that almost all of the Garda Lake samples share properties that distinguish them from the Vallecamonica samples, on the basis of the potential mobility of metals in the environment. It is also noteworthy that the Vallecamonica samples do not form a cluster, indicating greater variation among those samples, and likely greater variation in the origin of readily extractable metals in soil. This observation is consistent with the fact that the Vallecamonica samples were collected at different distances from the Mn-Fe alloy plants, likely capturing a wide range of influence of ferroalloy plant operations on surface soil metal levels.
Thus results of PCA not only substantiate the geologic origin of some metals, but also reveal specific differences in the anthropogenic origin of metals in soil between the two areas investigated here.
PCA highlights that anthropogenic source metals in soils in the two investigated areas are largely captured in the two first fractions, reflecting that anthropogenically contributed metals are weakly bound to soil constituents. The high chemical lability of metals reflected in the first two fractions gives an indication of metal availability, which in turn may reflect potential exposure risk connected with the presence of metals. In order to estimate the environmental risk associated with metal pollution in the Vallecamonica, and to identify those metals posing the greatest risk, soil samples were classified on the basis of their chemical lability, or extractability in soil. For this, the extractability was related to the metal quantity released into the mobile phases (based on the sums of the two first fractions) relative to the near-total metal content in soil (Obrador et al. 2001; Rodríguez et al. 2009). To compare the results obtained from the two areas, the f1+f2 median concentration values were considered to reflect the readily extractable fraction of metals in soil. The results of this analysis are reported in Figure 5. The criterion used for classification is the following: soils that can release in fractions f1+f2 more than 50% of their total metal content may be considered to pose greater environmental exposure risk than soils that release less for a given total soil metal content, because their released metals can readily enter the food chain (Rath et al. 2009).
Figure 5.
Environmental risk associated with metal pollution. Dotted line indicates dangerous value (Rath et al. 2009).
All metals in the Vallecamonica samples (except Cd, that shows very low concentration) were more mobile than in the Garda Lake samples. In particular, the concentrations of metals in f1+f2 of the Vallecamonica samples were respectively: Mn (99%) > Zn (84%) > Pb (71%) > Cd (55%) > Al (33%) > Fe (20%) > Cr (16%). With respect to the other metals Mn is mostly present in the readily extractable fractions, and this suggests that Mn in Vallecamonica soils poses the greatest potential risk of exposure compared to soils from the Garda Lake.
Despite the relative levels of metals in fractions f3 and f4 evidence a common geogenic origin of the relatively refractive metals in soil in both areas, the amounts of metals like Mn in the readily extractable fractions, f1+f2, evidence an anthropogenic origin of those metals in the Vallecamonica compared to the Garda Lake.
Our results are in agreement with recently reported data (Lucchini et al. 2012) that showed a significant association of total Mn levels in soil with some neuromotor changes among adolescents residing in the Vallecamonica. Moreover, the results show that in the Vallecamonica soil metals levels are elevated due both to anthropogenic inputs and contributions from geologic sources that are naturally enriched in metals.
Conclusions
Metal concentrations in soils of the Vallecamonica, a Northern Italy region impacted by Mn-ferroalloy plant emissions for over a century, were compared to that of the Garda Lake. The BCR sequential extraction procedure was applied to provide information on the environmental mobility and potential toxicity risk of metals in soil. This approach allowed us to distinguish different loadings and mobilities of metals in soils collected in the two areas.
The statistical method of PCA was applied for the evaluation of experimental data sets because it means to describe and visualize potentially meaningful relationships between data. The PCA approach was applied to the concentration values of metals in the less extractable (f3+f4) and readily extractable (f1+f2) fractions, allowing to assess the geogenic and anthropologic origins of metals in soils.
Results showed the presence of higher metals concentrations in soils of the Vallecamonica, closer to Mn-ferroalloy plants. Our results also suggest that Al, Fe, and Cr in soils from both the Vallecamonica and Garda Lake regions have mainly a geogenic origin. There also appears to be a geogenic origin for Cd and to some extent amounts of Mn in the Vallecamonica soils, with additional contributions from anthropogenic sources in the same area, i.e. Mn-ferroalloy plant emissions. For other metals, such as Pb and Zn, the contribution from anthropogenic sources appears to be prevalent in the Vallecamonica soils.
However, the high concentrations and readily extractability of Mn, which may suggest potential bioavailability for organisms, in the Vallecamonica soils create an environmental risk and potential health risk of exposure.
Besides the results showed that the anthropogenic contribution to metals concentrations in soils is prevalent for the Vallecamonica compared to the Garda Lake area.
Acknowledgments
This study was supported by Award Number R01ES019222 from the National Institute of Environmental Health Sciences (NIEHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the National Institutes of Health. We thank Tom Jursa and Rob Franks for substantial analytical assistance.
References
- Agency for Toxic Substances and Disease Registry. Public Health Assessment Guidance Manual-Appendix F. ATSDR; Atlanta, GA: 2005. http://www.atsdr.cdc.gov/HAC/phamanual/appf.html. [Google Scholar]
- Bontempi E, Zacco A, Benedetti D, Borgese L, Stosnach H, Depero LE, Finzi G, Apostoli P, Buttini P. Total reflection X-ray fluorescence (TXRF) for direct analysis of aerosol particle samples. Environ Technol. 2010;31:467–477. doi: 10.1080/09593330903513260. [DOI] [PubMed] [Google Scholar]
- Borgese L, Salmistraro M, Gianoncelli A, Zacco A, Lucchini R, Zimmerman N, Pisani L, Siviero G, Depero LE, Bontempi E. Airborne particulate matter (PM) filter analysis and modeling by total reflection X-ray fluorescence (TXRF) and X-ray standing wave (XSW) TALANTA. 2012;89:99–104. doi: 10.1016/j.talanta.2011.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese L, Zacco A, Pal S, Bontempi E, Lucchini R, Zimmerman N, Depero LE. A new non-destructive method for chemical analysis of particulate matter filters: The case of manganese air pollution in Vallecamonica (Italy) TALANTA. 2011;84:192–198. doi: 10.1016/j.talanta.2010.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazauskiene DM, Paulauskas V, Sabiene N. Speciation of Zn, Cu, and Pb in the soil depending on soil texture and fertilization with sewage sludge compost. J Soils Sediments. 2008;8:184–192. doi: 10.1007/s11368-008-0004-6. [DOI] [Google Scholar]
- Brigo L, Venerandi I. Bollettino della Società Geologica Italiana. 2005;24:493–510. [Google Scholar]
- Centers for Disease Control and Prevention. Preventing Lead Poisoning in Young Children. Atlanta, GA: 2005. [Google Scholar]
- Diaz-de Alba M, Galindo-Riãno MD, Casanueva-Marenco MJ, Garcia-Vargas M, Kosore CM. Assessment of the metal pollution, potential toxicity and speciation of sediment from Algeciras Bay (South of Spain) using chemometric tools. Journal of Hazardous Materials. 2011;190:177–187. doi: 10.1016/j.jhazmat.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Dong JD, Zhang YY, Wang YS, Wu ML, Zhang S, Cai CH. Chemometry use in the evaluation of the sanya bay water quality. Braz j oceanogr. 2010;58(4) doi.org/10.1590/S1679-87592010000400008. [Google Scholar]
- Favas PJC, Pratas J, Gomes MEP, Cala V. Selective chemical extraction of heavy metals in tailings and soils contaminated by mining activity: Environmental implications. Journal of Geochemical Exploration. 2011;111:160–171. doi: 10.1016/j.gexplo.2011.04.009. [DOI] [Google Scholar]; Journal of Environmental Analytical Chemistry. 51:135–151. doi: 10.1080/03067319308027619. [DOI] [Google Scholar]
- Kabata-Pendias A, Pendias H. Trace elements in soils and plants. CRC Press; Boca Raton, Florida: 1984. [Google Scholar]
- Keller C, Hammer D. Metal availability and soil toxicity after repeated croppings of Thlaspi caerulescens in metal contaminated soils. Environmental Pollution. 2004;131:243–254. doi: 10.1016/j.envpol.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Kersten M, Förstner U. Speciation of trace elements in sediments. In: Batley GE, editor. Trace Element Speciation: Analytical Methods and Problems. CRC Press; Boca Raton, FL: 1989. pp. 245–317. [Google Scholar]
- Kubova J, Stresko V, Bujdos M, Matus P, Medved Fractionation of various elements in CRMs and in polluted soils. J Anal Bioanal Chem. 2004;379: 108–114. doi: 10.1007/s00216-004-2505-5. [DOI] [PubMed] [Google Scholar]
- Lucchini RG, Albini E, Benedetti L, Borgese S, Coccaglio R, Malara EC, Parrinello G, Garattini S, Resola S, Alessio L. High prevalence of Parkinsonian disorders associated to manganese exposure in the vicinities of ferroalloy industries. Am J Ind Med. 2007;50:788–800. doi: 10.1002/ajim.20494. [DOI] [PubMed] [Google Scholar]
- Lucchini RG, Guazzetti S, Zoni S, Donna F, Peter S, Zacco A, Salmistraro M, Bontempi E, Zimmerman NJ, Smith DR. Tremor olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology. 2012 doi: 10.1016/j.neuro.2012.01.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid F, Reinoso R, Florido MC, Díaz Barrientos A, Ajmone-Marsan F, Davidson CM, Madrid L. Estimating the extractability of potentially toxic metals in urban soils: A comparison of several extracting solutions. Environmental Pollution. 2007;147:713–722. doi: 10.1016/j.envpol.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Mossop KF, Davidson CM. Comparison of original and modified BCR sequential extraction procedures for the fractionation of copper, iron, lead, manganese and zinc in soils and sediments. Analytica Chimica Acta. 2003;478:111–118. doi: 10.1016/S0003-2670(02)01485-X. [DOI] [Google Scholar]
- Obrador A, Rico MI, Alvarez JM, Novillo J. Influence of thermal treatment on sequential extraction and leaching behaviour of trace metals in a contaminated sewage sludge. Bioresour Technol. 2001;76:259–264. doi: 10.1016/S0960-8524(00)00101-2. [DOI] [PubMed] [Google Scholar]
- Pueyo M, Sastre J, Hernández E, Vidal M, López-Sánchez JF, Rauret G. Prediction of Trace Element Mobility in Contaminated Soils by Sequential Extraction. J Environ Qual. 2003;32:2054–2066. doi: 10.2134/jeq2003.2054. [DOI] [PubMed] [Google Scholar]
- Quevauviller P, Rauret G, Rubio R, López-Sánchez JF, Ure A, Bacon J, Muntau H. Certified reference materials for the quality control of EDTA- and acetic acid-extractable contents of trace elements in sewage sludge amended soils (CRMs 483 and 484) Fresenius J Anal Chem. 1997;357: 611–618. doi: 10.1007/s002160050222. [DOI] [Google Scholar]
- Rao RM, Sahuquillo A, Lopez Sanchez JF. A review of the different methods applied in environmental geochemistry for single and sequential extraction of trace elements in soils and related materials. Water Air Soil Pollut. 2008;189:291–333. doi: 10.1007/s11270-007-9564-0. [DOI] [Google Scholar]
- Rath P, Panda UC, Bhatta D, Sahu KC. Use of sequential leaching, mineralogy, morphology and multivariate statistical technique for quantifying metal pollution in highly polluted aquatic sediments-A case study: Brahmani and Nandira Rivers, India. Journal of Hazardous Materials. 2009;163:632–644. doi: 10.1016/j.jhazmat.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Rauret G, López-Sánchez JF, Bacon J, Gómez A, Muntau H, Quevauviller Ph. Report EUR 19774 EN. European Commission; Brussels: 2001. Certification of the Contents (Mass Fractions) of Cd, Cr, Cu, Ni, Pb and Zn in an Organic-Rich Soil Following Harmonised EDTA and Acetic Acid Extraction Procedures, BCR-700; p. 61. [Google Scholar]
- Rauret G, López-Sánchez JF, Sahuquillo A, Barahona E, Lachica M, Ure A, Muntau H. Quevauviller Ph (200)Indicative values for extractable contents (mass fractions) of Cd, Cr, Cu, Ni, Pb and Zn in sewage sludge amended soil (CRM 483) following the modified BCR-sequential extraction procedure (addendum to EUR-report 17127 EN) EUR 19503 EN. European Commission, BCR information, Reference materials. :22.
- Rauret G, López-Sánchez JF, Sahuquillo A, Rubio R, Davidson C, Ure A, Quevauviller Ph. Improvement of the BCR three-step sequential extraction procedure prior to the certification of new sediment and soil reference materials. Journal of Environmental Monitoring. 1999;1:57–61. doi: 10.1039/a807854h. [DOI] [PubMed] [Google Scholar]
- Rodríguez L, Ruiz E, Alonso-Azcárate J, Rincón J. Heavy metal distribution and chemical speciation in tailings and soils around a Pb-Zn mine in Spain. Journal of Environmental Management. 2009;90:1106–116. doi: 10.1016/j.jenvman.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Santamaria AB. Manganese exposure, essentiality and toxicity. Indian J Med Res. 2008;128:484–500. [PubMed] [Google Scholar]
- Sesana L, Polla G, Facchini U, De Capitani L. Radon-prone areas in the Lombard plain. J Environ Radioactiv. 2005;82:51–62. doi: 10.1016/j.jenvrad.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Singh KP, Malik A, Mohan D, Sinha S, Singh VK. Chemometric data analysis of pollutants in wastewater - a case study. Anal Chim Acta. 2005;532:15–25. doi: 10.1016/j.aca.2004.10.043. [DOI] [Google Scholar]
- Tessier A, Campbell PGC, Bisson M. Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry. 1979;51:844–851. doi: 10.1021/ac50043a017. [DOI] [Google Scholar]
- Tokalioglu S, Kartal A, Günes AA. Statistical Evaluation of Bioavailability of Metals to Grapes Growing in Contaminated Vineyard Soils Using Single Extractants. Int J Environ Anal Chem. 2004;84:691–705. doi: 10.1080/03067310410001688444. [DOI] [Google Scholar]
- Tokalioğlu S, Yilmaz V, Kartal S. An Assessment on Metal Sources by Multivariate Analysis and Speciation of Metals in Soil Samples Using the BCR Sequential Extraction Procedure. Clean – Soil, Air, Water. 2010;38:713–718. doi: 10.1002/clen.201000025. [DOI] [Google Scholar]
- Ure AM, Quevauviller Ph, Muntau H, Griepink B. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. 1993. Speciation of heavy metals in soils and sediments. [Google Scholar]
- Vanmechelen L, Groenemans R, Van Ranst E. Forest soil condition in Europe, Results of a Large-Scale Soil Survey. EC-LJNECE; Brussels, Geneva: 1997. [Google Scholar]
- Violante A, Cozzolino V, Perelomov L, Caporale AG, Pigna M. Mobility and bioavailability of heavy metals. J Soil Sci Plant Nutr. 2010;10(3): 268–292. doi: 10.4067/S0718-95162010000100005. [DOI] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Factor-Litvak P, Ahsan H, Levy D, Kline J, van Geen A, Mey J, Slavkovich V, Siddique AB, Islam T, Graziano JH. Arsenic and manganese exposure and children’s intellectual function. Neurotoxicology. 2011;32:450–457. doi: 10.1016/j.neuro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley A, Grant A. Assessment of the phase selectivity of the European-Community Bureau-of-Reference (BCR) sequential extraction procedure for metals in sediment. Anal Chim Acta. 1994;291:287–295. doi: 10.1016/0003-2670(94)80024-3. [DOI] [Google Scholar]
- Zacco A, Resola S, Lucchini R, Albini E, Zimmerman N, Guazzetti S, Bontempi E. Analysis of settled dust with X-ray Fluorescence for exposure assessment of metals in the province of Brescia, Italy. J Environ Monit. 2009;1:1579–1585. doi: 10.1039/b906430c. [DOI] [PMC free article] [PubMed] [Google Scholar]