Abstract
Background
Myeloperoxidase (MPO) is an endogenous oxidant enzyme that produces reactive oxygen species (ROS) and may be involved in lung carcinogenesis. The MPO−463G>A polymorphism influences MPO transcription and has been associated with lung cancer susceptibility. However, the association between the MPO−463G>A polymorphism and lung cancer risk remains controversial.
Method
To investigate the effect of this polymorphism on lung cancer susceptibility, we performed a meta-analysis based on 22 published case–control studies including 7,520 patients with lung cancer and 8,600 controls. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to assess the strength of the association.
Results
Overall, there was no evidence for significant association between MPO−463G>A polymorphism and lung cancer susceptibility (for AA versus GG: OR = 0.91, 95%CI = 0.67–1.24; for GA versus GG: OR = 0.87, 95% CI = 0.78–0.98; for AA/GA versus GG: OR = 0.90, 95% CI = 0.80–1.01; for AA versus GA/GG: OR = 0.96, 95% CI = 0.72–1.28). In the stratified analyses by ethnicity, source of controls and smoking status, we also did not find any significant association between them.
Conclusions
In summary, this meta-analysis suggests MPO−463G>A polymorphism may not be a risk factor for developing lung cancer. However, further prospective well-designed population-based studies with larger sample size are expected to validate the results.
Introduction
Lung cancer is one of the most common malignancies and the leading cause of cancer-related deaths in the United States and worldwide. It is estimated that in 2011, approximately 221,000 new cases will be diagnosed and 156,900 deaths due to lung cancer will occur in the United States [1]. The exact mechanism of lung carcinogenesis is still unclear. Lung cancer may be a multifactorial disease that resulted from complex interactions between genetic and environmental factors [2]. It has been estimated that cigarette smoking is responsible for 85–90% of lung cancer development [3]. Although smoking can account for the majority of lung cancer, most chronic smokers still do not develop lung cancer. This suggests that lung cancer susceptibility differs among individuals and might have genetic factors which may influence the risk of lung cancer among these who are exposed to tobacco smoke carcinogens.
Myeloperoxidase (MPO) as an endogenous oxidant lysosomal enzyme available in polymorphonuclear neutrophils and monocytes catalyzes the reaction between the chloride ion and hydrogen peroxide and generates hypochlorous acid and other reactive oxygen species (ROS) [4]. The reactive by-products generated by MPO can cause oxidative damage in vivo to biomolecules, such as DNA, protein and lipids, and cause cellular alterations that may lead to carcinogenesis.
The human MPO gene located on chromosome 17q23.1 consists of 12 exons and 11 introns [5] and there are at least 319 different polymorphism sites in the MPO gene (http://www.ncbi.nlm.nih.gov/projects/SNP). The most extensively studied single nucleotide polymorphism (SNP) was −463G>A polymorphism (rs2333227) located in the promoter region of the MPO gene. Since Austin et al first published the MPO−463G>A polymorphism in 1993 [5], many subsequent studies have consecutively reported the relationship between this polymorphism and different cancer types including esophagus, breast, bladder, brain and lung cancer and so forth. Among them, the relationship between lung cancer risk and the MPO−463G>A polymorphism was the most extensively studied, It has been previously suggested that there was an association between the GG+GA genotype of MPO and a decreased risk of lung cancer [6]–[10]. However, other studies have failed to confirm such the association [8], [11]–[24]. The exact relationship between MPO−463G>A polymorphism and susceptibility to lung cancer is inconclusive or conflicting. Up to now, there have been two relevant published meta-analysis studies involving the MPO−463G>A polymorphism and lung cancer risk [25], [26], among which one was published in Chinese and the other one was dealt with the meta-analysis on overall cancer susceptibilities. Unfortunately, those two meta-analyses all failed to adopt the most likely appropriate genetic model and lacked subgroups analyses such as smoking status and thus compromised the authentic values of statistical results. In addition, an increasing number of new studies between MPO−463G>A polymorphism and lung cancer risk are available.
So it is necessary and significant to perform a meta-analysis to explore the association between the MPO−463G>A polymorphism and lung cancer risk. Therefore, we performed a meta-analysis on all eligible case–control studies to estimate the overall lung cancer risk of MPO−463G>A polymorphism and to quantify heterogeneity among the individual eligible studies.
Methods
Search Strategy
Eligible literatures published before the end of September 2012 were identified by the search of PUBMED, EMBASE, ISI Web of Science databases using combinations of the following keywords: “Myeloperoxidase”, “MPO”, “polymorphism” or “variant” and “lung cancer” or “lung carcinoma” without restriction on language. All relevant publications were reviewed. Articles in reference lists were also hand-searched for potentially relevant publications.
Inclusion and Exclusion Criteria
Studies included had to meet all the following inclusion criteria: (a) the diagnosis of lung cancer patients were confirmed histologically or pathologically; (b) a case–control study on MPO−463G>A polymorphism and lung cancer; (c) sufficient available data for estimating an odds ratios (ORs) with 95% confidence intervals (CIs). Major reasons for exclusion of studies were as follows: (i) not for lung cancer study, (ii) only case population and (iii) duplicate of previous publication.
Data Extraction
Two investigators (JP Yang, B Wang) extracted the data independently including first author, year of publication, country, ethnicity (Caucasian, Asian and Mixed), source of controls (hospital-based studies, population-based studies), genotyping methods, matching variables, number of genotypes in cases and controls. Discrepancies were adjudicated by the third investigator (YF Zhou) until consensus was achieved on every item. Because two studies [7], [11] only provided the information of genotypes as “GG” and “AA/GA” without data for all three genotypes, we could only calculate the odds ratios (ORs) for the AA/GA versus GG model. We also abstracted the information of smoking status from available studies, and cigarette smoking status was strategically classified as never smokers, light smokers and heavy smokers.
Statistical Analysis
For control group of each study, the allelic frequency was calculated and the observed genotype frequencies of the MPO−463G>A polymorphism were assessed for Hardy-Weinberg equilibrium (HWE) by using the chi-square test. We calculated the strength of the association between MPO−463G>A polymorphism and lung cancer risk by odds ratios (ORs) corresponding to 95% confidence intervals (CIs). The pooled ORs were performed for homozygote model (GA versus GG), heterozygote model (AA versus GG), dominant model (AA/GA versus GG) and recessive model (AA versus GA/GG) respectively. Stratified analyses were also performed by ethnicity, source of controls and smoking status respectively. Heterogeneity was evaluated with a chi-square-based Q test (P<0.10 was considered significant) [27]. When heterogeneity was present, the random effects model was used to calculate the pooled ORs, whereas the fixed effects model was used [28]. Galbraith plot was used to determine the main sources of the heterogeneity [29], [30]. The one-way sensitivity analyses were performed to assess the stability of the results, namely, a single study in the meta-analysis was deleted each time to reflect the influence of the individual data set to the pooled ORs [31]. The publication bias of literatures was assessed using funnel plot and funnel plot asymmetry was assessed by the method of Egger’s linear regression test [32]–[34]. The significance of the intercept was determined by the t test suggested by Egger and P<0.05 was considered significant. All statistical analyses were performed with STATA software (version 12.0; STATA Corporation, College Station, TX), and all tests were two sided.
Results
Study Characteristics
For lung cancer susceptibility related to MPO−463G>A polymorphism, Study selection process was shown in Figure 1 . 22 case–control studies met the inclusion criteria including7,520 patients with lung cancer and 8,600 controls in this meta-analysis[6]–[24], [35]–[37]. The characteristics of included studies and distribution of the frequencies of MPO−463G>A polymorphism on lung cancer were summarized in Table 1 and Table 2 respectively. Overall, there were 9 studies of Caucasians, 6 studies of Asians, 7 of mixed population, and 11 studies of population-based, 11 studies of hospital-based. Six studies [8], [15], [16], [18], [22], [23] collected the information on possible confounding factors like smoking status. All cases were pathologically confirmed and Almost controls were mainly matched for age and sex. Most polymorphisms in the control subjects were in Hardy–Weinberg equilibrium.
Figure 1. Flow chart of study selection based on the inclusion and exclusion criteria.
Table 1. Main characteristics of studies included in the meta-analysis.
| First author | Year | Country | Ethnicity | Control source | Genotyping method | Matching |
| Arslan | 2011 | Turkey | Caucasian | PB | PCR–RFLP | NA |
| Klinchid | 2009 | Thailand | Asian | HB | PCR–RFLP | NA |
| Yoon | 2008 | Korea | Asian | PB | Taqman | age |
| Zienolddiny | 2008 | Norway | Caucasian | PB | Taqman | age and sex |
| Yang | 2007 | Korea | Asian | HB | Taqman | NA |
| Larsen | 2006 | Australia | Caucasian | HB | PCR–RFLP | age |
| Park | 2006 | Korea | Asian | HB | PCR–RFLP | age and sex |
| Chan | 2005 | China | Asian | HB | PCR–RFLP | age and sex |
| Skuladottir | 2005 | Denmark | Caucasian | PB | PCR–RFLP | age and sex |
| Harms | 2004 | USA | Mixed | PB | PCR–RFLP | NA |
| Liu | 2004 | USA | Mixed | PB | PCR–RFLP | NA |
| Chevrier | 2003 | France | Caucasian | HB | Taqman | age |
| Dally, H | 2002 | Germany | Caucasian | HB | PCR–RFLP | sex |
| Feyler | 2002 | France | Caucasian | HB | PCR–RFLP | age and sex |
| Kantarci | 2002 | USA | Mixed | HB | PCR–RFLP | sex and ethnicity |
| Lu | 2002 | China | Asian | PB | PCR–RFLP | age and sex |
| Schabath | 2002 | USA | Mixed | HB | PCR–RFLP | age,sex and ethnicity |
| Xu | 2002 | USA | Mixed | PB | PCR–RFLP | NA |
| Misra | 2001 | Finland | Caucasian | PB | Taqman | age |
| Cascorbi | 2000 | Germany | Caucasian | HB | PCR–RFLP | age and sex |
| Marchand | 2000 | USA | Mixed | PB | PCR–RFLP | age,sex and ethnicity |
| London | 1997 | USA | Mixed | PB | PCR–RFLP | age,sex and ethnicity |
Abbreviations HB: Hospital-based studies; PB: Population-based studies; PCR-RFLP: Polymerase chain reaction–restriction fragment length polymorphism; NA: Not available.
Table 2. Distribution of MPO−463G>A polymorphism among lung cancer cases and controls included in the meta-analysis.
| First author | Year | Sample size | cases | Controls | P for HWE | |||||
| Cases | Controls | GG | GA | AA | GG | GA | AA | |||
| Arslan | 2011 | 106 | 271 | 67 | 35 | 4 | 136 | 110 | 21 | 0.85 |
| Klinchid | 2009 | 88 | 81 | 59 | 29 a | 57 | 24 a | NA | ||
| Yoon | 2008 | 213 | 213 | 180 | 31 | 2 | 175 | 35 | 3 | 0.42 |
| Zienolddiny | 2008 | 258 | 297 | 150 | 74 | 34 | 179 | 109 | 9 | 0.11 |
| Yang | 2007 | 318 | 353 | 269 | 49 | 0 | 283 | 68 | 2 | 0.33 |
| Larsen | 2006 | 627 | 624 | 382 | 205 | 40 | 383 | 210 | 31 | 0.75 |
| Park | 2006 | 432 | 432 | 353 | 76 | 3 | 356 | 72 | 4 | 0.87 |
| Chan | 2005 | 75 | 162 | 44 | 28 | 3 | 118 | 42 | 2 | 0.41 |
| Skuladottir | 2005 | 122 | 396 | 75 | 47 a | 270 | 126 a | NA | ||
| Harms | 2004 | 110 | 119 | 56 | 47 | 7 | 59 | 56 | 4 | 0.03 |
| Liu | 2004 | 830 | 1119 | 490 | 296 | 44 | 692 | 386 | 41 | 0.15 |
| Chevrier | 2003 | 243 | 245 | 135 | 98 | 10 | 140 | 93 | 12 | 0.49 |
| Dally, H | 2002 | 625 | 340 | 429 | 173 | 23 | 218 | 105 | 17 | 0.35 |
| Feyler | 2002 | 150 | 172 | 98 | 42 | 10 | 96 | 63 | 13 | 0.55 |
| Kantarci | 2002 | 307 | 307 | 192 | 106 | 9 | 181 | 111 | 15 | 0.70 |
| Lu | 2002 | 314 | 320 | 248 | 60 | 6 | 227 | 87 | 6 | 0.48 |
| Schabath | 2002 | 375 | 378 | 235 | 126 | 14 | 202 | 157 | 19 | 0.10 |
| Xu | 2002 | 989 | 1128 | 599 | 343 | 47 | 697 | 390 | 41 | 0.13 |
| Misra | 2001 | 315 | 311 | 191 | 108 | 16 | 206 | 84 | 21 | <0.01 |
| Cascorbi | 2000 | 196 | 196 | 141 | 49 | 6 | 117 | 75 | 4 | 0.04 |
| Marchand | 2000 | 323 | 437 | 234 | 77 | 12 | 294 | 116 | 27 | <0.01 |
| London | 1997 | 339 | 703 | 353 | 136 | 16 | 401 | 243 | 59 | 0.01 |
Abbreviations HWE: Hardy–Weinberg Equilibrium; NA: not applicable; a: number of GA+AA.
Meta-analysis Results
Table 3 listed the results of the association between the MPO−463G>A polymorphism and lung cancer risk. Overall, there was no evidence for significant association between MPO−463G>A polymorphism and lung cancer susceptibility (for AA versus GG: OR = 0.91, 95%CI = 0.67–1.24; for AA/GA versus GG: OR = 0.90, 95%CI = 0.80–1.01; for AA versus GA/GG: OR = 0.96, 95%CI = 0.72–1.28) ( Figure 2 ). In the stratified analysis by ethnicity, source of controls and smoking status, we also did not find any significant association between MPO−463G>A polymorphism and lung cancer risk. MPO −463G>A polymorphism was a low protective susceptibility gene in lung cancer development in homozygote model (for GA versus GG: OR = 0.87, 95%CI = 0.78–0.98), however, when stratified by ethnicity, source of controls and smoking status, we also did not find any significant association between them (for Caucasian population: OR = 0.86, 95%CI = 0.71–1.05, for Asian population: OR = 0.92, 95%CI = 0.67–1.25, for mixed population: OR = 0.86, 95%CI = 0.73–1.02, for population-based studies: OR = 0.87, 95%CI = 0.74–1.03, for hospital-based studies: OR = 0.87, 95%CI = 0.74–1.02, for never smokers: OR = 0.77, 95%CI = 0.43–1.38, for light smokers: OR = 1.00, 95%CI = 0.79–1.27, for heavy smokers: OR = 0.92, 95% CI = 0.69–1.23).
Table 3. Meta-analyses of the MPO −463G>A polymorphism on lung cancer risk.
| Subgroup | Number | AA versus GG(heterozygote) | GA versus GG(homozygote) | AA/GA versus GG (dominant) | AA versus GA/GG (recessive) | ||||
| N/Cases/Controls | OR (95% CI) | Pvalue/Phet | OR (95% CI) | Pvalue/Phet | OR (95% CI) | Pvalue/Phet | OR (95% CI) | Pvalue/Phet | |
| Total | 22/7520/8600 | 0.91(0.67,1.24) | 0.56/<0.01 | 0.87(0.78,0.98) | 0.02/<0.01 | 0.90(0.80,1.01) | 0.07/<0.01 | 0.96(0.72,1.28) | 0.77/<0.01 |
| Ethnicity | |||||||||
| Caucasian | 9/2642/2848 | 1.03(0.64,1.67) | 0.89/<0.01 | 0.86(0.71,1.05) | 0.13/0.02 | 0.92(0.77,1.11) | 0.38/0.01 | 1.09(0.67,1.76) | 0.74/<0.01 |
| Asian | 6/1440/1561 | 0.92(0.46,1.85)a | 0.82/0.46 | 0.92(0.67,1.25) | 0.59/0.04 | 0.95(0.71,1.26) | 0.70/0.04 | 0.95(0.47,1.90)a | 0.87/0.56 |
| Mixed | 7/3439/4191 | 0.79(0.47,1.33) | 0.38/<0.01 | 0.86(0.73,1.02) | 0.09/0.01 | 0.84(0.68,1.04) | 0.11/<0.01 | 0.84(0.53,1.33) | 0.46/<0.01 |
| Study design | |||||||||
| PB | 11/4085/5310 | 0.96(0.58,1.60) | 0.87/<0.01 | 0.87(0.74,1.03) | 0.10/<0.01 | 0.91(0.76,1.09) | 0.28/<0.01 | 1.00(0.62,1.64) | 0.99/<0.01 |
| HB | 11/3436/3290 | 0.88(0.67,1.14)a | 0.32/0.46 | 0.87(0.74,1.02) | 0.09/0.03 | 0.88(0.75,1.03) | 0.11/0.03 | 0.92(0.71,1.20)a | 0.54/0.58 |
| Smoking status | |||||||||
| Never smokers | 2/174/709 | 1.07(0.23,5.00) | 0.93/NA | 0.77(0.43,1.38) | 0.38/NA | 0.79(0.54,1.14) | 0.21/NA | 1.17(0.25,5.40) | 0.84/NA |
| Light smokers | 6/825/943 | 1.05(0.60,1.84)a | 0.86/0.83 | 1.00(0.79,1.27)a | 0.98/0.37 | 0.92(0.74,1.13)a | 0.41/0.18 | 1.07(0.62,1.86)a | 0.81/0.84 |
| Heavy smokers | 5/744/688 | 1.39(0.75,2.60)a | 0.30/0.85 | 0.92(0.69,1.23)a | 0.57/0.60 | 0.82(0.66,1.03)a | 0.09/0.21 | 1.58(0.86,2.90)a | 0.15/0.47 |
Abbreviations N:Number of studies; NA: Not applicable; Phet : Probability of heterogeneity; a: Fixed-effects model was used when Phet≥0.1, otherwise, random model was used.
Figure 2. Forest plots of lung cancer risk associated with the MPO−463G>A polymorphism for AA/GA versus GG model in the stratified analyses by ethnicities.
Test of Heterogeneity
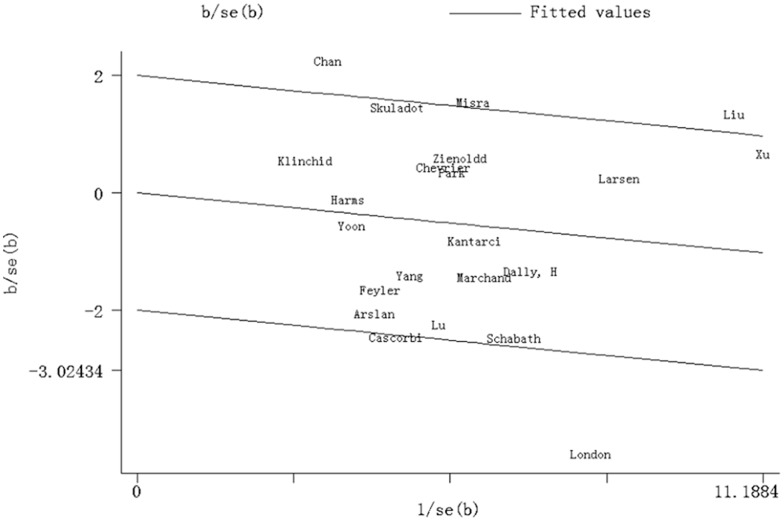
Significant heterogeneity existed in four genetic models of the MPO−463G>A polymorphism (AA versus GG, GA versus GG, AA/GA versus GG, AA versus GA/GG) ( Table 3 ). Galbraith plot analyses of all included studies were used to assess the potential sources of heterogeneity. Chan, Liu and London’s studies [10], [35] were found to be the main contributors of heterogeneity in the AA/GA versus GG model ( Figure 3 ). The significance of pooled ORs with 95%CIs in the AA/GA versus GG model in both overall comparison and subgroup analyses was not influenced by omitting those three studies (Data not shown).
Figure 3. Galbraith plot analysis of the amount of heterogeneity from all the included studies (AA/GA versus GG).
The Y-axis shows the ratio of the log OR to its standard error (SE), and the x-axis shows the reciprocal of the SE. At a 2 standard deviation distance parallel to the regression line, the 2 lines create an interval. Studies lacking in heterogeneity would lie within the 95% confidence interval.
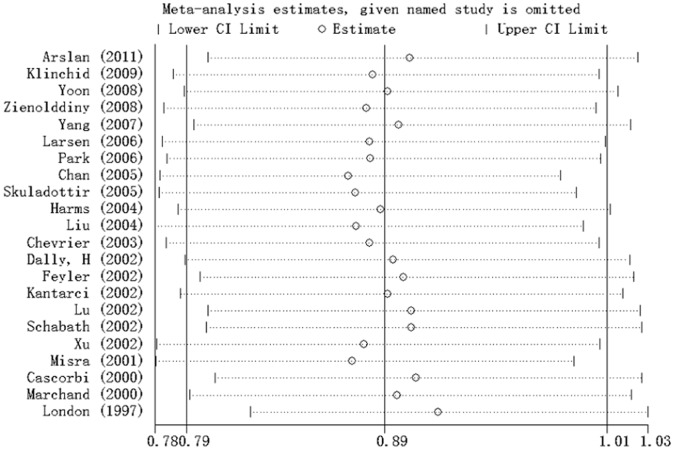
Sensitivity Analysis
Sensitivity analysis was performed through sequentially excluding individual studies. Statistically similar results were obtained after sequentially excluding each study in GA/AA versus GG model ( Figure 4 ) and the corresponding pooled ORs in the other genetic models were not materially altered (Data not shown), suggesting stability and liability of this meta-analysis.
Figure 4. Sensitivity analysis conducted to assess the influence of each study on the pooled ORs by individual studies omission in AA/GA versus GG model.
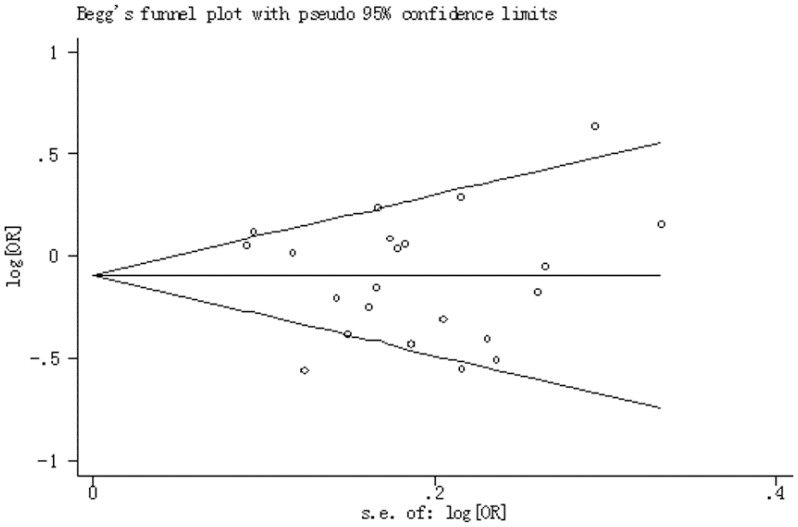
Publication Bias
The funnel plot and Egger’s test did not provide any obvious evidence of publication bias that examined the MPO−463G>A polymorphism and lung cancer risk. The shape of funnel plots did not reveal any evidence of funnel plot asymmetry ( Figure 5 ), Egger's test further provided statistical evidence of funnel plot symmetry for AA versus GG (P = 0.55), GA versus GG (P = 0.27), AA/GA versus GG (P = 0.56) and for AA versus GA/GG (P = 0.61).
Figure 5. Begg’s funnel plot between the MPO−463G>A polymorphism and lung cancer risk in AA/GA versus GG model.
Discussion
As we all know, lung cancer is a complex multifactorial and multistage process, in which both host genetic factors and environmental factors are involved [2]. Individual genetic susceptibility has been suggested to correlate with lung cancer risk. In the current meta-analysis, on the basis of 22 case-control studies providing the information on the MPO−463G>A polymorphism and lung cancer involving 7,520 cases and 8,600 controls, we did not find any significant association between the MPO−463G>A polymorphism and lung cancer risk in any genetic model and also the similar results stratified by ethnicity, source of controls and smoking status respectively. The one-way sensitivity analyses suggested the stability and liability of the results in this meta-analysis. Publication bias was not observed in this study. Our meta-analysis suggests that the MPO −463G>A polymorphism is not associated with lung cancer development.
Oxidative stress occurs when the excessive production of reactive oxygen species (ROS) overwhelms the antioxidant defense system. Increasing evidences suggests variability in these genes involved in oxidative stress may determine the level of oxidative stress in the organism and play a crucial role in carcinogenesis [4], [5]. Therefore, it is rational to speculate that certain genetic variants or polymorphisms in the genes involved in oxidative stress may have an impact on cancer risk. MPO is an endogenous oxidant enzyme that generates reactive oxygen species (ROS) that may be involved in carcinogenesis [4], [38]. MPO−463G>A polymorphism was located in the promoter region of the MPO gene. The G allele acts as a strong stimulatory protein 1 (SP1) transcription factors binding site, which reacts with SP1 to elevate MPO transcriptional activity [39]. Therefore the guanosine (G) to adenosine (A) nucleotide base substitution is associated with disruption of the SP1 binding site and thus reduces 25 times MPO gene expression and decreases the enzyme levels. So it is biologically plausible that MPO−463G>A polymorphism may modulate the risk of lung cancer. Therefore, many studies have investigated the role of MPO−463G>A polymorphism in the pathogenesis of lung cancer [6]–[24], [35]–[37]. However, the results remain conflicting rather than conclusive. There are several possible explanations for this discordance, such as small sample size, ethnic background, uncorrected multiple hypothesis testing, and publication bias. Meta-analysis is a statistical procedure for combining the results of several studies to produce a single estimate of the major effect with enhanced precision. So we performed a meta-analysis on 22 eligible case–control studies to estimate the overall lung cancer risk of MPO−463G>A polymorphism, whereas no significant associations were found between them in any genetic model in this meta-analysis.
Population stratification is an area of concern that can lead to spurious evidence for the association between a marker and cancer and suggest a possible role for ethnic differences in genetic backgrounds.
Previous studies have found a wide variation in the A allele frequency of the MPO−463G>A polymorphism across different ethnicities. The −463A allele frequency was 22.8% in European population, but approximately 14.7% in Asian population [40]. When stratified according to ethnicity in this meta-analysis study, no significant associations were found in any of the genetic models in the Caucasian, Asian and mixed population. Although hospital-based studies may have inherent selection biases, we also did not find any positive result in the stratified analyses by population-based and hospital-based studies. These results suggested that the different ethnicities and source of controls did not influence the association between the MPO−463G>A polymorphism and lung cancer risk.
Lung cancer have been characterized as causally related to cigarette smoking [41]. MPO transforms tobacco smoke procarcinogens, such as benzo(α)pyrene and arylamines, into highly carcinogenic intermediates, such as benzo(α)pyrene dio-epoxide. Since MPO−463G>A polymorphism may be associated with weaker transcriptional activity and decreases the enzyme levels, carcinogens contained in cigarette smoking will not be metabolically activated by MPO enzyme, therefore this polymorphism has been suggested to have a protective effect against the development of cancers related to smoking such as lung cancer [42], [43]. Whereas in our meta-analysis study, the MPO−463G>A polymorphism was shown to have no statistically significant protective effect in light smokers and heavy smokers. The exact mechanism for this inverse result was not clear. That may be due to the limited number of study subjects related to smoking which may have low power of statistical test, so further large-scale researches between MPO−463G>A polymorphism and risk of lung cancer in smokers are expected to confirm the results.
Several limitations of this meta-analysis should be addressed. First, when interpreting the results of this meta-analysis, heterogeneity was a potential problem and the origins of heterogeneity may include many factors, such as the differences in control characteristics and diverse genotyping methods; Second, the lack of detailed information such as age, sex and lifestyle of the patients in some studies, limited further stratification, and more accurate ORs would be corrected for age, sex and other factors that were associated with lung cancer risk. Nevertheless, our meta-analysis has some advantages. First, the well-designed search and selection method significantly increased the statistical power of this meta-analysis. Second, we could perform a subgroup analysis to address a possible interaction between smoking parameters and MPO−463G>A polymorphism. Third, the results did not show any evidence of publication bias.
In summary, our meta-analysis suggests that the MPO −463G>A polymorphism may not be a risk factor for lung cancer. However, lung cancer may be a multifactorial disease that resulted from complex interactions between genetic and environmental factors, we could not collect the detailed original data of MPO gene polymorphisms and then we were not able to investigate potential gene-gene, gene- environment interactions. Further prospective researches using adjusted individual data with large sample studies which study the relationship between MPO−463G>A polymorphism and the risk of lung cancer are necessary and expected, which would lead a better, comprehensive understanding of the association between MPO−463G>A polymorphism and lung cancer risk.
Supporting Information
MOOSE Checklist.
(DOC)
Funding Statement
This work was supported by the National Natural Science foundation of China (81071825/H1610). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Siegel R, Ward E, Brawley O, Jemal A (2011) Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 61: 212–236. [DOI] [PubMed] [Google Scholar]
- 2. Pharoah PD, Dunning AM, Ponder BA, Easton DF (2004) Association studies for finding cancer-susceptibility genetic variants. Nat Rev Cancer 4: 850–860. [DOI] [PubMed] [Google Scholar]
- 3.(2004) The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta GA. [PubMed] [Google Scholar]
- 4. Klebanoff SJ (1999) Myeloperoxidase. Proc Assoc Am Physicians 111: 383–389. [DOI] [PubMed] [Google Scholar]
- 5. Austin GE, Lam L, Zaki SR, Chan WC, Hodge T, et al. (1993) Sequence comparison of putative regulatory DNA of the 5′ flanking region of the myeloperoxidase gene in normal and leukemic bone marrow cells. Leukemia 7: 1445–1450. [PubMed] [Google Scholar]
- 6. Arslan S, Pinarbasi H, Silig Y (2011) Myeloperoxidase G-463A polymorphism and risk of lung and prostate cancer in a Turkish population. Molecular Medicine Reports 4: 87–92. [DOI] [PubMed] [Google Scholar]
- 7. Skuladottir H, Autrup H, Autrup J, Tjoenneland A, Overvad K, et al. (2005) Polymorphisms in genes involved in xenobiotic metabolism and lung cancer risk under the age of 60 years: A pooled study of lung cancer patients in Denmark and Norway. Lung Cancer 48: 187–199. [DOI] [PubMed] [Google Scholar]
- 8. Lu W, Qi J, Xing D, Tan W, Miao X, et al. (2002) Lung cancer risk associated with genetic polymorphism in myeloperoxidase (-463 G/A) in a Chinese population. Zhonghua zhong liu za zhi [Chinese journal of oncology] 24: 250–253. [PubMed] [Google Scholar]
- 9. Cascorbi I, Henning S, Brockmoller J, Gephart J, Meisel C, et al. (2000) Substantially reduced risk of cancer of the aerodigestive tract in subjects with variant–463A of the myeloperoxidase gene. Cancer Res 60: 644–649. [PubMed] [Google Scholar]
- 10. London SJ, Lehman TA, Taylor JA (1997) Myeloperoxidase genetic polymorphism and lung cancer risk. Cancer Research 57: 5001–5003. [PubMed] [Google Scholar]
- 11. Klinchid J, Chewaskulyoung B, Saeteng S, Lertprasertsuke N, Kasinrerk W, et al. (2009) Effect of combined genetic polymorphisms on lung cancer risk in northern Thai women. Cancer Genetics and Cytogenetics 195: 143–149. [DOI] [PubMed] [Google Scholar]
- 12. Zienolddiny S, Campa D, Lind H, Ryberg D, Skaug V, et al. (2008) A comprehensive analysis of phase I and phase II metabolism gene polymorphisms and risk of non-small cell lung cancer in smokers. Carcinogenesis 29: 1164–1169. [DOI] [PubMed] [Google Scholar]
- 13. Yoon KA, Kim JH, Gil HJ, Hwang H, Hwangbo B, et al. (2008) CyP1B1, CYP1A1, MPO, and GSTP1 polymorphisms and lung cancer risk in never-smoking Korean women. Lung Cancer 60: 40–46. [DOI] [PubMed] [Google Scholar]
- 14. Yang M, Choi Y, Hwangbo B, Lee JS (2007) Combined effects of genetic polymorphisms in six selected genes on lung cancer susceptibility. Lung Cancer 57: 135–142. [DOI] [PubMed] [Google Scholar]
- 15. Park JH, Park JM, Kim EJ, Cha SI, Lee EB, et al. (2006) Myeloperoxidase -463G>A polymorphism and risk of primary lung cancer in a Korean population. Cancer Detection and Prevention 30: 257–261. [DOI] [PubMed] [Google Scholar]
- 16. Larsen JE, Colosimo ML, Yang IA, Bowman R, Zimmerman PV, et al. (2006) CYP1A1 Ile462Val and MPO G-463A interact to increase risk of adenocarcinoma but not squamous cell carcinoma of the lung. Carcinogenesis 27: 525–532. [DOI] [PubMed] [Google Scholar]
- 17. Liu G, Zhou W, Wang LI, Park S, Miller DP, et al. (2004) MPO and SOD2 polymorphisms, gender, and the risk of non-small cell lung carcinoma. Cancer Letters 214: 69–79. [DOI] [PubMed] [Google Scholar]
- 18. Harms C, Salama SA, Sierra-Torres CH, Cajas-Salazar N, Au WW (2004) Polymorphisms in DNA repair genes, chromosome aberrations, and lung cancer. Environmental and Molecular Mutagenesis 44: 74–82. [DOI] [PubMed] [Google Scholar]
- 19. Chevrier I, Stucker I, Houllier AM, Cenee S, Beaune P, et al. (2003) Myeloperoxidase: new polymorphisms and relation with lung cancer risk. Pharmacogenetics 13: 729–739. [DOI] [PubMed] [Google Scholar]
- 20. Schabath MB, Spitz MR, Hong WK, Delclos GL, Reynolds WF, et al. (2002) A myeloperoxidase polymorphism associated with reduced risk of lung cancer. Lung Cancer 37: 35–40. [DOI] [PubMed] [Google Scholar]
- 21. Kantarci OH, Lesnick TG, Yang P, Meyer RL, Hebrink DD, et al. (2002) Myeloperoxidase -463 (G–>A) polymorphism associated with lower risk of lung cancer. Mayo Clin Proc 77: 17–22. [DOI] [PubMed] [Google Scholar]
- 22. Feyler A, Voho A, Bouchardy C, Kuokkanen K, Dayer P, et al. (2002) Point: Myeloperoxidase (-463)G -> A polymorphism and lung cancer risk. Cancer Epidemiology Biomarkers & Prevention 11: 1550–1554. [PubMed] [Google Scholar]
- 23. Dally H, Gassner K, Jager B, Schmezer P, Spiegelhalder B, et al. (2002) Myeloperoxidase (MPO) genotype and lung cancer histologic types: The MPO-463 A allele is associated with reduced risk for small cell lung cancer in smokers. International Journal of Cancer 102: 530–535. [DOI] [PubMed] [Google Scholar]
- 24. Misra RR, Tangrea JA, Virtamo J, Ratnasinghe D, Andersen MR, et al. (2001) Variation in the promoter region of the myeloperoxidase gene is not directly related to lung cancer risk among male smokers in Finland. Cancer Letters 164: 161–167. [DOI] [PubMed] [Google Scholar]
- 25. Hua F, Wang J, Gu J, Li S, Liu H, et al. (2010) A meta analysis on the relationship between myeloperoxidase G-463A genetic polymorphisms and lung cancer susceptibility. Chinese Journal of Lung Cancer 13: 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chu HY, Wang ML, Wang MM, Gu DY, Wu DM, et al. (2010) The MPO-463G>A polymorphism and cancer risk: a meta-analysis based on 43 case-control studies. Mutagenesis 25: 389–395. [DOI] [PubMed] [Google Scholar]
- 27. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 28. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 29. Galbraith RF (1988) A note on graphical presentation of estimated odds ratios from several clinical trials. Statistics in Medicine 7: 889–894. [DOI] [PubMed] [Google Scholar]
- 30. Bax L, Ikeda N, Fukui N, Yaju Y, Tsuruta H, et al. (2009) More than numbers: The power of graphs in meta-analysis. American Journal of Epidemiology 169: 249–255. [DOI] [PubMed] [Google Scholar]
- 31. Patsopoulos NA, Evangelou E, Ioannidis JP (2008) Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. International Journal of Epidemiology 37: 1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 33. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sterne JA, Gavaghan D, Egger M (2000) Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 53: 1119–1129. [DOI] [PubMed] [Google Scholar]
- 35. Chan EC, Lam SY, Fu KH, Kwong YL (2005) Polymorphisms of the GSTM1, GSTP1, MPO, XRCC1, and NQO1 genes in Chinese patients with non-small cell lung cancers: relationship with aberrant promoter methylation of the CDKN2A and RARB genes. Cancer Genetics and Cytogenetics 162: 10–20. [DOI] [PubMed] [Google Scholar]
- 36. Xu LL, Liu G, Miller DP, Zhou W, Lynch TJ, et al. (2002) Counterpoint: the myeloperoxidase -463G–>a polymorphism does not decrease lung cancer susceptibility in Caucasians. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 11: 1555–1559. [PubMed] [Google Scholar]
- 37. Marchand LL, Seifried A, Lum A, Wilkens LR (2000) Association of the myeloperoxidase -463G-> A polymorphism with lung cancer risk. Cancer Epidemiology Biomarkers and Prevention 9: 181–184. [PubMed] [Google Scholar]
- 38. Feig DI, Reid TM, Loeb LA (1994) Reactive oxygen species in tumorigenesis. Cancer Res 54: 1890s–1894s. [PubMed] [Google Scholar]
- 39. Piedrafita FJ, Molander RB, Vansant G, Orlova EA, Pfahl M, et al. (1996) An Alu element in the myeloperoxidase promoter contains a composite SP1-thyroid hormone-retinoic acid response element. J Biol Chem 271: 14412–14420. [DOI] [PubMed] [Google Scholar]
- 40. Chu H, Wang M, Wang M, Gu D, Wu D, et al. (2010) The MPO −463G>A polymorphism and cancer risk: a meta-analysis based on 43 case–control studies. Mutagenesis 25: 389–395. [DOI] [PubMed] [Google Scholar]
- 41.(2006) The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta GA. [PubMed] [Google Scholar]
- 42. Van Helden YG, Keijer J, Knaapen AM, Heil SG, Briede JJ, et al. (2009) Beta-carotene metabolites enhance inflammation-induced oxidative DNA damage in lung epithelial cells. Free Radic Biol Med 46: 299–304. [DOI] [PubMed] [Google Scholar]
- 43. Van Schooten FJ, Boots AW, Knaapen AM, Godschalk RW, Maas LM, et al. (2004) Myeloperoxidase (MPO) -463G->A reduces MPO activity and DNA adduct levels in bronchoalveolar lavages of smokers. Cancer Epidemiol Biomarkers Prev 13: 828–833. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MOOSE Checklist.
(DOC)