Abstract
Since stem cells emerged as a new generation of medicine, there are increasing efforts to deliver the stem cells to a target tissue via intravascular injection. However, the therapeutic stem cells lack a capacity to detect and adhere to the target tissue. Therefore, this study presents synthesis of a bioactive hyper-branched polyglycerol (HPG) which can non-invasively associate with stem cells and further guide them to target sites, such as inflamed endothelium. The overall process is analogous to the way in which leukocytes are mobilized to the injured endothelium.
Stem and progenitor cells are being extensively studied as a new generation of medical therapy because of their ability to sustainably produce therapeutic proteins and recreate new tissues.1–5 One popular cell transplantation strategy is to inject cells into the circulatory system. However, this approach often encounters difficulties in recruiting transplanted cells to the target tissue because therapeutic cells, such as mesenchymal stem cells, lack the capability to bind with target tissue. According to fundamental studies, leukocytes are mobilized to injured or pathologic tissues because they express transmembrane receptors that can specifically bind with proteins overexpressed by the tissue. To mimic the function of leukocytes, efforts are increasingly made to modify the therapeutic cell surface with peptides or antibodies that can associate with proteins overexpressed in target tissues.6–7 For example, the cell surface was first modified with protein linkers such as protein G using activated esters or thiol maleimides, and subsequently exposed to the intercellular adhesion molecules (ICAM) antibodies or E-selectin binding peptides;8–11 however, such sequential and chemical modification of the cell surface may negatively impact cellular viability and therapeutic activities. Such concern was not well addressed in these past studies.
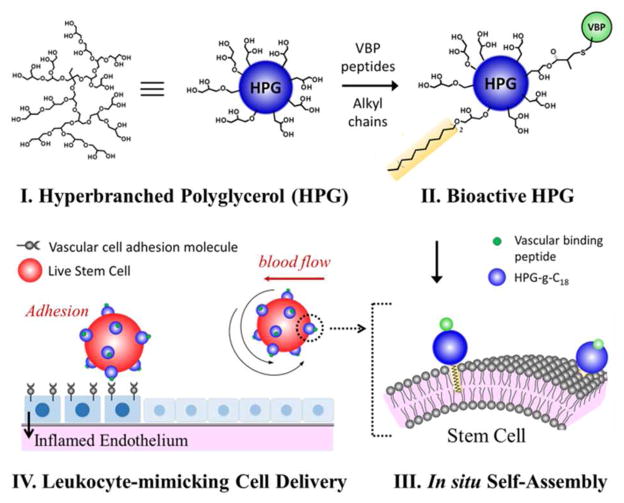
Therefore, this study presents a nano-sized cell carrier that can non-invasively modify cell surfaces with vasculature binding peptides (VBPs) via in situ self-assembly with cells, and regular cellular anchorage to target tissues (Scheme 1). In this study, we hypothesized that hyperbranched polyglycerol (HPG) covalently modified with octadecyl chains and VBPs would bind with cell membranes through hydrophobic chain insertion and display a controlled number of VBPs on the cell surface (I – III in Scheme 1). Additionally, the number of VBP-conjugated HPG on the cell membrane would control the adhesion of transplanted cells to a target tissue (IV in Scheme 1). The HPG possesses a compact, globular architecture which has a restricted conformation, minimizing intermolecular entanglements often encountered with linear polymers.12–18 Such a molecular architecture is more advantageous for presenting a larger number of octadecyl chains and VBPs than linear polymers.19
Scheme 1.
A bioactive hyperbranched polyglycerol (HPG) covalently modified with octadecyl chains and vasculature binding peptides (VBPs) was utilized as a novel cell-guidance molecule which can associate with stem cells and further guide them to target defective vasculature.
Additionally, the HPG consisting of polyether-polyol units is highly water-soluble, and minimally stimulates host responses (i.e., high biocompatibility).20–22 The number of octadecyl chains linked to the HPG would tune the binding affinity between the HPG and cells. In this study, an oligopeptide containing the VHSPNKK sequence was used as a model VBP, because it is known to bind with vascular endothelial adhesion molecule (VCAM) overexpressed by inflamed blood vessels.23, 26 We evaluated the association and dissociation kinetics of the bio-functionalized HPG on the VCAM-coated substrate, using surface plasmon resonance (SPR) spectroscopy. Further, we demonstrated using an in vitro circulation system that the resulting bio-functionalized HPG associates with mesenchymal stem cells (MSCs) and guides them to inflammatory tissue.
First, to develop a hyperbranched polyglycerol (HPG) capable of binding cell surfaces, HPG-g-C18 was prepared by a treating a 150 kDa HPG with octadecyl bromide (C18-Br) and base (Figure S1a and SI). 1H NMR analysis indicated that the average of seven C18 chains was linked per HPG (Figure S1b; see Eq. (1) in SI). The resulting HPG-g-C18 remained soluble in water and could be readily dissolved into cell suspensions. The capability of the HPG-g-C18 to associate with cells was evaluated by measuring an association rate constant (ka), a dissociation rate constant (kd), and the number of HPG-g-C18 adhered to a lipid bilayer simulating a cell membrane, using surface plasmon resonance (SPR) spectroscopy.24–25 Both ka and kd were quantified by fitting SPR curves to 1:1 Langmuir model using Eq. (1),
| (1) |
where RUmax is the maximal response capacity (RU) when the target surface is saturated with HPG-g-C18, and [A] is the concentration of the HPG-g-C18. As expected, the HPG-g-C18 exhibited a higher ΔRU and ka, and a smaller kd than the unmodified HPG (Table 1, Figure S2a). Additionally, HPG-g-C18 conjugated with fluorescein displayed a limited increase of ΔRU, compared to non-fluorescent HPG-g-C18.
Table 1.
SPR response unit changes (ΔRU) and calculated surface density of unmodified HPG and HPG-g-C18 towards the lipid bilayer.
| Carriers | Response unit change (ΔRU)[a] | Surface density (ng/mm2)[b] | Surface HPG number density (number/mm2)[c] |
|---|---|---|---|
| HPG | 180 | 0.18 | 7.2 × 108 |
| HPG-g-C18 | 1900 | 1.9 | 7.5 × 109 |
Response unit change (ΔRU) from the binding of HPG on the lipid bilayer.
Calculated from the response unit (RU).
Calculated from the RU and Mw of HPG (150 kDa) on the lipid bilayer.
Additionally, the underlying mechanism by which the C18 chains improve the association of HPG with the cell membrane was examined by quantifying the enthalpy (ΔH), entropy (ΔS), and Gibbs free energy (ΔG).27 The measured KA of HPG-g-C18 decreased with increasing temperature, and a plot of –ln(KA) vs. 1/T fits a linear regression (Table 2, Figure S3). The ΔG calculated from the linear regression using van’t Hoff equation, Eq. (2),
Table 2.
Quantified analysis of the effect of temperature on the association rate constant (ka), dissociation rate constant (kd), affinity constant (KA), and changes of enthalpy (ΔH), entropy (ΔS), and Gibbs free energy (ΔG) of HPG-g-C18 towards the lipid bi-layer.
| Temp. | Conc. (mg/ml) | Response unit change (ΔRU) | kd × 10−4 (1/s) | ka × 104 (1/M·s) | KA × 107 (1/M) |
|---|---|---|---|---|---|
| 298 K | 0.05 | 1900 | 3.98 | 3.03 | 7.6 |
| 303 K | 0.05 | 1300 | 4.73 | 2.37 | 5.0 |
| 310 K | 0.05 | 780 | 9.79 | 2.92 | 2.9 |
| (2) |
was approximately −11 kcal/mol. The ΔH and ΔS values were determined from the slope and abscissa, respectively, of the linear regression curve between ln (KA) and 1/T, using Eq. (3).
| (3) |
The ΔH and ΔS values were −14.2 kcal/mol and 11.7 cal/mol·K, respectively. These results indicate that the thermodynamically favorable association between HPG-g-C18 and lipid membrane is driven by the negative enthalpy.
Furthermore, mixing the HPG-g-C18 with CD44/CD90-positive and CD45-negative mesenchymal stem cells (MSCs) resulted in the coating of MSCs with HPGs after a ten-minute period. Thus, fluorescein-conjugated HPG-g-C18 was observed to stain the cell surface, whereas, MSCs mixed with the unmodified polyglycerol exhibited minimal fluorescence (Figure S4). The fluorescence intensity on the cell surface minimally changed over 72 hours indicating a long residence time for the HPG-g-C18 attached to the cell membrane (Figure S4). The MSCs associated with HPG-g-C18 displayed minimal difference of metabolic activity, as compared to uncoated cells (Figure S5).
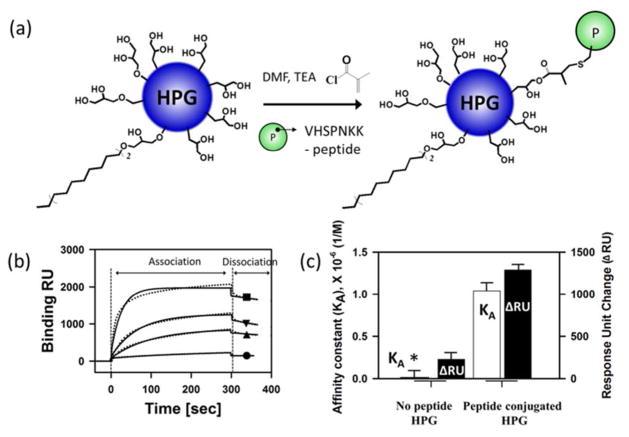
Next, to target damaged tissue, the HPG-g-C18 was modified with a VCAM-binding peptide containing the VHSPNKK sequence, termed VHSPNKK peptide. For the peptide conjugation, certain hydroxyl groups of HPG-g-C18 were modified to present acrylate groups (Figure 1a). These Michael-acceptor groups could be conjugated to the thiol group of the VHSPNKK peptide upon treatment with base. Using the UV absorbance of the tryptophan residue of the VHSPNKK peptide, it was found that the HPG-g-C18 contained on average ten peptide groups per alkyl groups.
Figure 1.
Bioconjugation of HPG-g-C18 with VHSPNKK peptides and SPR analysis of the binding VHSPNKK-HPG-g-C18 with VCAM. (a) Chemical reaction scheme to conjugate HPG-g-C18 with VHSPNKK peptides. (b) SPR response curves of the VHSPNKK-HPG-g-C18’s association of and dissociation with the VCAM-coated substrate. (c) SPR analysis of the affinity constant (KA) and the response unit change (ΔRU) of VHSPNKK-HPG-g-C18 for the VCAM-coated substrate. In (b), -●- represents peptide-free polyglycerol with 5.0 μM. The concentration of VHSPNKK-HPG-g-C18 incorporated into the flow of SPR unit was varied from 1.2 (-▲-), 5.0 (-▼-), and 10 μM (-■-). The dotted lines (··) show binding RU obtained. The solid lines (-) represent global fits to 1:1 Lang-muir model (A + B = AB). The scores of X2 (Chi2) in 1.2 and 5.0 μM are <10, indicating that the model used adequately describes the observed binding.
The capability of the VHSPNKK-HPG-g-C18 to bind with a VCAM-coated substrate was evaluated using SPR. As expected, the unmodified HPG-g-C18 displayed a minimal adhesion to the substrate, as marked with almost zero value of the response unit change (ΔRU) (Figure 1b). In contrast, the VHSPNKK-HPG-g-C18 exhibited an affinity constant six-fold larger than the peptide-free HPG-g-C18. Accordingly, the number of VHSPNKK-HPG-g-C18 bound to the VCAM-coated substrate, represented by the response unit change, increased in proportion to the number of HPG added to the SPR unit. Ultimately, the number of HPG molecules bound to the VCAM substrate was 10 times larger than the peptide-free HPG-g-C18 after a 300 sec time period (Figure 1c). However, the VHSPNKK-HPG-g-C18 exhibited a minimal adhesion to a substrate pre-exposed to free VSHPNKK peptide (Figure S6). Taken together, these results indicate that the VHSPNKK peptide plays a critical role in the targeted adhesion of HPG to VCAM-coated substrate.
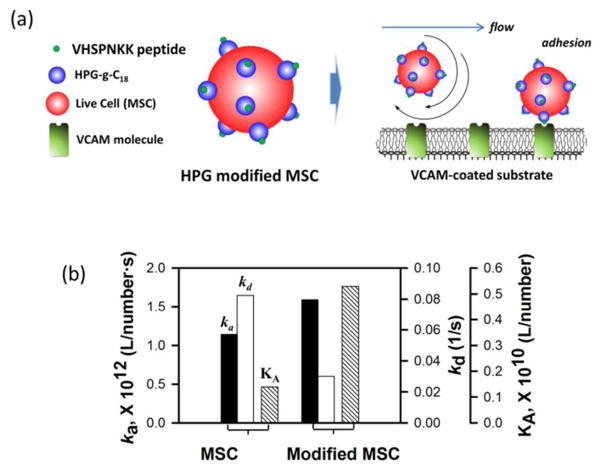
Finally, we examined the ability of VHSPNKK-HPG-g-C18 to guide mesenchymal stem cells (MSCs) to a target VCAM-coated substrate using SPR (Figure 2a). Interestingly, MSCs coated with VHSPNKK-HPG-g-C18 displayed a 1.5-fold increase in the association rate constant (ka) and 70 % decrease in the dissociation rate constant (kd), as compared to uncoated MSCs (Figure 2b, Figure S7). Additionally, the affinity constant (KA) of MSCs associated with the VHSPNKK-HPG-g-C18 was three times larger than the KA value of uncoated MSCs.
Figure 2.
In vitro evaluation of the function of VHSPNKK-HPG-g-C18 to regulate adhesion of MSC to an inflamed endothelium. (a) Schematic of the SPR analysis to characterize adhesion of MSC to a target VCAM-coated substrate. (b) Association rate constant (ka), dissociation rate constant (kd), and affinity constant (KA) of uncoated MSCs and MSCs associated with VHSPNKK-HPG-g-C18, characterized by SPR response curves.
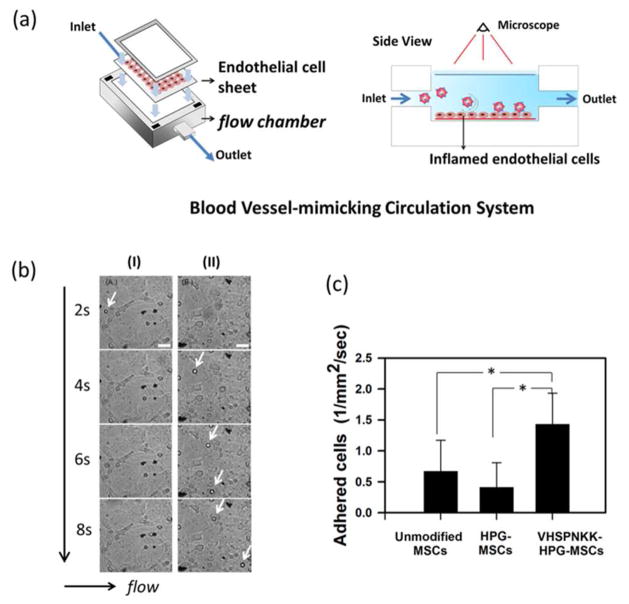
Furthermore, we examined the ability of VHSPNKK-HPG-g-C18 to direct the adhesion of CD44/CD90-positive and CD45-negative adipose-derived MSCs to an inflamed endothelium using a blood vessel-mimicking circulation system (Figure S8, Figure 3a). The inflamed endothelium was prepared by exposing an endothelial cell sheet to tumor necrosis factor (TNF)-α, which induced endothelial cells to overexpress VCAM.28 A flow rate of MSC-suspending fluid was kept constant at 1.0 ml/min, in order to keep shear stress on endothelium to be comparable to coronary artery wall shear stress. Both uncoated MSCs and MSCs associated with peptide-free HPG-g-C18 displayed minimal cell adhesion to the inflamed cells (Figure 3b). In contrast, MSCs coated with VHSPNKK-HPG-g-C18 displayed significantly smaller rolling velocities compared to uncoated cells, and actively adhered to the inflamed endothelium (Figure 3b, Figure S9). Sphere-shaped MSCs with a 10 μm diameter were readily distinguished from any debris in the cell culture media and endothelial cells fully spread on a substrate. Ultimately, there was a two-fold increase in the number of MSCs adhered to the endothelium when using VSPNKK-HPG-g-C18 (Figure 3c). In addition, the MSCs associated with VHSPNKK-HPG-g-C18 molecules displayed limited adhesion to the endothelial cells not exposed to TNF-α. These results support the finding that the peptide-conjugated HPG is capable of delivering cells exclusively to the target inflammatory tissue.
Figure 3.
In vitro evaluation of the function of VHSPNKK-HPG-g-C18 to regulate adhesion of MSC to an inflamed endothelium. (a) Schematic of the MSC delivery to a target inflamed endothelium using an in vitro circulation system. (b) Images of the inflamed endothelial cells exposed to the flow of uncoated MSCs (I) and MSCs associated with VHSPNKK-HPG-g-C18 (II). Arrows indicate the MSCs anchored to the endothelial cells. The scale bar represents 50 μm. (c) Quantification of the number of MSCs adhered to the target inflamed endothelial cells during a given time period. * represents a statistical significance of the difference between conditions (* P < 0.05).
In summary, this study demonstrates a novel cell-guidance molecule which can associate with cells in a minimally invasive manner and further guide them to target sites, such as inflamed endothelium. The HPG modified with octadecyl chains associated with MSCs in a thermodynamically favorable manner. Subsequent modification of the HPG-g-C18 with VHSPNKK peptides provided a HPG molecule with the ability to bind with VCAM-coated substrate. Ultimately, coating stem cell surfaces with the VHSPNKK-HPG-g-C18 significantly enhanced the cellular affinity for the VCAM protein, and therefore successfully directed the anchorage of cells to the target inflamed endothelium. The overall process is analogous to the way in which leukocytes are mobilized to the injured endothelium.29 Additionally, this approach to functionalize cell membrane with VHSPNKK-HPG-g-C18 via self-assembly should be superior to prior, multi-step chemical modifications of cell membranes.
We envisage that the strategy described herein, using a HPG carrier to bind cells and display targeting groups for delivery, would be highly useful for guiding a wide array of therapeutic cells to target tissues following intravascular injection. Such enhanced cell adhesion and subsequent increased cell quantities at target tissues would greatly improve the outcome of cell therapies. In addition, this functionalized HPG may prove useful for guiding various nano- or micro-sized drug carriers to target tissues. These possibilities are the focus of our current studies.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health (1R21 HL097314 and 1R01 HL109192 to H.J.K. and S.C.Z. and 1R25CA154015A to R.J.D.). We would also like to acknowledge the Biomedical Research Center, a joint venture of Carle Foundation Hospital and University of Illinois, Urbana-Champaign, for services provided in support of this research.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript
The authors declare no competing financial interests.
Experimental materials, instruments and procedures; synthesis and characterization of VHSPNKK-HPG-g-C18; analysis of the association of HPG-g-C18 with cells; surface plasmon resonance (SPR) analysis, and flow chamber assay; Supplemental videos for the adhesion of modified MSCs to an inflamed endothelium. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Asahara T, Kawamoto A. Am J Physiol Cell Physiol. 2004;287:572–579. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- 2.Kinnaird T, Stabile E, Burnett MS, Epstein SE. Circ Res. 2004;95:354–363. doi: 10.1161/01.RES.0000137878.26174.66. [DOI] [PubMed] [Google Scholar]
- 3.Minguell JJ, Erices A. Exp Biol Med. 2006;231:39–49. doi: 10.1177/153537020623100105. [DOI] [PubMed] [Google Scholar]
- 4.Perin EC, Geng YJ, Willerson JT. Circulation. 2003;107:935–938. doi: 10.1161/01.cir.0000057526.10455.bd. [DOI] [PubMed] [Google Scholar]
- 5.Bhranti SS, Paul AC, Eduardo KM, Michael AS, Jeremy JM. Nano Lett. 2007;7:3071–3079. [Google Scholar]
- 6.Ko IK, Kean TJ, Dennis JE. Biomaterials. 2009;30:3702–3710. doi: 10.1016/j.biomaterials.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarkar D, Vemula PK, Zhao W, Gupta A, Karnik R, Karp JM. Biomaterials. 2010;31:5266–5271. doi: 10.1016/j.biomaterials.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behm CZ, Kaufmann BA, Carr C, Lankford M, Sanders JM, Rose CE, Kaul S, Lindner JR. Circulation. 2008;117:2902–2911. doi: 10.1161/CIRCULATIONAHA.107.744037. [DOI] [PubMed] [Google Scholar]
- 9.Sakhalar HS, Dalal MK, Salem AK, Ansari R, Fu J, Kiani MF, Kurjiaka DT, Hanes J, Shakesheff KM, Goetz DJ. Proc Natl Acad Sci. 2003;100:15895–15900. doi: 10.1073/pnas.2631433100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickerson JB, Blackwell JE, Ou JJ, Shinde Patil VR, Goetz DJ. Biotechnol Bioeng. 2001;73:500–509. doi: 10.1002/bit.1085. [DOI] [PubMed] [Google Scholar]
- 11.(a) Eniola AO, Rodgers SD, Hammer DA. Biomaterials. 2002;23:2167–2177. doi: 10.1016/s0142-9612(01)00349-0. [DOI] [PubMed] [Google Scholar]; (b) Debanjan S, Joel AS, Joseph AP, Weian Z, Sebastian S, Dawn PS, Luke JM, Juan PR, Praveen KV, Rukmani S, Sriram K, Rohit K, Charles PL, Jeffrey MK. Blood. 2011;118:e184–191. [Google Scholar]
- 12.Sunder A, Hanselmann R, Frey H, Mülhaupt R. Macromolecules. 1999;32:4240–4246. [Google Scholar]
- 13.Sunder A, Mülhaupt R, Haag R, Frey H. Macromolecules. 2000;33:253–254. [Google Scholar]
- 14.Mumprecht S, Schurch C, Schwaller J, Solenthaler M, Ochsenbein AF. Blood. 2009;114:1528–1536. doi: 10.1182/blood-2008-09-179697. [DOI] [PubMed] [Google Scholar]
- 15.Vellai T, Takacs-Vellai K. Adv Exp Med Biol. 2010;694:69–80. doi: 10.1007/978-1-4419-7002-2_7. [DOI] [PubMed] [Google Scholar]
- 16.Stephan MT, Irvine DJ. Nano Today. 2011;6:309–325. doi: 10.1016/j.nantod.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmerman SC, Quinn JR, Burakowska E, Haag R. Angew Chem Int Ed. 2007;46:8164–8167. doi: 10.1002/anie.200702580. [DOI] [PubMed] [Google Scholar]
- 18.(a) Sisson AL, Steinhilber D, Rossow T, Welker P, Licha K, Haag R. Angew Chem Int Ed. 2009;48:7540–7545. doi: 10.1002/anie.200901583. [DOI] [PubMed] [Google Scholar]; (b) Wei T, Yong L, Binbin J, Songrui Y, Wei H, Yongfeng Z, Deyue Y. J Am Chem Soc. 2012;134:762–764. [Google Scholar]
- 19.Wilms D, Stiriba SE, Frey H. Accounts Chem Res. 2010;43:129–141. doi: 10.1021/ar900158p. [DOI] [PubMed] [Google Scholar]
- 20.Kainthan RK, Janzen J, Levin E, Devine DV, Brooks DE. Biomacromolecules. 2006;7:703–709. doi: 10.1021/bm0504882. [DOI] [PubMed] [Google Scholar]
- 21.Kainthan RK, Hester SR, Levin E, Devine DV, Brooks DE. Biomaterials. 2007;28:4581–4590. doi: 10.1016/j.biomaterials.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Joseph-son L, Weissleder R. Circ Res. 2005;18:327–336. doi: 10.1161/01.RES.0000155722.17881.dd. [DOI] [PubMed] [Google Scholar]
- 23.Stabenfeldt SE, Gossett JJ, Barker TH. Blood. 2010;116:1352–1359. doi: 10.1182/blood-2009-11-251801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(a) Kim BY, Jeong JH, Park K, Kim JD. J Control Release. 2005;102:525–538. doi: 10.1016/j.jconrel.2004.10.032. [DOI] [PubMed] [Google Scholar]; (b) Jeong JH, Kim BY, Lee SJ, Kim JD. Chem Phys Lett. 2006;421:373–377. [Google Scholar]
- 25.Lai MH, Jeong JH, DeVolder RJ, Brockman C, Schroeder C, Kong HJ. Adv Funct Mater. 2012;22:3239–3246. doi: 10.1002/adfm.201102664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou CC, Hsiao HY, Hong QS, Chen CH, Peng YW, Chen HW, Yang PC. Nano Lett. 2008;8:437–445. doi: 10.1021/nl0723634. [DOI] [PubMed] [Google Scholar]
- 27.Day YSN, Baird CL, Rich RL, Myszka DG. Protein Science. 2002;11:1017–1025. doi: 10.1110/ps.4330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CW, Lin WN, Lin CC, Luo SF, Wang JS, Pouyssegur J, Yang CM. J Cell Physiol. 2006;207:174–186. doi: 10.1002/jcp.20549. [DOI] [PubMed] [Google Scholar]
- 29.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb RR. Cell. 1990;23:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.