Abstract
OBJECTIVE
There is debate as to whether fibronectin type III domain containing 5 (FNDC5) and its protein product irisin are therapeutic targets for obesity-associated maladies. Thus, we sought to examine FNDC5 mRNA within skeletal muscle of obese/diabetic-prone Otsuka Long-Evans Tokushima Fatty (OLETF) rats versus lean/healthy Long Evans Tokushima Otsuka (LETO) rats. We hypothesized that FNDC5 expression would be greater in obese (OLETF) versus lean (LETO) animals.
MATERIALS/METHODS
Triceps muscle of 30–32 week old OLETF and LETO rats were assayed for FNDC5 and PGC1α mRNA levels. Body composition and circulating biomarkers of the OLETF and LETO rats were also correlated with skeletal muscle FNDC5 mRNA expression patterns in order to examine potential relationships that may exist.
RESULTS
OLETF rats exhibited twice the amount of triceps FNDC5 mRNA compared to LETO rats (p < 0.01). Significant positive correlations existed between triceps muscle FNDC5 mRNA expression patterns versus fat mass (r = 0.70, p = 0.008), as well as plasma leptin (r = 0.82, p < 0.001). PGC1α mRNA levels were also highly correlated with FNDC5 mRNA (r = 0.85, p < 0.001). In subsequent culture experiments, low and high physiological doses of leptin had no effect on PGC1α mRNA or FNDC5 mRNA levels in C2C12 myotubes. Paradoxically, circulating irisin concentrations tended to be higher in a second cohort of LETO versus OLETF rats (p = 0.085).
CONCLUSION
These results reveal a positive association between total body adiposity and skeletal muscle FNDC5 gene expression. Of interest, circulating irisin levels tended to be lower in OLETF rats. Further research is needed to examine whether other adipose tissue-derived factors up-regulate FNDC5 transcription and/or inhibit irisin biosynthesis from FNDC5.
Keywords: FNDC5, irisin, obesity, skeletal muscle
INTRODUCTION
Fibronectin type III domain containing 5 (FNDC5) has garnered recent attention. The proteolytic FNDC5 cleavage product, irisin, was reported by Boström et al. [1] to a) be up-regulated in a proliferator-activated receptor gamma coactivator 1-α (PGC1α)- and exercise-dependent fashion in rodent and human skeletal muscle [1], and b) increase thermogenesis and decrease bodyweights in mice [1]; leading them to propose that FNDC5 and irisin may be novel therapeutic targets in the treatment of obesity. However, Timmons et al. [2] reported that skeletal muscle FNDC5 mRNA was shown to increase only within a subset of highly active human elderly subjects following 6 weeks of exercise [2]. Moreover, FNDC5 mRNA for all subjects correlated with body mass index (BMI), but not with changes in aerobic capacity. Huh et al. [3] similarly reported that circulating irisin increases acutely following a sprint-interval exercise bout in healthy humans. However, these same authors reported that surgically-induced weight loss in patients with a BMI > 50 decreased skeletal muscle FNDC5 mRNA as well as circulating irisin leading the authors to conclude that irisin could not be directly involved with health improvements given its positive association with body fat [3]. Thus, we hypothesized that triceps muscle FNDC5 mRNA would be elevated in OLETF rats, and would correlate with fat mass, lean body mass, and plasma leptin.
METHODS
Animals and experimental design
Experimental protocols were approved by the University of Missouri Animal Care and Use Committee. Four week-old, male LETO (n = 7) and OLETF rats (n = 7) (Tokushima Research Institute, Otsuka Pharmaceutical) were received and individually housed in with 0600–1800 light and 1800-0600 dark cycles at 21°C. Animals were given ad libitum access to standard chow (Formulab 5008, Purina Mills). On the day of sacrifice, at 30–32 weeks of age, rats were fasted for 12 h and anesthetized with an intraperitoneal injection of sodium pentobarbital (100 mg/kg). While animals were under anesthesia, body composition was assessed by dual energy x-ray absorptiometry (DXA; Hologic QDR-1000, calibrated for rodents), whole triceps muscles harvested, and animals killed by exsanguination.
Real-time PCR for mRNA expression
For triceps muscle RNA extraction, methods were done as previously described [4]. mRNAs for FNDC5, PGC1α, and18S were assayed using with SYBR green-based chemistry (Applied Biosystems) real-time PCR and gene-specific primers. Primer efficiency curves for all genes ranged between 90–110%.
Plasma cytokine and irisin concentrations
EDTA-treated plasma samples were assayed for plasma cytokine concentrations as described [5] in OLETF (n = 7) and LETO (n = 7) rats. EDTA-treated plasma samples were also assayed for plasma irisin concentrations in a separate set of OLETFs (n = 10) and LETOs (n = 10) using an ELISA kit (Phoenix Pharmaceuticals) due to limited plasma samples from the first cohort of rats. Of note, these animals were the same age as animals used in the first cohort (30–32 weeks of age) and were sacrificed in an identical manner as the first cohort [fasted for 12 h and anesthetized with an intraperitoneal injection of sodium pentobarbital (100 mg/kg)].
Myotube cell culture adipokine treatment and FNDC5 mRNA expression
C2C12 myoblasts (American Type Culture Collection CRL-1722) were maintained under standard conditions and differentiation was induced 24 h after growth reached 80–90% confluency with differentiation media [DM; DMEM, 2% (vol/vol) horse serum]. DM was replaced every 24 h for 124 h. Myotubes were then treated with lower (8 ng/ml) and higher (150 ng/ml) dosages of leptin (Sigma) for 24 h and 48 h. After treatments, myotubes were lysed with Trizol, cDNA was made from isolated RNA per the methods described above. 25 ng of cDNA was assayed for FNDC5, PGC1α and GAPDH mRNAs using the aforementioned SYBR green chemistry methods. Murine FNDC5 primers were used as previously used by Bostrom et al. [1].
Statistics
Between-group comparisons for all variables were made using independent samples t-tests. Univariate correlations were performed using Pearson correlation tests. In vitro myotube FNDC5 and PGC1α mRNA expression patterns were made between leptin-treated and control conditions using one-way ANOVAs. Significance was set at p < 0.05 and data are presented as mean ± SE.
RESULTS
Most general health markers presented in Table 1 were more favorable in LETO versus OLETF rats indicating that LETO rats exhibited a generally healthier phenotype.
Table 1.
Between-treatment differences in body composition and serum markers
| Variable | OLETF SED (n=7) | LETO SED (n=6) |
|---|---|---|
| Body composition variables | ||
| Bodyweight (g) | 693 ± 14 | 474 ± 19*** |
| DXA bfat (%) | 37.0 ± 1.6 | 14.9 ± 0.6*** |
| Lean mass (g) | 435 ± 8 | 407 ± 17 |
| fat mass (g) | 257 ± 15 | 72 ± 5*** |
| Serum markers | ||
| Glucose (mg/dl) | 290 ± 14 | 151 ± 5*** |
| Insulin (mg/dl) | 6.2 ± 0.6 | 2.8 ± 0.6** |
| HDL-c (mg/dl) | 33 ± 2 | 26 ± 1* |
| LDL-c (mg/dl) | 50 ± 7 | 61 ± 4 |
| Triglycerides (mg/dl) | 319 ± 47 | 40 ± 5*** |
| Plasma adipokines | ||
| Leptin (ng/ml) | 102 ± 11 | 13 ± 2*** |
| IL-1β (pg/ml) | 17 ± 13 | ND |
| IL-6 (pg/ml) | 825 ± 222 | ND |
| IL-10 (pg/ml) | 927 ± 332 | 34 ± 10* |
| IL-12p70 (pg/ml) | 125 ± 66 | 12 ± 5 |
| TNF-α (pg/ml) | 8.9 ± 4.2 | 3.9 ± 1.9 |
| MCP-1 (pg/ml) | 49 ± 10 | 56 ± 28 |
| RANTES (pg/ml) | 938 ± 69 | 1218 ± 334 |
All data are presented as mean ± SE.
Abbreviations: DXA bfat = dual x-ray absorptiometry body fat percentage, ND = not detectable;
indicate a significant difference between groups (p < 0.05),
indicate a significant difference between groups (p < 0.01),
indicate a significant difference between groups (p < 0.001).
Triceps FNDC5 and PGC1α mRNA expression patterns
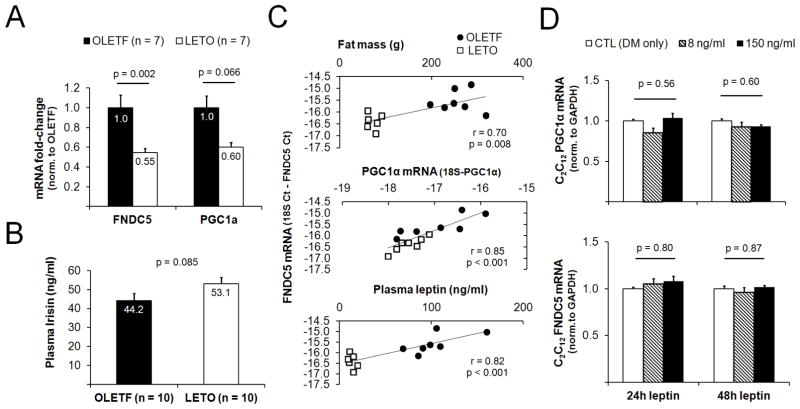
FNDC5 and PGC1α mRNAs were ~50% and ~45% less in the triceps muscle of LETO versus OLETF rats, respectively (Fig 1A).
Figure 1.
Relationship between muscle FNDC5 mRNA and adiposity. Obese OLETF rats possessed a greater triceps muscle FNDC5 mRNA expression compared to LETO rats (A). In a second cohort of rats, LETO rats tended to present more circulating irisin compared to OLETF rats OLETF (B). In all rats from panel A, positive correlations existed between triceps muscle FNDC5 mRNA expression versus fat mass, triceps muscle PGC1α and plasma leptin (C). Despite the positive association between circulating leptin and triceps muscle FNDC5 mRNA expression, 24-h and 48- treatments with low (8 ng/ml) and high (150 ng/ml) physiological doses of leptin did not alter the mRNA expression of FNDC5 (mRNA precursor to irisin) in C2C12 myoblasts (D). In panel D, all data are normalized to DM-only-treated cells and are presented as mean ± SE (n = 4 wells per treatment).
Correlations between biomarkers and triceps FNDC5 mRNA expression patterns
Triceps FNDC5 mRNA expression was positively associated with total body fat mass (r = 0.70, p = 0.008; Fig 1B), although there was no association between triceps FNDC5 mRNA expression total lean body mass (r = −0.06, p = 0.76). There was no association with circulating insulin and triceps FNDC5 mRNA expression (r = 0.40, p = 0.20). We have previously examined differences in multiple plasma adipokines between the same OLETF and LETO animals as the current study [5]. Circulating leptin exhibited the strongest correlation with triceps muscle FNDC5 mRNA expression patterns relative to the other adipokines (r = 0.82, p < 0.001; Fig 1C), although other adipokines were also associated with triceps muscle FNDC5 mRNA expression patterns [(IL-1β: r = 0.54, p = 0.054); IL-6: (r = 0.61, p = 0.03); and IL-10: (r = 0.64, p = 0.02)]. Plasma leptin was also correlated triceps PGC1α mRNA expression levels (r = 0.42, p = 0.04). Finally, triceps muscle FNDC5 mRNA expression was also positively associated with triceps muscle PGC1α mRNA (r = 0.85, p < 0.001; Fig 1D).
Effects of leptin treatment on myotube FNDC5 and PGC1α mRNA levels
Given the strong correlation between triceps FNDC5 mRNA expression and circulating leptin, we next assessed whether leptin-treated C2C12 myotubes exhibited a dose-response increase in FNDC5 gene expression. Contrary to our hypothesis, FNDC5 and PGC1α mRNA expression was not altered in leptin-treated C2C12 myotubes (Fig 1E).
Plasma irisin in a second set of OLETF and LETO rats
Due to limited plasma from the original animals, a second set of OLETF (n = 10) and LETO (n = 10) rats were used to assay plasma irisin. While circulating irisin was not statistically different between these cohorts, LETO rats tended to present greater concentrations of circulating irisin (p = 0.085, Fig. 1). Within this second cohort of animals (n = 20 total), there were no correlations between plasma irisin and body weight (r = −0.19, p = 0.42), or DXA body fat percentage (r = −0.24, p = 0.32).
DISCUSSION
Novel findings of this study are that total body fat and plasma leptin levels are positively associated with higher skeletal muscle FNDC5 mRNA expression in the obese OLETF rat. These data agree with previous studies by Timmons et al. [2], as well as Huh et al. [3], who reported positive associations between BMI and FNDC5 mRNA expression in human skeletal muscle. Likewise, Huh et al. [3] demonstrated that weight loss after bariatric surgery decreased human vastus lateralis muscle FNDC5 mRNA expression as well as circulating leptin concentrations. We also report that OLETF rats tended to express more triceps PGC-1α mRNA, which is a known regulator of FNDC5 gene expression. Given that: a) the obese OLETF animals exhibited greater circulating leptin, and b) leptin signaling can increase PGC1α mRNA expression [6–7], we hypothesized that an increase in PGC-1α and consequently, FNDC5 mRNA expression patterns occurred in an exercise-independent manner in the examined OLETF rats. Indeed, plasma leptin was positively correlated with triceps FNDC5 mRNA expression levels, as well as PGC1α mRNA expression. In leptin-deficient ob/ob mice leptin administration increases expression of PGC1α protein in skeletal muscle through AMPK-mediated signaling [8]. Hence, we further speculated that leptin-mediated “crosstalk” between adipose tissue and skeletal muscle might constitute a novel compensatory mechanism to increase thermogenesis and stimulate fat loss via irisin-mediated signaling in the obese OLETF rats. In contrast to this expectation, longer-term (24 h and 48 h) leptin treatments at low and high physiological doses did not alter PGC1α mRNA or FNDC5 mRNA levels in C2C12 myotubes in culture. An increase in triceps muscle FNDC5 gene expression with elevated circulating leptin concentrations may be coincidental with an increase in adiposity, although the apparent lack of an effect of leptin on FNDC5 mRNA expression in skeletal muscle does not rule out a potential role of leptin in regulating circulating irisin levels in vivo. Future in vivo studies are necessary to further address whether leptin regulates FNDC5 protein expression and/or irisin production. Despite the aforementioned findings with leptin, we propose that an unknown obesity-induced adipose tissue-derived factor could regulate skeletal muscle FNDC5 gene expression in an attempt to increase thermogenesis and stimulate fat loss via irisin-mediated signaling in the obese OLETF rats. Further research is needed to test this hypothesis.
Interestingly, in a second set of animals circulating irisin tended to be greater in leaner/healthier LETO versus fatter/unhealthier OLETF rats, although no correlations existed between plasma irisin and body weight or DXA body fat percentage. This finding is in agreement with Huh et al. [3] who demonstrated that BMI was not correlated with circulating irisin in humans. Therefore, while muscle FNDC5 gene expression may be upregulated in obese skeletal muscle, it remains possible that post-translational proteolytic cleavage mechanisms responsible for the conversion of FNDC5 to irisin are impaired in the muscle of obese/diseased OLETFs.
Limitations to the current study include using a separate cohort of animals to assess plasma irisin as well a relatively small sample size. Nonetheless, our findings provide guidance for future studies addressing: a) whether one or multiple adipose tissue-derived factors increase FNDC5 gene expression, and/or b) whether obesity affects the post-translational modification of FNDC. Nonetheless, despite initial excitement over irisin as a novel myokine [1], our data combined with recent human data [2, 3] suggest that its role in obesity-associated metabolic pathologies is somewhat ambiguous at the present time.
Acknowledgments
The authors gratefully acknowledge Pam Thorne, Lauren Oberle and Grace Meers for their technical assistance. The authors also would like to thank Phoenix Pharmaceuticals for their technical assistance with the Irisin ELISA. Funding was provided by NIH T32-AR048523 (N.T.J., J.S.M and M.D.R.), AHA 11POST5080002 (J.P.), anonymous gifts (F.W.B.), VA-CDA-IK2 BX001299-01 (R.S.R.), and NIH RO1HL036088 (M.H.L.). This work was supported in part with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO.
List of abbreviations
- FNDC5
fibronectin type III domain containing 5
- OLETF
Otsuka Long-Evans Tokushima Fatty
- LETO
Long Evans Tokushima Otsuka
- PGC1α
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
Footnotes
AUTHOR CONTRIBUTIONS
MDR: tissue collection, specimen processing, data procurement and analysis, and writing of the manuscript
DSB: specimen processing, data procurement and analysis, and assisted in writing of the manuscript
JMC: data analysis and assisted in writing of the manuscript
NTJ: serum cytokine analysis, data analysis and assisted in writing of the manuscript
JP: data analysis and assisted in writing of the manuscript
TEC: critical involvement in cell culture experiments and data analysis thereafter
JSM: data analysis and assisted in writing of the manuscript
VJD: assisted in study design, data analysis and assisted in writing of the manuscript
FWB: data analysis and assisted in writing of the manuscript
RSR: procured grant funding, serum biochemical analysis, assisted in writing of the manuscript
MHL: procured grant funding, critically designed all aspects of the study, and assisted in writing of the manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bostrom P, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–8. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timmons JA, et al. Is irisin a human exercise gene? Nature. 2012;488(7413):E9–10. doi: 10.1038/nature11364. discussion E10-1. [DOI] [PubMed] [Google Scholar]
- 3.Huh JY, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012 doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts MD, et al. Early depression of Ankrd2 and Csrp3 mRNAs in the polyribosomal and whole-tissue fractions in skeletal muscle with decreased voluntary running. J Appl Physiol. 2012 doi: 10.1152/japplphysiol.01419.2011. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins NT, et al. Effects of Endurance Exercise Training, Metformin, and their Combination on Adipose Tissue Leptin and IL-10 Secretion in OLETF Rats. J Appl Physiol. 2012 doi: 10.1152/japplphysiol.00936.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo GF, et al. Alteration of mitochondrial oxidative capacity during porcine preadipocyte differentiation and in response to leptin. Mol Cell Biochem. 2008;307(1–2):83–91. doi: 10.1007/s11010-007-9587-2. [DOI] [PubMed] [Google Scholar]
- 7.Yasari S, et al. Exercise training decreases plasma leptin levels and the expression of hepatic leptin receptor-a, -b, and, -e in rats. Mol Cell Biochem. 2009;324(1–2):13–20. doi: 10.1007/s11010-008-9979-y. [DOI] [PubMed] [Google Scholar]
- 8.Sainz N, et al. Leptin administration favors muscle mass accretion by decreasing FoxO3a and increasing PGC-1alpha in ob/ob mice. PLoS One. 2009;4(9):e6808. doi: 10.1371/journal.pone.0006808. [DOI] [PMC free article] [PubMed] [Google Scholar]