Abstract
Background
Chemokines provide critical immune cell homing and activation signals that if altered could affect the inflammatory milieu and cellular composition of lymphoid tissues. During HIV-1 and SIV infection, the virus triggers an increase in inflammation/activation, leading to immunodeficiency and development of opportunistic infections, such as in the lungs – a massive interface between the host and the environment.
Methods
Chemokine, cytokine, and chemokine receptor expression profiles were determined using real-time RT-PCR and in situ hybridization in hilar lymph nodes from cynomolgus macaques at different stages after infection with SIV/DeltaB670. Immunostaining of tissue sections and flow cytometric analysis of cryopreserved cells were used to examine cellular compositions of lymph nodes.
Results
IFN-γ, type 1 chemokine and cognate chemokine receptor mRNAs were up-regulated, whereas type 2 and homeostatic chemokine and chemokine receptor mRNAs were down-regulated in hilar lymph nodes after SIV infection. Local SIV and IFN-γ levels were positively correlated with type 1 chemokine levels, but negatively correlated with type 2 and homeostatic chemokine levels. Using in situ hybridization, Pneumocystis carrini rRNA was detected in lung-draining lymph nodes from animals with P. carrini pneumonia. Changes in the cellular composition of hilar lymph nodes included decreased proportions of CD4+ cells and dendritic cells, and increased proportions of CD8+, CXCR3+ and CCR5+ cells.
Conclusions
SIV infection of cynomolgus macaques dramatically alters the cellular homing signals of lung-draining lymph nodes, which correlated with changes in the immune cellular composition. These changes could contribute to the loss of immune function that defines AIDS.
Keywords: SIV, AIDS, chemokine, interferon, chemokine receptor, real-time RT-PCR
INTRODUCTION
Secondary lymphoid tissues are key compartments for the generation of cellular and humoral immune responses and are critical locations of HIV and SIV replication and of soluble and cell-associated antigen sampling of peripheral tissues. Hilar lymph nodes (HiLNs) drain lung tissues, and identifying changes in the HiLN immune environment could provide insight into the mechanisms by which HIV-1 infection drives pulmonary disease as well as overall immunodeficiency. In addition, given the different antigens and cells draining different tissues, the changes that arise in lung draining LNs might differ from those draining skin or intestine.
Inflammation and activation are critical components of HIV-1 and SIV pathogenesis.1 As major players in inflammation, chemokines mediate cell trafficking during immune inductive and effector activities, and changes in their expression in lymphoid tissues could contribute to the pathogenesis of HIV-1 and SIV.2,3 Homeostatic chemokines, such as CCL21, are involved in the constitutive trafficking of naïve T-lymphocytes and DCs to LNs, whereas inflammatory chemokines such as CXCL9 are involved in normal and chronic inflammatory events. Our previous studies have shown that proinflammatory CXCR3 ligands are up-regulated in SIV-infected peripheral LNs, whereas homeostatic lymphoid chemokine CCL21 and Th2 recruiting chemokine CCL17 are down-regulated.3 The altered expression of these chemokines and receptors, and upstream and downstream cytokines, could contribute to local and general immunodeficiency due to changes in the cellular compositions of lymphoid tissues and local immune activities during SIV infection, such as loss of regulatory T cells (Treg).3
In this study, we used the SIV/macaque model to investigate relationships between SIV infection and changes in chemokine and cytokine expression in HiLNs, using real-time RT-PCR. As observed for peripheral LNs, we found that IFN-γ and type 1 chemokine mRNAs were up-regulated, but type 2 and homeostatic chemokine mRNAs were down-regulated during SIV infection. We also identified changes in the cellular compositions of lung-draining LNs during SIV infection and AIDS. Interestingly, we also discovered P. carinii mRNA in the HiLNs and peripheral LNs of macaques with SIV infection and Pneumocyctis carinii pneumonia (PCP). Our findings indicate that SIV infection leads to dramatic changes in the cytokine and chemokine networks in lung-draining LNs, which affect recruitment, retention and/or the steady state proportions of immune cells in macaque HiLNs.
MATERIALS AND METHODS
Animals and Tissue Processing
These studies were carried out under the approval and guidance of the University of Pittsburgh Institutional Animal Care and Use Committee. The University of Pittsburgh is accredited by the American Association for the Accreditation of Laboratory Animal Care International. Adult cynomolgus macaques (Macaca fascicularis) were in this study. Macaques were inoculated intrarectally with the pathogenic SIV/DeltaB670 isolate,4 and comprehensive details regarding their clinicovirological states have been described5. Two of the macaques sacrificed 2 weeks after infection were subsequently found to likely have resisted transmission of the virus, and for group comparisons these animals were grouped with the uninfected controls. Tissues were obtained at necropsy and fixed by immersion in fresh 4% paraformaldehyde (PF)/PBS as described.6,7 HiLN single cell suspensions were generated as described.7 Plasma and tissue SIV RNA loads were assessed by real-time RT-PCR as described.7,8
In situ Hybridization and Immunohistochemistry
In situ hybridization (ISH) with 35S-labeled riboprobes and immunohistochemistry (IHC) with monoclonal and polyclonal antibodies were performed as described.2,6 Autoradiographic exposure times, following ISH with 35S-labeled riboprobes specific for SIV or individual chemokines were 7-10d. Antibodies used for IHC were specific for CD209/DC-SIGN (BD Pharmingen, DCN46) or CD183/CXCR3 (BD, 1C6). Following IHC, CD209/DC-SIGN + cells were manually counted in five random high-powered fields (hpf; x600). Quantitative image analysis (QIA) was performed with the CXCL9 and CXCL10 ISHs using a Nikon Eclipse LV100 microscope for reflected dark field illumination camera (access kindly provided by Nikon). Images were captured with a Nikon DS-Ri1 camera. Up to five random images from each tissue section were captured and analyzed using the NIS-Elements AR software package (Nikon) by adjusting the threshold level until all surface areas covered by silver grains were thresholded, measured, and exported to an Excel spreadsheet. Simultaneous detection of DC-SIGN mRNA by ISH and CD68 or HLA-DR protein by IHC was performed as described,6,9 with IHC performed immediately following the final ISH washes. Antibodies for IHC were specific for CD68 (Dako, 1:50) and HLA-DR (Dako, 1:25). Autoradiographic exposure times were 13d.
Real-time RT-PCR
RNA extraction, template preparation and real-time RT-PCR for host mRNAs were performed using a 2-step protocol as described.3
Flow Cytometry
Cryopreserved, thawed HiLN cell suspensions were stained with fluorochrome-labeled antibodies specific for CD4 (BD, L200), CD8 (BD, SK1), CD20 (BD, L27), CD14 (Beckman Coulter, RM502), CXCR3 (BD, 1C6) or CCR5 (BD, 3A9). Stained cells were analyzed using an XL cytometer (Beckman Coulter). Staining with isotype control antibodies was performed in parallel, and data were analyzed using Expo32 software (Beckman Coulter).
Stimulation of Macaque HiLN Cells
Cryopreserved HiLN cells from SIV-naïve macaques were thawed, counted, and plated (5×105 viable cells/ml) in complete RPMI-1640 culture medium. Cells were treated with IFN-γ (R&D Systems; 20ng/ml), LPS (InvivoGen; 1ug/ml) or Poly (I:C) (InvivoGen; 25ug/ml) for 0, 6 and 24 hr of culture (37°C, 5% CO2). Cells were harvested and stored as pellets at −80°C until RNA extraction. Real-time RT-PCRs were performed in duplicate as described3 and all values were normalized first to the endogenous control β-GUS and then the 0hr calibrator sample.
Statistical Analyses
All statistical analysis outputs were derived using Prism software (GraphPad, La Jolla, CA). Given the numbers of animals available in the different groups, Mann-Whitney nonparametric comparisons were performed for group comparisons. Pairwise comparisons of the real-time RT-PCR measurements were performed (Spearman’s rank correlation). A P-value <0.05 was considered significant. Treatment groups presented in Figure 3 were compared using the paired t-test since no non-parametric method for paired data can be properly applied to samples of size less than six.
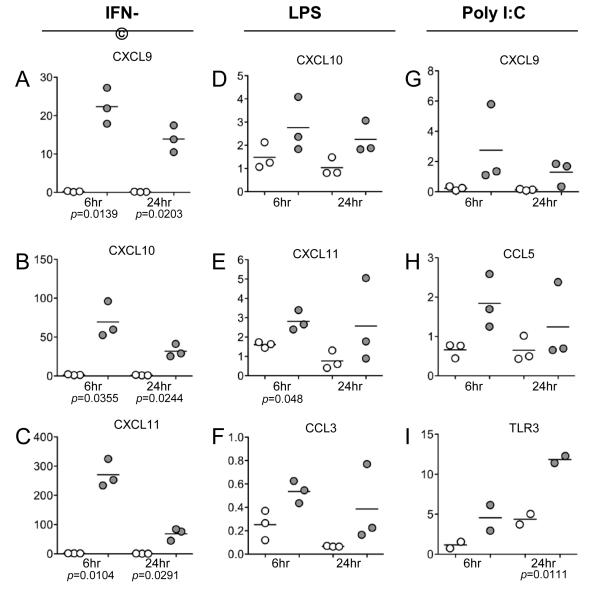
FIGURE 3.
Induction of chemokine and TLR3 mRNAs following stimulation of HiLN cells with IFN-γ, LPS or poly I:C. Using real-time RT-PCR, the relative mRNA levels of the indicated genes were detected in HiLN cells following exposure to IFN-γ, LPS or Poly I:C. In (A-C) are shown the induction of CXCR3 ligand mRNAs with IFN-γ at 6 and 24 hr post-stimulation. In (D-F) are shown the induction of CXCR3 and CCR5 ligand mRNAs with LPS at 6 and 24 hr post-stimulation. In (G-I) are shown the induction of CXCR3 and CCR5 ligand and TLR3 mRNAs with poly I:C at 6 and 24 hr post-stimulation. These data are combined analyses of HiLN cells from three different uninfected animals (only two for TLR3). Open symbols represent the untreated control cells and grey-filled symbols represented the paired treated cells. The mean values are shown superimposed upon the individual data points. Paired t-tests were used to compare the control from treated cultures and all p-values < 0.05 are provided.
RESULTS
Hilar LN Chemokine Expression Profiles Are Altered by SIV Infection
To identify changes in chemokine expression in lung-draining HiLNs during the course of SIV infection, we examined HiLNs from four uninfected and 12 SIV-infected adult cynomolgus macaques with plasma viral loads ranging from 1.8×102 to 3.6×106 copies/ml. These animals and their clinicovirologic states have been described in an analysis of lung tissues, and we note that two of the animals sacrificed two weeks after infection were found to have been exposed but uninfected, and therefore grouped with the uninfected controls.5 Changes in RNA levels of chemokines, chemokine receptors and cytokines were measured for a large number of targets, first by standard RT-PCR analysis (not shown), then by real-time RT-PCR on a subset of 18 chemokines. These analyses revealed that type 1 chemokine mRNA expression levels were up-regulated in HiLNs during SIV infection. Expression of CXCR3 ligand (CXCL9-11) mRNAs was higher (5.4 to 11.8-fold) in SIV-infected macaques or macaques developing AIDS compared to uninfected controls (Table 1). In addition, CCR5 ligand (CCL3-5) mRNA levels were higher (1.8 to 5.3-fold) in the entire group of SIV-infected animals or the group developing AIDS compared to controls (Table 1). In contrast, five chemokine mRNAs were decreased in expression as disease progressed, including type 2 chemokine CCL17, homeostatic chemokine CCL21, as well as CXCL12, CCL25, and CCL20 (Table 1). In situ hybridization (ISH) was used to probe HiLN sections with 35S-labeled riboprobes specific for a subset of mRNAs. As expected the expression of ligands for CXCR3 and CCR5 was low in the HiLNs from uninfected macaques, and was upregulated in acutely infected or AIDS-developing macaques (Table 2 and Figure 1). Quantitative image capture and analysis (QIA) of the CXCL9 and CXCL10 ISHs supported the real-time RT-PCR measurements, indicating a 2.1-fold increase in CXCL9 and 3.6-fold increase in CXCL10 levels for all SIV infected macaques compared to controls. In contrast, CCL21 and CCL20 mRNA expression levels decreased as the infection progressed (Table 2 and Fig. 1). Overall, the ISH and real-time RT-PCR data were concordant, although not completely. Consistent with expected animal-to-animal variability, a small number of animals scored as outliers, such as M5902 (Table 2). In addition, CCL2 levels did not appear to change by real-time RT-PCR (Table 1), whereas histologic scores of ISHs indicated that CCL2 levels increased (Table 2). Reasons for this could include cross-hybridization of the ISH probe to a related chemokine and/or the qualitative nature of the histologic scoring.
TABLE 1.
Measurement of Host Response mRNA Expression Levels in HiLNs by Real-time RT-PCR
| UI (n=6) | SIV (n=10) | AIDS (n=6) | Fold-change | MW P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | Median | Range | SIV/UI | AIDS/UI | SIV/UI | AIDS/UI | |
| Chemokines: Increased | ||||||||||
| CXCL9a | 2.4b | 1.0 – 10.6 | 25.4b | 5.0 – 44.8 | 28.4b | 5.0 – 44.8 | 10.6 | 11.8 | 0.003 | 0.008 |
| CXCL10 | 1.0 | 0.9 – 2.7 | 5.4 | 2.1 – 13.8 | 5.4 | 2.1 – 7.2 | 5.4 | 5.4 | 0.003 | 0.008 |
| CXCL11 | 1.4 | 0.9 – 4.9 | 9.0 | 3.5 – 52.3 | 8.2 | 5.6 – 19.2 | 6.4 | 5.9 | 0.002 | 0.005 |
| CCL3 | 1.8 | 1.0 – 2.9 | 7.7 | 3.1 – 19.0 | 9.5 | 7.1 – 14.7 | 4.3 | 5.3 | 0.001 | 0.005 |
| CCL4 | 1.8 | 0.8 – 3.1 | 3.3 | 1.9 – 7.7 | 4.0 | 2.2 – 7.7 | 1.8 | 2.2 | nsc | ns |
| CCL5 | 1.3 | 0.5 – 2.0 | 3.5 | 0.4 – 6.3 | 3.8 | 0.4 – 6.3 | 2.7 | 2.9 | 0.034 | ns |
| CCL18 | 1.5 | 0.8 – 4.9 | 5.1 | 1.2 – 40.1 | 9.9 | 1.7 – 40.1 | 3.4 | 6.6 | 0.033 | 0.015 |
| CCL23 | 1.2 | 0.6 – 1.7 | 2.5 | 1.0 – 12.2 | 2.7 | 1.2 – 5.4 | 2.1 | 2.2 | ns | ns |
| Chemokines: Decreased | ||||||||||
| CCL17 | 1.3 | 1.0 – 1.8 | 0.3 | 0.02 – 0.9 | 0.3 | 0.02 – 0.8 | 0.2 | 0.2 | 0.001 | 0.005 |
| CCL21 | 0.7 | 0.6 – 1.0 | 0.1 | 0.0 – 0.5 | 0.1 | 0.0 – 0.3 | 0.1 | 0.1 | 0.001 | 0.005 |
| CCL25 | 1.1 | 0.6 - 1.6 | 0.4 | 0.01 - 1.2 | 0.4 | 0.04 - 1.2 | 0.4 | 0.4 | 0.02 | 0.045 |
| CXCL12 | 1.2 | 0.5 - 1.9 | 0.6 | 0.3 - 1.3 | 0.5 | 0.3 - 1.3 | 0.5 | 0.4 | 0.02 | 0.045 |
| CCL20 | 1.0 | 0.4 - 5.9 | 0.5 | 0.1 - 2.0 | 0.4 | 0.1 - 0.8 | 0.5 | 0.4 | ns | 0.045 |
| Chemokine Receptors: Increasedd | ||||||||||
| CCR5 | 1.6 | 0.2 - 2.5 | 3.3 | 0.3 - 8.7 | 5.3 | 0.3 - 8.7 | 2.1 | 3.3 | ns | ns |
| CXCR3 | 2.0 | 1.0 - 2.8 | 4.1 | 0.1 - 7.1 | 4.1 | 0.1 - 5.7 | 2.0 | 2.0 | 0.04 | ns |
| Chemokine Receptors: Decreasedd | ||||||||||
| CCR4 | 1.3 | 0.6 - 2.6 | 0.4 | 0.3 - 1.6 | 0.4 | 0.3 - 0.7 | 0.3 | 0.3 | 0.011 | 0.008 |
| CCR6 | 0.9 | 0.3 - 1.1 | 0.3 | 0.1 - 0.5 | 0.2 | 0.1 - 0.3 | 0.3 | 0.2 | 0.005 | 0.01 |
| CCR7 | 1.2 | 1.0 - 2.6 | 0.5 | 0.03 - 2.5 | 0.5 | 0.03 - 1.1 | 0.4 | 0.4 | 0.011 | 0.008 |
| CCR8 | 0.6 | 0.03 - 2.2 | 0.1 | 0.0 - 0.09 | 0.02 | 0.0 - 0.2 | 0.2 | 0.03 | ns | 0.037 |
| CCR9 | 1.0 | 0.5 - 1.3 | 0.2 | 0.1 - 0.8 | 0.2 | 0.1 - 0.3 | 0.2 | 0.2 | 0.01 | 0.007 |
| Cytokines | ||||||||||
| IFN-γ | 1.7 | 0.9 - 8.7 | 10.6 | 3.4 - 17.0 | 11.0 | 4.4 - 17.0 | 6.2 | 6.5 | 0.008 | 0.02 |
| IL-10 | 1.2 | 0.8 - 4.5 | 2.1 | 1.1 - 3.7 | 2.3 | 1.7 - 3.7 | 1.8 | 1.9 | ns | 0.046 |
| TNFα | 1.0 | 0.4 - 1.4 | 0.6 | 0.03 - 1.1 | 0.3 | 0.03 - 1.1 | 0.6 | 0.3 | ns | ns |
| IFN-α2 | NDe | ND | ND | ND | ND | ND | ND | |||
| IFN-β | 1.1 | 0.2 - 3.7 | 1.4 | 0.4 - 2.9 | 1.4 | 0.4 - 2.9 | 1.3 | 1.3 | ns | ns |
| TGF-β1 | 1.3 | 1.0 - 1.7 | 1.0 | 0.2-1.4 | 0.9 | 0.2-1.1 | 0.8 | 0.7 | 0.025 | 0.011 |
| Other Immune-relevant Markers | ||||||||||
| TLR3 | 1.9 | 1.0 - 4.5 | 3.5 | 2.1 - 11.8 | 3.8 | 2.1 - 6.5 | 1.8 | 2.0 | 0.037 | ns |
| DC-SIGN | 0.7 | 0.4 - 1.1 | 0.2 | 0.1 - 0.4 | 0.2 | 0.1 - 0.4 | 0.3 | 0.3 | 0.001 | 0.004 |
| FOXP3 | 0.9 | 0.7 - 2.0 | 0.3 | 0.1 - 0.9 | 0.3 | 0.1 - 0.3 | 0.3 | 0.3 | 0.004 | 0.005 |
| RORγt | 1.2 | 0.5 - 2.9 | 0.2 | 0.1 - 1.0 | 0.1 | 0.1 - 0.2 | 0.2 | 0.1 | 0.011 | 0.016 |
Inclusion criteria for this table were either a greater than 2-fold increase after SIV infection or a p-value <0.05 from a Mann-Whitney nonparametric test (all SIV infected animals versus uninfected controls, or AIDS-developing animals versus uninfected controls). The mRNAs encoding chemokines CCL2, CCL19, CX3CL1, CXCL13, XCL1, CCR1, CCR3, CCR11, CXCR1, CXCR4, CXCR5 and CX3CR1 were also measured but did not change in expression after SIV infection according to these criteria.
The median values provided are for the indicated group (UI, uninfected and two macaques determined to be exposed but uninfected [n=6]; all SIV infected animals, including two acutely infected, two chronically infected, and six AIDS-developing macaques [n=10]; or all animals developing AIDS [n=6]), with the individual animal’s expression values normalized to the β-GUS endogenous control and then normalized to the same uninfected control “calibrator” sample.
Not significant (P>0.05).
Chemokine receptor mRNAs for CCR2, CCR10, CXCR2 and XCR1 mRNAs were not detected.
ND, not detected.
TABLE 2.
In situ hybridization signals for cellular and pathogen RNAs in macaque HiLNs
| Uninfected | Acute | Clinical latency | AIDS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6202 | 7102 | 13402 | 13502 | 5702 | 7402 | 6002 | 7902 | 13002 | 13102 | 5802 | 5902 | 7002 | 12402b | 12602b | 12802b | |
| CXCL9/Mig | ++a | ++ | ++ | ++ | ++ | ++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| CXCL10/IP-10 | + | ++ | ++ | + | ++ | ++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ++ |
| CXCL11/I-TAC | + | + | + | + | + | + | +++ | +++ | ++ | +++ | +++ | + | +++ | +++ | +++ | ++ |
| CCL2/MCP1 | +/− | + | ++ | + | ++ | ++ | +++ | +++ | +++ | +++ | +++ | ++ | +++ | +++ | +++ | +++ |
| CCL3/MIP-1α | + | + | + | +/− | + | + | +++ | +++ | ++ | +++ | +++ | − | ++ | +++ | +++ | ++ |
| CCL5/RANTES | ++ | ++ | + | − | − | − | − | +++ | +++ | +++ | +++ | − | +++ | +++ | +++ | +++ |
| CCL20/MIP-3α | +++ | +++ | +++ | ++ | +++ | +++ | +++ | +++ | ++ | +++ | + | +/− | + | ++ | + | ++ |
| CCL21/6Ckine | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| IFN-γ | − | +/− | +/− | + | + | +/− | ++ | +++ | ++ | ++ | + | + | ++ | +++ | ++ | ++ |
| IL-10 | + | + | + | + | + | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | +++ | +++ | ++ |
| P.carinii | − | − | ndc | nd | nd | nd | − | nd | − | nd | − | − | − | +++ | ++ | +++ |
| SIV | − | − | − | − | − | − | +++ | +++ | + | +++ | +++ | ++ | + | ++ | + | ++ |
ISH was performed with antisense and control sense 35S-labeled riboprobes specific for the indicated host gene-specific mRNA, SIV mRNA, or P. carinii rRNA. -, no signal detected; +/-, rare mRNA+ cells in entire tissue section; +, 1-5 mRNA+ cells per 200X field; ++, 6-10 mRNA+ cells per 200X field; +++, >10 mRNA+ cells per 200X field.
These three macaques had clear histologic evidence of severe P. carinii pneumonia (PCP), as detailed in ref. 5.
nd, not done.
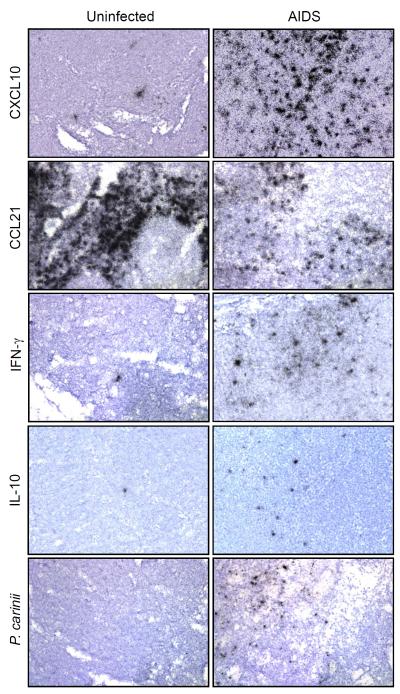
FIGURE 1.
In situ hybridization detection of chemokine, cytokine and pathogen RNAs in HiLN tissue sections from SIV-infected and uninfected macaques. ISH was performed using the indicated 35S-labeled mRNA probes. These are representative images from individual animals from the indicated disease state. Original magnifications: X200.
In parallel, we examined the levels of 18 chemokine receptor mRNAs in HiLNs using real-time RT-PCR, of which 14 were detectable. Only CXCR3 and CCR5 increased after SIV infection (Table 1), whereas CCRs 4, 6, 7, 8, and 9 decreased (Table 1). These changes in chemokine receptor expression profiles paralleled the changes in the corresponding ligands.
To understand potential mechanisms driving changes in chemokine expression, we measured TNF-α, IFN-α2, IFN-β1, IFN-γ, IL-10 and TGF-β1 levels in HiLNs using real-time RT-PCR. IFN-γ mRNA levels increased significantly during SIV infection and AIDS stage (P=0.008, P=0.02, Table 1). IL-10 mRNA levels also increased after SIV infection, with statistical significance during AIDS (P=0.046, Table 1). In contrast, IFN-β mRNA levels did not change, TGF-β1 and TNF-α mRNA levels decreased during SIV infection, and IFN-α2 mRNA levels were not detectable. Using ISH, IFN-γ and IL10 mRNA levels were confirmed to be upregulated, primarily within the T lymphocyte rich paracortices (Fig. 1).
To extend these analyses, we profiled additional immunologically-relevant mRNAs. SIV infection leads to increased TLR3 expression and reduced FOXP3 expression in lymphoid tissues.3,7 In HiLNs the same host response(s) after SIV infection were observed, with TLR3 increasing 2-fold and DC-SIGN and FOXP3 decreasing 3-fold relative to controls (Table 1). Given that CCL20 and CCR6 were decreased after infection, and CCR6 is expressed by Th17/Tc17 cells, we measured the mRNA levels of RORγt, a Th17/Tc17 differentiation factor. RORγt levels were decreased after SIV infection (Table 1) and correlated with multiple immune markers, including CCR6 (see table, Supplemental Digital Content 1).
Altogether, these results indicated that SIV infection altered immune profiles in lung-draining LNs with induction of IFN-γ, pro-inflammatory type 1chemokines, and TLR3, and reduction of homeostatic and type 2 chemokines, FOXP3, Th17/Tc17 marker RORγt and DC-SIGN, consistent with observations in rhesus macaques and in peripheral LNs. These data further supports the interpretation that SIV drives a pro-inflammatory, type 1 polarized response in mucosal-draining lymphoid tissues, in addition to peripheral LNs.
Correlation Analyses Reveal Associations amongst Host Response Genes and SIV Levels
To unveil possible relationships between changes in host response genes and local SIV levels, we performed correlation analyses. In HiLNs, SIV mRNA levels were higher in the animals with acute SIV infection (mean value of 38.8, after normalization to β-GUS) than those in clinical latency (mean 11.1) or AIDS (mean 18.3). ISH also revealed that the numbers of SIV+ cells were highest during acute infection (Table 2). As the HiLNs drain the lungs, and a number of these animals had high P. carinii levels in lung tissues (Table 2),5 we used ISH to probe for P. carinii rRNA in HiLNs (Fig. 1 and Table 2) and found strong signals in those animals with clear PCP, which could drive a more potent inflammatory response.
Pairwise correlation analyses revealed that SIV RNA levels in HiLNs positively correlated with levels of IFN-γ, and type 1 chemokine (CXCL9-11 and CCL3) mRNAs, but negatively correlated with levels of type 2 chemokine (CCL17), homeostatic chemokine (CCL21), CCL25, CCR4 and CCR6 mRNAs (see table, Supplemental Digital Content). Host response genes that positively or negative correlated with IFN-γ mRNA levels moved similarly in relation to local SIV levels. Also, IFN-γ mRNA levels correlated positively with CXCR3, CCR1 and CCR5 mRNA levels, but correlated negatively with CCR6 mRNA levels. CCL5 mRNA levels correlated positively with CCR5 mRNA levels, and CCL19 and CCL21 mRNA levels correlated positively with CCR7 mRNA levels. These data revealed that there was an association between local SIV levels, IFN-γ, and type 1 (IFN-γ-inducible) chemokines in HiLNs. In addition, DC-SIGN mRNA levels, which decreased during the course of infection, were correlated positively with CCL21, CCL25, CCR6 and CCR7 mRNA levels, but negatively correlated with SIV, IFN-γ, and CCL3 mRNA levels. Amongst the other informative correlations, FOXP3 mRNA levels correlated positively with TGF-β1, DC-SIGN, CCL21, CCR6 and CCR7 mRNA levels, but correlated negatively with SIV, IFN-γ and CCL3 mRNA levels, consistent with roles for chemokines and cytokines in modulating local Treg levels, as in peripheral LNs.3
Immune Cell Composition Is Altered in HiLNs during SIV Infection
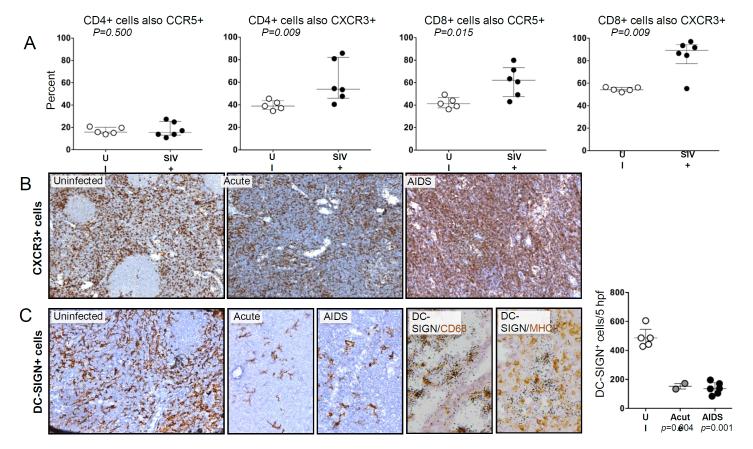
To determine whether in these HiLNs, SIV infection also led to major changes in the proportions of key immune cells, we used flow cytometry to detect T lymphocytes, B lymphocytes, and macrophages in HiLN cell populations from naïve and SIV-infected macaques. As expected, the percentage of CD4+ lymphocytes decreased by 2.9-fold during AIDS, and the percentage of CD8+ lymphocytes increased by 2.0-fold during AIDS. The percentage of CD20+ B lymphocytes slightly increased (21% to 30%), whereas the percentage of CD14+ macrophages (0.7% to 1.4%) did not change. Staining for cell surface CCR5 and CXCR3 revealed that the percentages of CD4+ T cells co-expressing CCR5+ did not change after SIV infection, although the percentage co-expressing CXCR3+ increased (Fig. 2A). In contrast, the percentages of CD8+ T cells co-expressing either CCR5 or CXCR3 increased after SIV infection (Fig. 2A). These findings are consistent with upregulation of CXCR3 and CCR5 ligands in these tissues, and the potential infection and loss of CCR5+ CD4+ T cells.
FIGURE 2.
Flow cytometric and IHC measurement of cell types in HiLNs from SIV-infected and uninfected macaques. A: Flow cytometry was performed on available samples of viably cryopreserved HiLN cells using fluorophore-conjugated antibodies to CD3, CD20, CD4, CD8, CCR5 and/or CXCR3. Shown are the percentages of CD3+/CD20− CD4+ or CD8+ lymphocytes that were also either CCR5+ or CXCR3+ from individual animals, with the group medians and interquartile ranges. Groups were compared using the Mann-Whitney nonparametric test. B: Immunohistochemistry (IHC) was performed to detect CXCR3+ cells in HiLN tissue sections, with antigen-expressing cells staining brown. Original magnification, X100 and X200 (C). C: IHC was performed to detect DC-SIGN+ cells in HiLN tissue sections (original magnifications x200). In addition, ISH for DC-SIGN mRNA was performed simultaneously on HiLN sections stained for either CD68 or MHC-II (original magnificaitons×400). The average density of DC-SIGN+ cells per 0.3mm2 was determined for individual HiLNs from the indicated disease states, with the median and interquartile ranges denoted (to the right). Group comparisons were made using the Mann-Whitney nonparametric test.
Immunohistochemical staining for CXCR3 further confirmed an increase in CXCR3 levels in the HiLNs during AIDS (Fig. 2B). In contrast to CXCR3, the C-type lectin receptor DC-SIGN/CD209 expressed by DCs and macrophages showed reduced staining by IHC (Fig. 2C), consistent with mRNA measurements (Table 1). The DC-SIGN mRNA+ cells localized predominantly to the medullary sinuses, as observed in peripheral LNs by us10 and others,11 and were most likely moderately-activated macrophages as they co-stained strongly for CD68 but only weakly or not at all for MHC class II (Fig. 2C). The reduction in DC-SIGN correlated positively with CCL21 levels and negatively with local SIV levels (see table, Supplementary Digital Content 1) suggesting that indirect effects from virus and reduced DC-homing signals contribute to the loss of DC-SIGN+ cells.
IFN-γ, LPS and Poly I:C Induce Type 1 Chemokines from Cultured HiLN Cells
To identify potential regulators of the changes in HiLNs, we used IFN-γ and the TLR ligands lipopolysaccharide (LPS) and poly I:C to stimulate cultured HiLN cells and measure their responses. All of these stimuli induced the expression of CXCR3 ligands after 6hr and 24hr of treatment (Fig. 3). In addition, LPS and poly I:C induced expression of CCR5 ligands, whereas poly I:C also induced TLR3 (Figs. 3D-I). Coupled with the correlations between local SIV levels and changes to the host response profiles (above), these findings support the interpretation that SIV, host cytokine responses, and pathogen-associated microbial patterns (PAMPs) all could contribute to changes in host responses in HiLNs.
DISCUSSION
Using the well-established SIV/macaque model, we have defined the changes in the chemokine networks that arise in HiLNs during the course of SIV infection. These comprehensive measurements revealed that IFN-γ, and type 1 chemokine and chemokine receptor mRNAs were up-regulated, in contrast to type 2 and homeostatic chemokines and chemokine receptor mRNAs that were down-regulated during SIV infection and AIDS. In parallel, we identified changes in the immune cellular components in HiLNs that were associated with the alternations in the cytokine and chemokine networks in HiLNs as a consequence of infection. This study is the most detailed analysis to date of the chemokine network in LNs and indicates that HiLNs exhibit behaviors consistent with peripheral LNs3 and across nonhuman primate species,12 altogether strengthening the body of data that reveal a type 1 polarization of the chemokine/cytokine network in lymphoid tissues after SIV infection.
Chemokine receptor expression patterns are different on T cell subsets. Th1 cells express CXCR3 and CCR5, Th2 cells express CCR3, CCR4 and CCR8,13-15 Treg express CCR4 and CCR7,16 and Th17 cells express CCR6.17 Therefore, altered chemokine levels would be expected to change the cellular components and immune environments in HiLNs. Here we found that CXCR3 and CCR5 mRNA levels, and proportions of CCR5+ and CXCR3+ cells were increased in HiLNs during SIV infection and AIDS, whereas chemokine receptors CCR4 and CCR6-9 mRNA levels and the densities of DC-SIGN+ medullary macrophages were decreased in SIV-infected HiLNs. Chemokines also can control the cytokine polarization of local environments through the recruitment of specific subsets of T cells. The induction in expression of type 1 chemokines and reduction in type 2 and homeostatic chemokines in lymphoid tissues during SIV infection would increase recruitment of Th1/Tc1 cells and decrease recruitment of Th2/Tc2 cells. In addition, reduction in the CCR6 ligand CCL20 likely contributed to a concordant reduction in the levels of CCR6 and the Th17/Tc17 transcription factor ROR-γt. Increased CXCL9-11 expression in HiLNs could result from increased IFN-γ levels subsequent to SIV-specific T cell responses, which could further induce recruitment of IFN-γ-producing CXCR3+ T lymphocytes and maintain a positive feedback loop.9 Our ex vivo studies confirmed that IFN-γ induces HiLN cells to upregulate CXCR3 ligands (Figs. 3A-C).
DCs are potent antigen presenting cells (APCs) and express chemokine receptors CCR7, CCR4, and CCR6 that control their migration to draining LNs. Given the changes we observed in the profile of chemokines in HiLNs, as well as in expression of IL-10, which can reduce the migration of DCs to draining LNs,18 decreased levels of DC-SIGN in SIV-infected HiLNs could be driven in part by decreases in levels of chemokines that attract DCs and by increases in IL-10 expression. However, lack of strong correlations between IL-10 levels and chemokine levels does not support a direct role for IL-10 in controlling chemokine levels (Supplementary Digital Content). Finally, if monocytes undergo apoptosis upon arrival in LNs during SIV infection, as has been shown for DCs,19 this might contribute to the SIV-driven loss of DC-SIGN+ cells in LNs.
Regulatory T cells play key roles in immune regulation through multiple modes of suppression.20,21 The balance of Treg numbers in HiLN tissues during SIV infection could modulate local levels of immune activation and/or immune suppression. Given that CCR4 and CCR7 are expressed on Treg,16 the decreased CCR4 and CCR7 ligand (CCL17 and CCL21) expression in HiLNs during SIV infection could contribute to loss of Treg (FoxP3 levels). In addition, TGF-β1 drives induction and differentiation of Treg,22 and its loss would also contribute to loss of Treg. As a consequence, immune activation in HiLNs during SIV infection might be due in part to the loss of Treg,3 subsequent to modification of the local chemokine expression profile.
Two additional likely roles for chemokines in the immunopathogenesis of SIV in HiLN and other lymphoid tissues include both their effects on the pool of ligands available to bind/block/signal CCR5 and their overall anti-apoptotic activity. CCR5 ligands inhibit viral replication in vitro.23 However, here and as published by LaFranco-Scheuch et al.,24 increased local expression of CCR5 ligands positively correlated with increased local SIV levels, likely due to a more dominant proinflammatory effect through recruitment of CCR5+ cells that become targets for infection. Consistent with this interpretation, whereas the proportion of HiLN CD8+ T cells co-expressing CCR5 increased after infection, the proportion of CD4+ T cells co-expressing CCR5 did not increase (Fig. 2). In addition, CXCL12, CCL21, and CCL25 are anti-apoptotic for different cell populations.25-28 Expression of these chemokines was reduced in HiLN tissues during SIV infection, which could contribute to increased apoptosis of multiple immune cell types. CCL25 and CXCL12 reduce apoptosis of cells induced by SIV in vitro.2
Interestingly, we also found P. carinii mRNA in the HiLNs of macaques with PCP. P. carinii is an important opportunistic pulmonary pathogen in HIV infection and AIDS patients. It has been reported that some AIDS patients have extrapulmonary P. carinii infections,29-34 mostly commonly in LNs, suggesting lymphatic dissemination. In immunocompromised rats P. carinii DNA is found in multiple tissues including LNs35 and it has been suggested that heavy organism load and destruction of lung tissue might be a contributing factor in dissemination of P. carinii.35 The macaques in our study that had high lung and HiLN P. carinii burdens also had a generally higher induction of proinflammatory immune mediators (Table 2). Conceivably, systemic dissemination of pulmonary pathogens could contribute to the systemic activation associated with the progressive development of AIDS attributed in part to intestinal microbial translocation.
The overall implications of the findings with HiLN tissues from SIV-infected macaques suggest that SIV infection leads to changes in cytokine and chemokine networks and development of a polarized, inflammatory milieu. These changed environments in HiLN tissues are polarized toward Th1, IFN-γ production due to increased recruitment of CCR5+ and CXCR3+ Th1/Tc1 cells,13,15 and due to decreased recruitment of the cells expressing CCR4/CCR7 (Th2/Tc2 cells), CCR6 (Th17/Tc17 cells), DCs and Treg.14,16 The net overall effect would be less balanced immune response profiles, enhanced immune activation, and ongoing SIV replication. Immunotherapies that modulate these immune mediators might reduce potentially damaging inflammation in lymphoid tissues.
Supplementary Material
ACKNOWLEDGEMENTS
We thank D. McClemens-McBride of the University of Pittsburgh Primate Facility for Infectious Disease Research for assistance with project coordination and animal care; we were kindly provided reagents and plasmids by Drs. K. Norris and F. Villinger. This work was supported by NIH grant RO1 HL 072682 to D.E.K. and RO1 AI060422 to T.A.R.
Supported by the National Institute of Health grants RO1 HL072682 and RO1 AI060422.
Footnotes
The authors have no conflicts of interest to disclose.
Conceived and designed the experiments: T.A.R., S.Q., and D.E.K.; Performed the experiments: S.Q., B.A.J, C.M.L., C.R.K., A.T., and M.M.C; Analyzed the data: S.Q., T.A.R., and P.M.T.; Wrote the article: S.Q., T.A.R.; Critical review of article: B.A.J, A.T., D.E.K., P.M.T., and M.M.C.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sodora DL, Silvestri G. Immune activation and AIDS pathogenesis. AIDS. 2008 Feb 19;22(4):439–446. doi: 10.1097/QAD.0b013e3282f2dbe7. [DOI] [PubMed] [Google Scholar]
- 2.Qin S, Sui Y, Murphey-Corb MA, Reinhart TA. Association between decreased CXCL12 and CCL25 expression and increased apoptosis in lymphoid tissues of cynomolgus macaques during SIV infection. J.Med.Primatol. 2008;37(Suppl 2):46–54. doi: 10.1111/j.1600-0684.2008.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin S, Sui Y, Soloff AC, et al. Chemokine and cytokine mediated loss of regulatory T cells in lymph nodes during pathogenic simian immunodeficiency virus infection. J.Immunol. 2008;180(8):5530–5536. doi: 10.4049/jimmunol.180.8.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphey-Corb M, Martin LN, Rangan SR, et al. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986;321(6068):435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- 5.Qin S, Fallert Junecko BA, Trichel AM, et al. Simian immunodeficiency virus infection alters chemokine networks in lung tissues of cynomolgus macaques: association with Pneumocystis carinii infection. Am J Pathol. 2010;177(3):1274–1285. doi: 10.2353/ajpath.2010.091288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fallert BA, Reinhart TA. Improved detection of simian immunodeficiency virus RNA by in situ hybridization in fixed tissue sections: combined effects of temperatures for tissue fixation and probe hybridization. J.Virol.Methods. 2002;99(1-2):23–32. doi: 10.1016/s0166-0934(01)00378-0. [DOI] [PubMed] [Google Scholar]
- 7.Sanghavi SK, Reinhart TA. Increased expression of TLR3 in lymph nodes during simian immunodeficiency virus infection: implications for inflammation and immunodeficiency. J.Immunol. 2005;175(8):5314–5323. doi: 10.4049/jimmunol.175.8.5314. [DOI] [PubMed] [Google Scholar]
- 8.Fuller DH, Rajakumar PA, Wilson LA, et al. Induction of mucosal protection against primary, heterologous simian immunodeficiency virus by a DNA vaccine. J.Virol. 2002;76(7):3309–3317. doi: 10.1128/JVI.76.7.3309-3317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinhart TA, Fallert BA, Pfeifer ME, et al. Increased expression of the inflammatory chemokine CXC chemokine ligand 9/monokine induced by interferon-gamma in lymphoid tissues of rhesus macaques during simian immunodeficiency virus infection and acquired immunodeficiency syndrome. Blood. 2002;99(9):3119–3128. doi: 10.1182/blood.v99.9.3119. [DOI] [PubMed] [Google Scholar]
- 10.Choi YK, Fallert BA, Murphey-Corb MA, Reinhart TA. Simian immunodeficiency virus dramatically alters expression of homeostatic chemokines and dendritic cell markers during infection in vivo. Blood. 2003 Mar 1;101(5):1684–1691. doi: 10.1182/blood-2002-08-2653. [DOI] [PubMed] [Google Scholar]
- 11.Granelli-Piperno A, Pritsker A, Pack M, et al. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J Immunol. 2005 Oct 1;175(7):4265–4273. doi: 10.4049/jimmunol.175.7.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaefer TM, Fuller CL, Basu S, et al. Increased expression of interferon-inducible genes in macaque lung tissues during simian immunodeficiency virus infection. Microbes Infect. 2006 Jun;8(7):1839–1850. doi: 10.1016/j.micinf.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J.Exp.Med. 1998;187(1):129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Ambrosio D, Iellem A, Bonecchi R, et al. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J.Immunol. 1998;161(10):5111–5115. [PubMed] [Google Scholar]
- 15.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 16.Lim HW, Broxmeyer HE, Kim CH. Regulation of trafficking receptor expression in human forkhead box P3+ regulatory T cells. J.Immunol. 2006;177(2):840–851. doi: 10.4049/jimmunol.177.2.840. [DOI] [PubMed] [Google Scholar]
- 17.Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol. 2008 Jan 1;180(1):214–221. doi: 10.4049/jimmunol.180.1.214. [DOI] [PubMed] [Google Scholar]
- 18.Demangel C, Bertolino P, Britton WJ. Autocrine IL-10 impairs dendritic cell (DC)-derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. Eur J Immunol. 2002 Apr;32(4):994–1002. doi: 10.1002/1521-4141(200204)32:4<994::AID-IMMU994>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Brown KN, Wijewardana V, Liu X, Barratt-Boyes SM. Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS.Pathog. 2009;5(5):e1000413. doi: 10.1371/journal.ppat.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodor J, Fehervari Z, Diamond B, Sakaguchi S. Regulatory T cell-mediated suppression: potential role of ICER. J Leukoc Biol. 2007 Jan;81(1):161–167. doi: 10.1189/jlb.0706474. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006 Aug;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 22.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25-cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007 Feb 15;178(4):2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 23.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270(5243):1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 24.LaFranco-Scheuch L, Abel K, Makori N, Rothaeusler K, Miller CJ. High beta-chemokine expression levels in lymphoid tissues of simian/human immunodeficiency virus 89.6-vaccinated rhesus macaques are associated with uncontrolled replication of simian immunodeficiency virus challenge inoculum. J.Virol. 2004;78(12):6399–6408. doi: 10.1128/JVI.78.12.6399-6408.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JW, Ferris RL, Whiteside TL. Chemokine C receptor 7 expression and protection of circulating CD8+ T lymphocytes from apoptosis. Clin.Cancer Res. 2005;11(21):7901–7910. doi: 10.1158/1078-0432.CCR-05-1346. [DOI] [PubMed] [Google Scholar]
- 26.Qiuping Z, Jei X, Youxin J, et al. CC chemokine ligand 25 enhances resistance to apoptosis in CD4+ T cells from patients with T-cell lineage acute and chronic lymphocytic leukemia by means of livin activation. Cancer Res. 2004;64(20):7579–7587. doi: 10.1158/0008-5472.CAN-04-0641. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Sanchez N, Riol-Blanco L, de la Rosa G, et al. Chemokine receptor CCR7 induces intracellular signaling that inhibits apoptosis of mature dendritic cells. Blood. 2004;104(3):619–625. doi: 10.1182/blood-2003-11-3943. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki Y, Rahman M, Mitsuya H. Diverse transcriptional response of CD4(+) T cells to stromal cell-derived factor (SDF)-1: cell survival promotion and priming effects of SDF-1 on CD4(+) T cells. J.Immunol. 2001;167(6):3064–3073. doi: 10.4049/jimmunol.167.6.3064. [DOI] [PubMed] [Google Scholar]
- 29.Anuradha Sinha A. Extrapulmonary Pneumocystis carinii infection in an AIDS patient: a case report. Acta Cytol. 2007;51(4):599–601. doi: 10.1159/000325806. [DOI] [PubMed] [Google Scholar]
- 30.Carter TR, Cooper PH, Petri WA, Jr., Kim CK, Walzer PD, Guerrant RL. Pneumocystis carinii infection of the small intestine in a patient with acquired immune deficiency syndrome. Am J Clin.Pathol. 1988;89(5):679–683. doi: 10.1093/ajcp/89.5.679. [DOI] [PubMed] [Google Scholar]
- 31.Cohen OJ, Stoeckle MY. Extrapulmonary Pneumocystis carinii infections in the acquired immunodeficiency syndrome. Arch.Intern.Med. 1991;151(6):1205–1214. [PubMed] [Google Scholar]
- 32.Lubat E, Megibow AJ, Balthazar EJ, Goldenberg AS, Birnbaum BA, Bosniak MA. Extrapulmonary Pneumocystis carinii infection in AIDS: CT findings. Radiology. 1990;174(1):157–160. doi: 10.1148/radiology.174.1.2294543. [DOI] [PubMed] [Google Scholar]
- 33.Rabodonirina M, Cotte L, Boibieux A, et al. Detection of Pneumocystis carinii DNA in blood specimens from human immunodeficiency virus-infected patients by nested PCR. J Clin.Microbiol. 1999;37(1):127–131. doi: 10.1128/jcm.37.1.127-131.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raviglione MC. Extrapulmonary pneumocystosis: the first 50 cases. Rev Infect Dis. 1990;12(6):1127–1138. doi: 10.1093/clinids/12.6.1127. [DOI] [PubMed] [Google Scholar]
- 35.Chary-Reddy S, Graves DC. Identification of extrapulmonary Pneumocystis carinii in immunocompromised rats by PCR. J Clin.Microbiol. 1996;34(7):1660–1665. doi: 10.1128/jcm.34.7.1660-1665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.