Abstract
People with sensorineural hearing loss have substantial difficulty understanding speech under degraded listening conditions. Behavioral studies suggest that this difficulty may be caused by changes in auditory processing of the rapidly-varying temporal fine structure (TFS) of acoustic signals. In this paper, we review the presently known effects of sensorineural hearing loss on processing of TFS and slower envelope modulations in the peripheral auditory system of mammals. Cochlear damage has relatively subtle effects on phase locking by auditory-nerve fibers to the temporal structure of narrowband signals under quiet conditions. In background noise, however, sensorineural loss does substantially reduce phase locking to the TFS of pure-tone stimuli. For auditory processing of broadband stimuli, sensorineural hearing loss has been shown to severely alter the neural representation of temporal information along the tonotopic axis of the cochlea. Notably, auditory-nerve fibers innervating the high-frequency part of the cochlea grow increasingly responsive to low-frequency TFS information and less responsive to temporal information near their characteristic frequency (CF). Cochlear damage also increases the correlation of the response to TFS across fibers of varying CF, decreases the traveling-wave delay between TFS responses of fibers with different CFs, and can increase the range of temporal modulation frequencies encoded in the periphery for broadband sounds. Weaker neural coding of temporal structure in background noise and degraded coding of broadband signals along the tonotopic axis of the cochlea are expected to contribute considerably to speech perception problems in people with sensorineural hearing loss.
Keywords: auditory nerve, sensorineural hearing loss, temporal fine structure, temporal envelope, neural coding, phase locking
1. Introduction
Sensorineural hearing loss involving damage and dysfunction within the cochlea is a common, sometimes preventable disorder that can profoundly affect communication ability and hence, the social wellbeing of affected individuals. The National Institute on Deafness and Other Communication Disorders estimates that 15% of Americans between the ages of 20 and 69 have hearing loss due to overexposure to loud sounds. The incidence of reported hearing loss increases with age, rising from 18% in individuals aged 45 to 64 to 30% aged 65–74 and 47% aged 75 and older. While amplification from a hearing aid can improve speech perception in quiet by restoring audibility of previously imperceptible acoustic cues, listeners with sensorineural hearing loss often still struggle to understand speech under degraded conditions with background noise and reverberation (e.g., Duquesnoy, 1983; Woods et al., 2010)
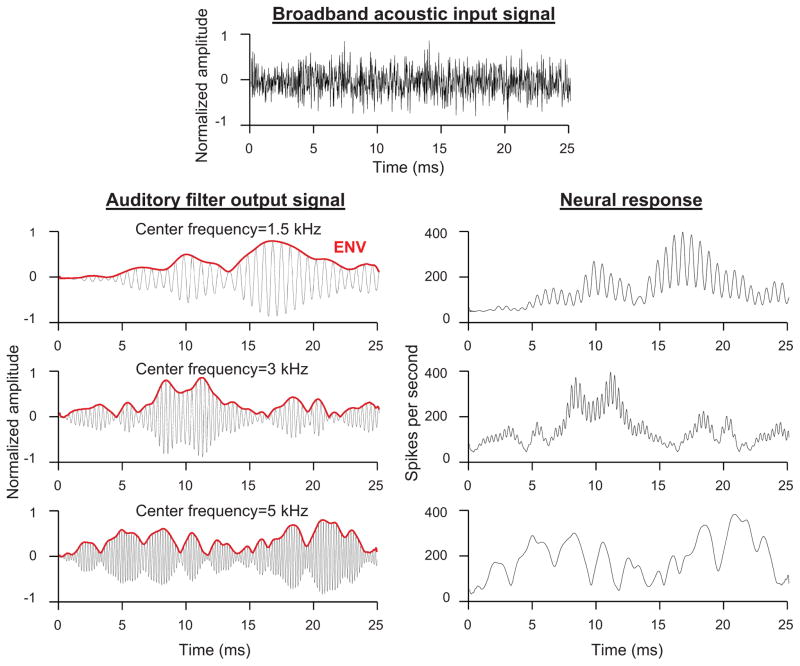
The mammalian cochlea is tonotopically arranged such that high-frequency sounds evoke greatest vibration of the basilar membrane at the base of the structure near its input from the stapes while lower-frequency sounds evoke greatest vibration from progressively more apical locations (Geisler, 1998). The system is commonly modeled as a bank of overlapping band-pass “auditory filters” centered on characteristic frequencies (CFs) distributed across the frequency range of normal hearing, each representing a place on the basilar membrane. Sharply tuned filters with high CFs represent basal cochlear locations, while more broadly tuned filters (on a log frequency scale) with lower CFs represent more apical cochlear locations. The auditory filters decompose broadband sound into a series of narrowband output signals (one per auditory filter), each of which contains two kinds of temporal information: slow changes in overall amplitude envelope (ENV) and faster variations in temporal fine structure (TFS; Fig. 1). Each output signal is encoded by a discrete population of auditory-nerve fibers through variation in spike rate over time. For frequencies below 4–5 kHz, both the TFS and ENV of the output signal are encoded through neural phase locking, or variation in spike rate with the temporal structure of the signal. At higher frequencies, in contrast, the TFS coding is greatly diminished and the auditory-nerve fibers encode primarily the ENV (Johnson, 1980; Joris and Yin, 1992).
Fig. 1.
Schematic of the spectral decomposition of broadband sounds and coding of TFS and ENV information in the cochlea. Broadband acoustic input signal (top) and output signals (left) of auditory filters with center frequencies between 1.5 and 5 kHz. Each output signal consists of a slowly varying amplitude envelope (ENV; red) and a quickly varying temporal fine structure (TFS, black). Auditory filter output signals are encoded in the spike rate of auditory-nerve fibers (right). In fibers with characteristic frequencies (CFs) below 4–5 kHz, spike rate varies with both the TFS and ENV of the auditory filter output signal. In fibers with higher CFs, spike rate varies primarily with the ENV of the output signal, due to the low-pass membrane filtering of the inner hair cells.
Recent behavioral studies suggest that speech perception problems in people with sensorineural hearing loss may be caused by diminished sensitivity to the temporal structure of acoustic signals (reviewed by Moore 2008). Lorenzi and colleagues (Lorenzi et al. 2006, 2009; Ardoint et al. 2010) compared the ability to use temporal information to perceive speech between subjects with varying configurations of sensorineural hearing loss and subjects with normal hearing. Consonant stimuli were decomposed into component narrowband signals (designed to fall within the bandwidth of a single auditory filter) and filtered to contain only ENV information, only TFS information, or both TFS and ENV information (i.e., left intact). The component signals were recombined and presented to subjects for identification. All study subjects, including listeners with hearing loss, could accurately identify consonants when they were intact or contained only ENV information. However, listeners with hearing loss had considerable difficulty identifying consonants containing only TFS information while normal-hearing subjects did not. A reduced ability to use TFS cues was even observed for listeners with only mild to moderate degrees of hearing loss (Ardoint et al. 2010). Furthermore, the listeners with hearing loss who were least able to identify consonants based on TFS information were also least able to perceive sentences presented in fluctuating background noise (Lorenzi et al., 2006). The results suggest that cochlear hearing loss degrades auditory processing of TFS information and that TFS information is critical for accurate perception of speech under degraded listening conditions. Processing of ENV information, in contrast, appears to be less vulnerable to hearing loss and is sufficient for perception of speech in quiet.
A follow-up study examined perception of target speech in the presence of a competing background talker in subjects with moderate hearing loss and normal hearing (Hopkins et al., 2008). The extent of TFS information in the target speech was manipulated by decomposing the signal into component narrowband signals as above and removing TFS from a variable number of filtered signals (beginning with the highest-frequency filter). Component signals were recombined and the speech reception threshold signal-to-noise ratio was measured. Speech reception thresholds in the listeners with hearing loss improved less with the addition of TFS information than in normal-hearing control listeners. These findings further suggest that cochlear hearing loss degrades processing of TFS information and that TFS information is critical for listening in background noise.
Although these ideas are not without controversy (e.g., Oxenham and Simonson, 2009; Swaminathan and Heinz, 2012), they have fueled a great interest in the physiological basis of this problem. A more definitive understanding of the physiological effects of sensorineural hearing loss on temporal coding would be a major asset to efforts aimed at restoring speech perception in people with cochlear hearing loss. In this paper, we review the existing neurophysiological literature on the effects of sensorineural hearing loss on neural coding of TFS information and ENV information in the peripheral auditory system of mammals. We consider coding of narrowband stimuli first followed by coding of broadband signals that stimulate auditory-nerve fibers tuned to a broader range of CFs and have acoustic structure more similar to human speech. In general, the effects of cochlear hearing loss on temporal coding of narrowband stimuli are relatively subtle under quiet conditions. More striking changes in neural coding of temporal information emerge in background noise, where listeners with hearing loss struggle most, and in responses to broadband signals across fibers of varying CF.
2. Basic characteristics of sensorineural hearing loss
Sensorineural hearing loss can arise from numerous sources including exposure to ototoxic drugs or excessively loud sounds, aging, congenital disorders, and infection. One or more of several component systems of the cochlea may suffer damage including (1) sensory inner and outer hair cells, which transduce and amplify basilar-membrane vibration, (2) spiral ganglion neurons, which serve as the only information channel from the cochlea to the auditory brainstem (Kujawa and Liberman, 2006, 2009), and (3) the stria vascularis, which provides the energy necessary for both inner hair cell transduction and cochlear amplification via the outer hair cells (Schuknecht and Gacek, 1993; Schmiedt et al., 2002). Our knowledge of the effects of sensorineural hearing loss on temporal coding is based on neurophysiological data from noise-exposed animals with mixed inner and outer hair cell damage and, to a lesser extent, from kanamycin-exposed animals with primarily outer hair cell damage. Noise exposures have generally been acute, that is, conducted once at high SPL for several hours. Noise and kanamycin-induced hearing losses are generally associated with reductions in cochlear sensitivity and broadened frequency tuning – louder sound pressure levels are required to evoke the same level of response activity from auditory-nerve fibers, and fibers respond to a broader range of acoustic frequencies (Young, 2012).
3. Effects of sensorineural hearing loss on temporal coding of narrowband stimuli
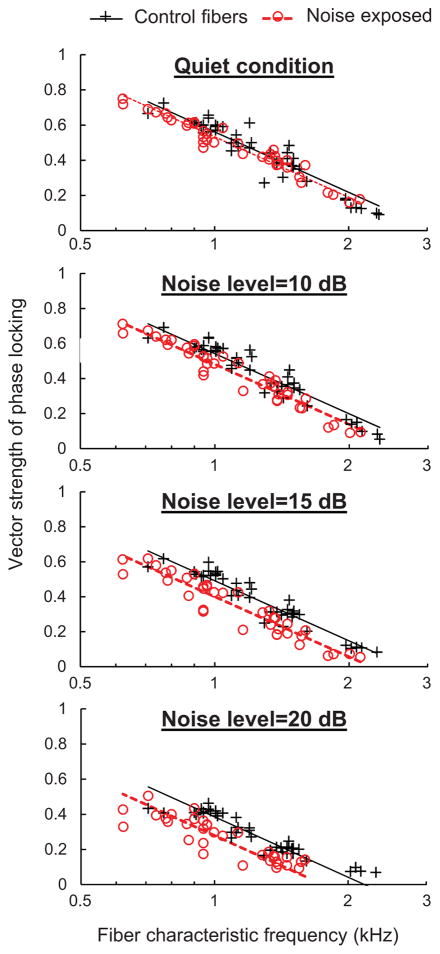
A handful of studies have examined the effects of sensorineural hearing loss on phase locking to the TFS of pure tones in the peripheral auditory system. Two early studies in guinea pigs and chinchillas used the ototoxic drug kanamycin to selectively damage cochlear outer hairs cells of the Organ of Corti (Harrison and Evans, 1979; Woolf et al., 1981). While outer hair cell damage reduced cochlear sensitivity in both studies as expected based on the role of outer hair cells in cochlear amplification, the first study in guinea pigs showed no effect of ototoxic damage on phase locking to tones (Harrison and Evans, 1979) while the second study in chinchillas showed a significant reduction, particularly near the upper frequency limit of phase locking (Woolf et al., 1981). Another more recent investigation showed no measurable effect of noise-induced hearing loss on phase locking to tones in cats (Miller et al., 1997) and similarly, a recent study in our lab showed only a small negative effect of noise-induced hearing loss on phase locking to tones in chinchillas (Henry and Heinz, 2012; Fig. 2). Taken as a whole, these studies suggest that cochlear hearing loss has relatively little impact on the fundamental ability of peripheral auditory neurons to follow the fast fluctuations (i.e., the TFS) of narrowband stimuli.
Fig. 2.
Phase locking to the TFS of pure tones in quiet and in background noise. Vector strength of phase locking to pure tones in chinchilla auditory-nerve fibers is plotted as a function of characteristic frequency. Control and noise-exposed fibers show similar phase locking to tones under quiet conditions. A significant reduction in the vector strength of phase locking in the noise-exposed population emerges under noisy conditions. Noise level is the root-mean-square amplitude of the noise expressed in dB relative to the root-mean-square amplitude of the tone. (Modified from Henry and Heinz, 2012, and originally published in Nature Neuroscience by Nature Publishing Group).
In another recent study in our lab, we investigated the effects of sensorineural hearing loss on temporal coding of narrowband amplitude modulated signals containing both TFS information and ENV information (Kale and Heinz, 2010; 2012). As in the pure-tone studies described above, noise-induced hearing loss in chinchillas had no measurable effect on phase locking to the TFS of sinusoidally amplitude modulated tones and single-formant stimuli. Intriguingly however, this cochlear damage enhanced phase locking to the amplitude ENV of both stimulus types to some degree (Kale and Heinz, 2010). Enhancement of ENV coding occurred across a broad range of modulation frequencies; however, the corner frequency of the temporal modulation transfer function was not extended as would be expected if coding of temporal modulation were limited only by the frequency tuning of auditory filters (Kale and Heinz, 2012). In summary, sensorineural hearing loss is associated with stronger neural coding of ENV modulations, but not with an increase in the range of modulation frequencies encoded for these narrowband stimuli.
The physiological results described above are consistent with behavioral studies in humans showing that sensorineural hearing loss is associated with stronger perception of ENV cues under some stimulus conditions, but without a clear increase in the range of modulation frequencies encoded. In one study for example, listeners with unilateral cochlear hearing loss required less modulation depth in their impaired ear than their normal ear to achieve a sensation of equal modulation depth in both ears, consistent with enhanced ENV representation in the damaged ear (Moore et al., 1996). Other studies of modulation detection thresholds (i.e., minimum detectable modulation depth) have shown either better detection thresholds in listeners with sensorineural hearing loss under some stimulus conditions (Bacon and Gleitman, 1992; Fullgrabe et al., 2003) or similar performance between subject groups (e.g., Moore et al. 1992). Changes in ENV coding with sensorineural hearing loss presumably arise from multiple factors including an increase in the slope of the input-output function of the basilar membrane (i.e., reduced compressive growth; Glasberg and Moore, 1992; Moore et al., 1996) and inner hair cell dysfunction (i.e., physical damage to stereocilia; Heinz and Young, 2004; Kale and Heinz, 2010).
An important limitation of the neurophysiological studies described above is that they evaluated neural coding of temporal information under quiet laboratory conditions only, while perceptual difficulties in people with sensorineural hearing loss are commonly exacerbated under degraded listening conditions. To help fill in this gap, we studied the effects of noise-induced hearing loss in chinchillas on phase locking to the TFS of pure tones in background noise (Henry and Heinz, 2012). As found in previous work (e.g., Abbas, 1981), auditory-nerve fibers in normal-hearing animals exhibited robust phase locking to TFS that decreased in vector strength with the addition of masking noise. This result, which indicates a decrease in the proportion of spikes synchronized to the tone with increasing noise level (and concomitant increase in spikes driven by the noise), is primarily attributable to the decrease in the signal-to-noise ratio with increasing masker level (Abbas, 1981). Phase locking in animals with noise-induced sensorineural hearing loss to equal-sensation-level tones was only slightly diminished compared to controls under quiet conditions, consistent with the studies described above, but substantially reduced in background noise (Fig. 2). This result shows that neural coding of signals in the impaired peripheral auditory system is less resilient to background noise than normal, and also highlights the importance of further advancement in technologies that boost signal-to-noise ratios for people with sensorineural hearing loss (e.g., FM devices and noise-reduction algorithms).
Across the noise-exposed population, fibers with the broadest tuning curves experienced the greatest reductions in phase locking in background noise (Henry and Heinz, 2012). Broader tuning allows more background noise into the receptive field of the neuron, thereby decreasing phase locking to the TFS of the relevant signal to a greater degree. Although normal-hearing auditory-filter bandwidths generally increase with overall sound level, the decrease in vector strength with increasing noise level in normal-hearing fibers is predominantly related to decreased signal-to-noise ratio, with very little dependence on overall level when signal-to-noise ratio is held constant (Abbas, 1981). Hence, this degraded coding of TFS in background noise with cochlear hearing loss appears to be a consequence of broader cochlear tuning, rather than simply higher overall sound levels. Theoretically, broader tuning should also decrease phase locking to ENV information in background noise in impaired fibers relative to controls for the same reason (i.e., more noise energy enters the receptive field of the neuron); however, ENV coding in background noise has not yet been studied. Finally, it should be noted that weaker cochlear suppression following sensorineural hearing loss (e.g., Miller et al. 1997) could be thought to also contribute to differences in the effect of masking level on coding of TFS. However, the contributions of weaker suppression are likely to be in the opposite direction of the effect of broader cochlear tuning, and therefore do not explain our results (consistent with the lack of a significant effect of overall level on phase locking to tones in noise (Abbas, 1981)). Weaker suppression leads to less of a reduction in the basilar-membrane response to tones with the addition of noise, and therefore, less of a decrease in signal-to-noise ratio with masking.
In addition to decreasing the resiliency of neural coding in background noise, sensorineural hearing loss also alters the temporal dynamics of auditory-nerve fiber responses to pure tones. Auditory-nerve fiber responses in normal-hearing animals show a rapid increase in spike rate shortly after the onset of the tone followed by gradual adaptation to a lower spike rate (Westerman and Smith, 1984). Following the offset of the tone, the spike rate drops precipitously before gradually recovering to the spontaneous firing rate observed in the absence of stimulation. Noise-induced sensorineural hearing loss in chinchillas increased the amplitude of the onset response and decreased its latency when measured at equal sensation levels (Fig. 3; Scheidt et al., 2010). Furthermore, noise-induced cochlear damage increased the rate of adaptation following the onset response and decreased the recovery rate following stimulus offset. This increase in the amplitude of onset responses and the slower recovery from adaptation with sensorineural hearing loss may contribute to the enhanced ENV coding with cochlear damage observed in studies using narrowband modulated stimuli described above (Kale and Heinz, 2010; 2012). Changes in the latency of the onset response appear to be a consequence of broader cochlear tuning. A broadly tuned system has a more transient impulse response with less filter buildup time than a more narrowly tuned system (Goldstein et al., 1971; Ruggero, 1994).
Fig. 3.
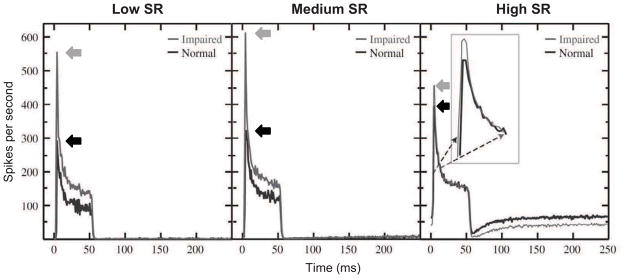
Post-stimulus time histograms of auditory-nerve fiber responses to 50-ms tones presented at characteristic frequency (CF). Fibers from chinchillas with noise-induced hearing loss show greater onset response amplitude (left-pointing arrows), reduced onset latency, faster adaptation during stimulation, and slower recovery following stimulus offset. Black and gray lines show mean responses of normal-hearing fibers and noise-exposed fibers, respectively, for populations of neurons with CFs between 1 and 4 kHz. Separate panels present data from fibers falling into different categories of spontaneous firing rate (SR): low (SR<1), medium (1<SR<18), and high (SR >18). Modified and reprinted from Scheidt et al. (2010), with permission from Elsevier.
4. Effects of sensorineural hearing loss on temporal coding of broadband stimuli
The studies discussed above all examined neural coding of narrowband stimuli that evoke activity from spatially restricted regions of the normal-hearing cochlea. Broadband human speech, by contrast, can stimulate activity from much of the cochlea at any given moment. In an attempt to gain further insight into the physiological basis of degraded speech perception in people with sensorineural hearing loss, a number of recent animal studies have examined the effects of cochlear damage on temporal coding of broadband signals along the tonotopic axis of the cochlea.
Recently in our lab, we used a spectro-temporal manipulation procedure to study the effects of noise-induced hearing loss on coding of broadband Gaussian noise and speech stimuli across auditory-nerve fibers of varying CF (Heinz et al., 2010). The spectro-temporal manipulation procedure relies on scaling invariance of cochlear mechanics to predict the TFS response of a set of “virtual” fibers with effective CFs spanning a ~1-octave range from the recorded responses of a single AN fiber to a stimulus presented at multiple sampling frequencies (Heinz 2007; Larsen et al., 2008; Wang and Delgutte, 2012). This method helps overcome several limitations of traditional across-CF studies of temporal coding, including sparse sampling of CFs, variability in the response properties across fibers with the same CF, and variability in estimates of the CF for any given fiber (Chintanpalli and Heinz, 2007). We used cross-correlation analyses to compare TFS responses across the set of virtual fibers with different effective CFs. Both the control and noise-exposed populations showed a decrease in the correlation between the TFS responses of two different AN fibers with increasing CF separation, as expected for a simple bank of filters. In the noise-exposed group, however, the correlation decreased much more slowly with increasing CF separation, presumably due to greater overlap in the receptive fields for TFS in broadly tuned fibers with different CFs. These results suggest that sensorineural hearing loss reduces the overall number of independent information channels in the peripheral auditory system. Other effects of noise-induced hearing loss on across-CF temporal coding, also probably due to broader tuning, were that neural responses had shorter latencies at any given CF, and estimated traveling-wave delays between TFS responses of fibers with different CFs were smaller. Taken together, the results indicate a substantial deviation in neural coding of TFS across fibers of varying CF, which based on spatiotemporal coding theories could impact a number of aspects of perception including discrimination of speech, pitch, intensity, and signals in noise (Shamma, 1985; Heinz et al., 2001; Carney et al., 2002; Larsen et al., 2008).
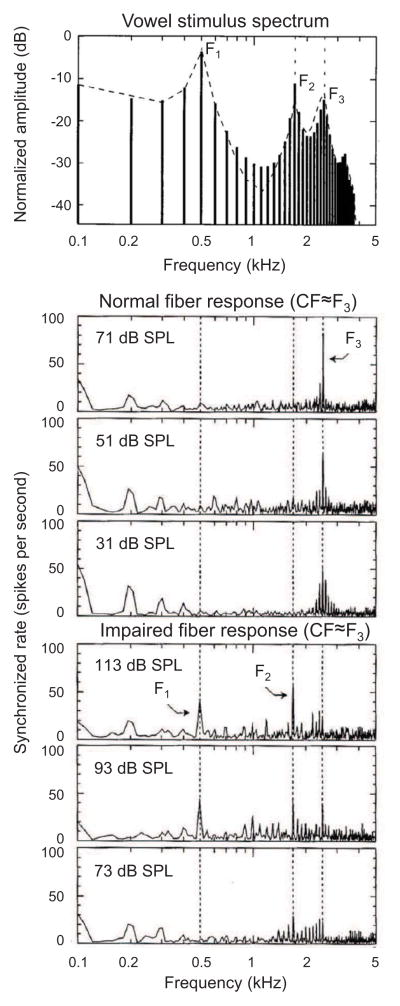
Two studies have examined changes with sensorineural hearing loss in the neural coding of vowels in the peripheral auditory system of mammals. One study examined coding of the vowel /ε/ across a large population of auditory-nerve fibers in cats with noise-induced hearing loss (Miller et al., 1997). Whereas normal-hearing fibers with characteristic frequencies near a formant phase lock preferentially to that formant (i.e., formants are encoded through “synchrony capture”; Young and Sachs, 1979; Sachs and Young, 1980), fibers in noise-exposed animals exhibited a marked reduction in synchrony capture of formants and a concomitant increase in phase locking to lower-frequency spectral components of the stimulus (Miller et al., 1997; Fig. 4). Similarly, another study in guinea pigs with kanamycin-induced hearing loss showed that when the two vowels /a/ and /i/ were presented simultaneously, synchrony capture of formants 1 and 2 (/a/: 730 and 1090 Hz; /i/: 270 and 2290 Hz) decreased in favor of stronger phase locking to the lower fundamental frequencies of the vowels (100 and 125 Hz; Palmer and Moorjani, 1993). These studies suggest that sensorineural hearing loss increases the neural representation of low-frequency TFS information while decreasing the representation of higher-frequency TFS information.
Fig. 4.
Loss of synchrony capture of vowel formants with noise-induced hearing loss. Representative neural responses in cats to the vowel /ε/ (top) from a control auditory-nerve fiber (middle plots) and noise-exposed fiber (lower plots). The characteristic frequencies (CFs) of the fibers were near F3. While the control fiber shows strong synchrony capture of TFS information near F3 (i.e., near CF), the noise-exposed fiber shows synchrony to a broader range of lower frequency TFS information. Modified and reprinted from Miller et al. 1997, with permission from the Acoustical Society of America.
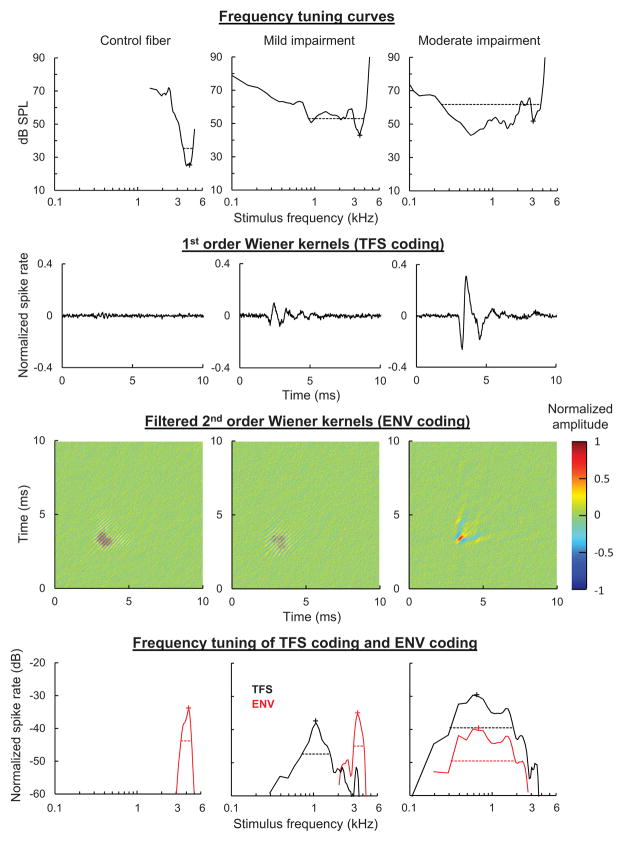
A recent study conducted in our lab provides further evidence that sensorineural hearing loss increases the representation of low-frequency TFS information while decreasing the representation of higher-frequency temporal information. We used Wiener-kernel analysis of neural responses to broadband Gaussian noise to examine the coding of TFS information and ENV information in auditory-nerve fibers of varying CF (Henry et al., in prep). The first-order Wiener kernel is the reverse-correlation (revcor) function, or average waveform of the stimulus occurring before a spike, which describes temporal coding of TFS information (de Boer and de Jongh, 1978). The second-order Wiener kernel is the second-order reverse-correlation function, the details of which are described elsewhere (Lewis et al. 2002; Recio-Spinoso et al. 2005). Briefly, the second-order kernel is a time domain representation of the spectro-temporal receptive field (Lewis and van Dijk, 2004), or average spectrogram of the stimulus occurring before a spike. Following removal of energy in the first and third quadrants of its 2-dimensional spectrum and decomposition into eigenvectors, the second-order Wiener kernel describes temporal coding of primarily ENV information (Recio-Spinoso et al. 2005).
Previous Wiener-kernel studies conducted in normal-hearing mammals show that auditory-nerve fibers tuned to CFs below 4–5 kHz encode both TFS information and ENV information present in a narrow frequency band of the stimulus near CF (Lewis et al., 2002; Recio-Spinoso et al. 2005). Neurons with higher CFs, in contrast, phase lock primarily to ENV information near CF due to the roll-off in phase locking to TFS that occurs with increasing frequency. In chinchillas with noise-induced sensorineural hearing loss, we found striking changes in the coding of temporal information in fibers with CFs above 2.5 kHz (Henry et al., in prep). While control fibers phase locked primarily to ENV information near CF as in previous studies, noise-exposed fibers routinely showed additional robust phase locking to low-frequency TFS information (0.5–1.5 kHz; Fig. 5). In the most severely impaired fibers, coding of ENV information near CF was lost as phase locking shifted entirely to the low-frequency band (0.5–1.5 kHz) of the stimulus. Fibers tuned to lower CFs exhibited less substantial downward shifts in the frequency tuning of TFS coding and ENV coding with cochlear damage.
Fig. 5.
Effect of cochlear hearing loss on the frequency tuning of temporal responses to TFS and ENV information. Representative frequency tuning curves (top) of chinchilla auditory-nerve fibers with varying degrees of noise-induced hearing loss. Characteristic frequencies (CFs) ranged from 3 to 4 kHz. Wiener kernels (middle panels) show the temporal response to TFS information (1st order kernels) and ENV information (filtered 2nd order kernels). Frequency-domain representations of the kernels (bottom panels) show that control fibers in this CF range encode primarily ENV information (red) based on stimulus energy near CF. Mild hearing loss introduced additional phase locking to low-frequency TFS information (black curve), while with moderate impairment both the TFS and ENV information encoded were centered at lower frequencies well below CF.
The results of the Wiener-kernel study can explain the abnormally strong masking of speech in background noise in people with sensorineural hearing loss (e.g., Duquesnoy, 1983). While many listeners with cochlear damage can discriminate vowels in background noise with a high degree of accuracy, they struggle to discriminate consonants due in part to a decreased ability to use high-frequency cues (Owens et al., 1968; Dubno et al., 1982; Woods et al., 2010). Our results suggest that when high-frequency cues are presented with low-frequency noise, normal-hearing fibers in the high-frequency part of the cochlea will encode only the temporal (ENV) structure of the high-frequency cues while mildly impaired fibers encode the temporal structure of both the high-frequency cue and low-frequency noise. The additional response to the low-frequency noise degrades the representation of the relevant cue and hence, should degrade perception. With moderate to severe hearing loss, the outlook for perception is particularly bleak because high-frequency cues are not encoded at all. Instead, high-frequency fibers phase lock exclusively to temporal information in the low-frequency band of the signal and any background noise that may be present. The representation of high-frequency cues in high-CF neurons could potentially be improved through high-pass filtering by an amplification device such as a hearing aid (Miller et al., 1999). It is less clear, however, how the response of these neurons to lower-frequency speech information (e.g., vowels) could be suppressed without diminishing the representation of this information in impaired low-CF-fibers (e.g., in cases such as flat hearing losses).
These physiological results may also help explain why listeners with hearing loss can perceive speech based on ENV cues similarly to normal-hearing listeners, but are less able to use TFS information. We observed changes in coding of TFS information more frequently and in less severe instances of hearing loss than coding of ENV information. Whereas ENV information was encoded in the normal tonotopic fashion following hearing loss in most cases, TFS information was not. Instead, low-frequency TFS information was encoded in all cochlear fibers regardless of their CF and higher-frequency TFS information (2.5–5 kHz) was never encoded. This abnormal and diminished representation of TFS information appears sufficient to explain the inability to perceive speech containing only TFS information and the inability to use TFS information to improve speech reception in noise in people with cochlear hearing loss.
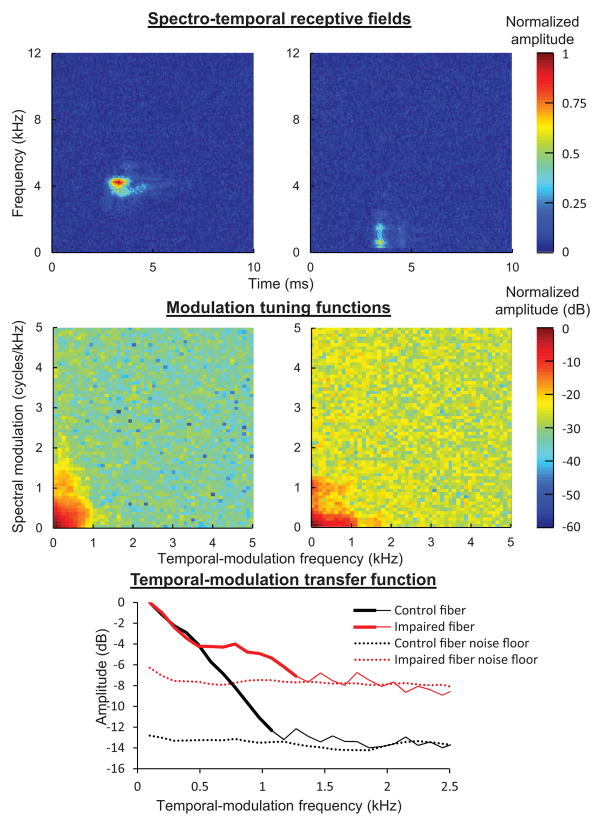
In a recent extension of the Wiener-kernel study, we examined the effects of noise-induced hearing loss on neural sensitivity to temporal modulations of varying modulation frequency (i.e., temporal-modulation transfer functions; Henry, K.S., Kale, S., and Heinz, M.G., unpublished data). Briefly, we computed the spectro-temporal receptive field from the filtered second-order Wiener kernel using a 1-dimensional Fourier transform (Lewis and van Dijk, 2004; Fig. 6). The modulation tuning function was calculated from the spectro-temporal receptive field with a 2-dimensional Fourier transform and finally, the modulation tuning function was collapsed across spectral-modulation frequencies to yield a temporal-modulation transfer function (as in Woolley et al., 2005). Note that in contrast to previous studies of temporal-modulation tuning in hearing-impaired animals based on responses to sinusoidally amplitude modulated tones (Kale and Heinz, 2012), this Wiener-kernel based approach quantifies temporal-modulation tuning from the response to random fluctuations in a continuous Gaussian noise stimulus. Similar to the previous work based on responses to sinusoidal amplitude modulation, all fibers were found to exhibit a roll-off in coding of temporal modulation with increasing modulation frequency, the corner frequency of which was greater in fibers with higher CFs. In contrast to these previous findings, however, temporal-modulation transfer functions from the noise-exposed population of fibers were broader than from control fibers of the same CF (Fig. 6). This result shows that cochlear hearing loss can extend the range of temporal-modulation frequencies that are encoded in the peripheral auditory system in response to broadband stimuli. The fate and perceptual consequences of these encoded modulations as they ascend the auditory pathway are unclear to date.
Fig. 6.
Derivation of temporal-modulation transfer functions from second-order Wiener kernels. Spectro-temporal receptive fields (top), modulation tuning functions (middle), and temporal-modulation transfer functions (bottom) for the control fiber and moderately impaired fiber shown in Fig. 5 (CFs between 3 to 4 kHz). Noise floors (dotted lines in bottom panel) were calculated from the temporal regions before (0 to 2 ms) and after (last 3 ms, e.g., 7 to 10 ms) the response in the spectro-temporal receptive fields. Noise-induced hearing loss enhanced the coding of faster temporal modulations.
5. Conclusions
In summary, existing research shows that sensorineural hearing loss has relatively subtle effects on temporal coding of narrowband sounds in quiet. In background noise, however, cochlear damage substantially diminishes phase locking to the TFS of pure tones. Sensorineural hearing loss is also associated with significant changes in temporal coding of broadband sounds compared to control fibers, including (1) overrepresentation of low-frequency TFS information, (2) underrepresentation of high-frequency temporal information, (3) overrepresentation of fast temporal ENV modulations, and (4) stronger correlation and less difference in timing of the TFS response between auditory-nerve fibers with different CFs. Stronger coding of low-frequency TFS information with cochlear hearing loss at the cost of high-frequency temporal information and the reduction in neural phase locking to TFS in background noise both help to explain why listeners with hearing loss struggle to perceive speech in background noise. The greater degradation of TFS coding than ENV coding with hearing loss, in turn, might contribute to the reduced ability of people with hearing loss to use TFS information to discriminate speech. While these neurophysiological results help to explain several common perceptual difficulties in listeners with cochlear hearing loss, they also leave unanswered questions for future research. For example, how does sensorineural hearing loss affect coding of ENV information in background noise and coding of broadband sounds in background noise, and what are the consequences of changes in temporal coding in the periphery to the representation of temporal information in the central auditory system? Also, how does the loss of afferent fibers due to synaptic degeneration (Kujawa and Liberman, 2009) affect temporal coding in central neurons? How does cochlear hearing loss affect coding of reverberant signals? How is temporal coding affected by other common forms of sensorineural hearing loss, including metabolic hearing loss, inner-hair-cell dysfunction, and cochlear hearing loss induced by chronic exposure to noise? Finally, to what extent can signal processing strategies incorporating dynamic filtering and noise-reduction algorithms counteract the changes in temporal coding with hearing loss documented so far. Future research in these areas will be important to help inform efforts aimed at restoring speech perception in people with sensorineural hearing loss.
Highlights.
Phase locking to the fine structure of narrowband stimuli is not degraded in quiet
Degraded phase locking to fine structure emerges in background noise
Envelope coding is enhanced following cochlear hearing loss
Loss of tonotopicity has a significant effect on the temporal coding of broadband stimuli
Across-fiber temporal coding is degraded following sensorineural hearing loss
Acknowledgments
Research in our lab and writing of this manuscript was supported by grants R01-DC009838 to MGH and F32-DC012236 to KSH from the National Institute on Deafness and other Communication Disorders.
Abbreviations
- AN
auditory nerve
- CF
characteristic frequency
- ENV
envelope
- FM
frequency modulation
- SPL
sound pressure level
- SR
spontaneous rate
- TFS
temporal fine structure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas PJ. Auditory-nerve fiber responses to tones in a noise masker. Hear Res. 1981;5:69–80. doi: 10.1016/0378-5955(81)90027-7. [DOI] [PubMed] [Google Scholar]
- Ardoint M, Sheft S, Fleuriot P, Garnier S, Lorenzi C. Perception of temporal fine-structure cues in speech with minimal envelope cues for listeners with mild-to-moderate hearing loss. Int J Audiol. 2010;49:823–831. doi: 10.3109/14992027.2010.492402. [DOI] [PubMed] [Google Scholar]
- Bacon SP, Gleitman RM. Modulation detection in subjects with relatively flat hearing losses. J Speech Hear Res. 1992;35:642–653. doi: 10.1044/jshr.3503.642. [DOI] [PubMed] [Google Scholar]
- Carney LH, Heinz MG, Evilsizer ME, Gilkey RH, Colburn HS. Auditory phase opponency: a temporal model for masked detection at low frequencies. Acust -Acta Acust. 2002;88:334–347. [Google Scholar]
- Chintanpalli A, Heinz MG. The effect of auditory-nerve response variability on estimates of tuning curves. J Acoust Soc Am. 2007;122:EL203–EL209. doi: 10.1121/1.2794880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer E, de Jongh HR. On cochlear encoding: potentialities and limitations of the reverse-correlation technique. J Acoust Soc Am. 1978;63:115–135. doi: 10.1121/1.381704. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Dirks DD, Langhofer LR. Evaluation of hearing-impaired listeners using a nonsense-syllable test II. Syllable recognition and consonant confusion patterns. J Speech Hear Res. 1982;25:141–148. doi: 10.1044/jshr.2501.141. [DOI] [PubMed] [Google Scholar]
- Duquesnoy AJ. Effect of a single interfering noise or speech source upon the binaural sentence intelligibility of aged persons. J Acoust Soc Am. 1983;74:739–743. doi: 10.1121/1.389859. [DOI] [PubMed] [Google Scholar]
- Fullgrabe C, Meyer B, Lorenzi C. Effect of cochlear damage on the detection of complex temporal envelopes. Hear Res. 2003;178:35–43. doi: 10.1016/s0378-5955(03)00027-3. [DOI] [PubMed] [Google Scholar]
- Geisler CD. From Sound to Synapse: Physiology of the Mammalian Ear. Oxford University Press; New York: 1998. [Google Scholar]
- Glasberg BR, Moore BCJ. Effects of envelope fluctuations on gap detection. Hear Res. 1992;64:81–92. doi: 10.1016/0378-5955(92)90170-r. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Baer T, Kiang NYS. A theoretical treatment of latency, group delay, and tuning characteristics for auditory-nerve responses to clicks and tones. In: Sachs MB, editor. Physiology of the Auditory System. National Education Consultants; Baltimore, MD: 1971. pp. 133–141. [Google Scholar]
- Harrison RV, Evans EF. Some aspects of temporal coding by single cochlear fibres from regions of cochlear hair cell degeneration in the guinea pig. Arch Otorhinolaryngol. 1979;224:71–78. doi: 10.1007/BF00455226. [DOI] [PubMed] [Google Scholar]
- Heinz MG. Spatiotemporal encoding of vowels in noise studied with the responses of individual auditory nerve fibers. In: Kollmeier B, Klump G, Hohmann V, Langemann U, Mauermann M, Uppenkamp S, Verhey J, editors. Hearing – From Sensory Processing to Perception. Springer-Verlag; Berlin: 2007. pp. 107–115. [Google Scholar]
- Heinz MG, Young ED. Response growth with sound level in auditory-nerve fibers after noise-induced hearing loss. J Neurophysiol. 2004;91:784–795. doi: 10.1152/jn.00776.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz MG, Colburn HS, Carney LH. Rate and timing cues associated with the cochlear amplifier: level discrimination based on monaural cross-frequency coincidence detection. J Acoust Soc Am. 2001;110:2065–2084. doi: 10.1121/1.1404977. [DOI] [PubMed] [Google Scholar]
- Heinz MG, Swaminathan J, Boley JD, Kale S. Across-fiber coding of temporal fine-structure: Effects of noise-induced hearing loss on auditory-nerve responses. In: Lopez-Poveda EA, Palmer AR, Meddis R, editors. The Neurophysiological Bases of Auditory Perception. Springer; New York: 2010. pp. 621–630. [Google Scholar]
- Henry KS, Heinz MG. Diminished temporal coding with sensorineural hearing loss emerges in background noise. Nat Neurosci. 2012;15:1362–1364. doi: 10.1038/nn.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KS, Kale S, Heinz MG. Sensorineural hearing loss disrupts neural coding of temporal information along the tonotopic axis of the peripheral auditory system in prep. [Google Scholar]
- Hopkins K, Moore BCJ, Stone MA. Effects of moderate cochlear hearing loss on the ability to benefit from temporal fine structure information in speech. J Acoust Soc Am. 2008;123:1140–1153. doi: 10.1121/1.2824018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DH. The relationship between spike rate and synchrony in responses of auditory-nerve fibers to single tones. J Acoust Soc Am. 1980;68:1115–1122. doi: 10.1121/1.384982. [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin TC. Responses to amplitude-modulated tones in the auditory nerve of the cat. J Acoust Soc Am. 1992;91:215–232. doi: 10.1121/1.402757. [DOI] [PubMed] [Google Scholar]
- Kale S, Heinz MG. Envelope coding in auditory nerve fibers following noise-induced hearing loss. J Assoc Res Otolaryngol. 2010;11:657–673. doi: 10.1007/s10162-010-0223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale S, Heinz MG. Temporal modulation transfer functions measured from auditory-nerve responses following sensorineural hearing loss. Hear Res. 2012;286:64–75. doi: 10.1016/j.heares.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26:2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen E, Cedolin L, Delgutte B. Pitch representation in the auditory nerve: two concurrent complex tones. J Neurophysiol. 2008;100:1301–1319. doi: 10.1152/jn.01361.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis ER, van Dijk P. New variations on the derivation of spectro-temporal receptive fields for primary auditory afferent axons. Hear Res. 2004;189:120–136. doi: 10.1016/s0378-5955(03)00406-4. [DOI] [PubMed] [Google Scholar]
- Lewis ER, Henry KR, Yamada WM. Tuning and timing in the gerbil ear: Wiener-kernel analysis. Hear Res. 2002;174:206–221. doi: 10.1016/s0378-5955(02)00695-0. [DOI] [PubMed] [Google Scholar]
- Lorenzi C, Gilbert G, Carn H, Garnier S, Moore BCJ. Speech perception problems of the hearing impaired reflect inability to use temporal fine structure. P Natl Acad Sci USA. 2006;103:18866–18869. doi: 10.1073/pnas.0607364103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi C, Debruille L, Garnier S, Fleuriot P, Moore BCJ. Abnormal processing of temporal fine structure in speech for frequencies where absolute thresholds are normal. J Acoust Soc Am. 2009;125:27–30. doi: 10.1121/1.2939125. [DOI] [PubMed] [Google Scholar]
- Miller RL, Schilling JR, Franck KR, Young ED. Effects of acoustic trauma on the representation of the vowel /ε/ in cat auditory nerve fibers. J Acoust Soc Am. 1997;101:3602–3616. doi: 10.1121/1.418321. [DOI] [PubMed] [Google Scholar]
- Miller RL, Calhoun BM, Young ED. Contrast enhancement improves the representation of /ε/-like vowels in the hearing-impaired auditory nerve. J Acoust Soc Am. 1999;106:2693–2708. doi: 10.1121/1.428135. [DOI] [PubMed] [Google Scholar]
- Moore BCJ. The role of temporal fine structure processing in pitch perception, masking, and speech perception for normal-hearing and hearing-impaired people. J Assoc Res Otolaryngol. 2008;9:399–406. doi: 10.1007/s10162-008-0143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BCJ, Shailer MJ, Schooneveldt GP. Temporal modulation transfer functions for band-limited noise in subjects with cochlear hearing loss. Br J Audiol. 1992;26:229–237. doi: 10.3109/03005369209076641. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Wojtczak M, Vickers DA. Effect of loudness recruitment on the perception of amplitude modulation. J Acoust Soc Am. 1996;100:481–489. [Google Scholar]
- Owens E, Talbott CB, Schubert ED. Vowel discrimination in hearing-impaired listeners. J Speech Hear Res. 1968;11:648–655. doi: 10.1044/jshr.1103.648. [DOI] [PubMed] [Google Scholar]
- Oxenham AJ, Simonson AM. Masking release for low-and high-pass-filtered speech in the presence of noise and single-talker interference. J Acoust Soc Am. 2009;125:457–468. doi: 10.1121/1.3021299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AR, Moorjani PA. Responses to speech signals in the normal and pathological peripheral auditory system. Prog Brain Res. 1993;97:107–115. doi: 10.1016/s0079-6123(08)62268-2. [DOI] [PubMed] [Google Scholar]
- Recio-Spinoso A, Temchin AN, van Dijk P, Fan Y, Ruggero MA. Wiener-kernel analysis of responses to noise of chinchilla auditory-nerve fibers. J Neurophsyiol. 2005;93:3615–3634. doi: 10.1152/jn.00882.2004. [DOI] [PubMed] [Google Scholar]
- Ruggero MA. Cochlear delays and traveling waves: Comments on ‘Experimental look at cochlear mechanics’. Audiology. 1994;33:131–142. doi: 10.3109/00206099409071874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs MB, Young ED. Effects of nonlinearities on speech encoding in the auditory nerve. J Acoust Soc Am. 1980;68:858–875. doi: 10.1121/1.384825. [DOI] [PubMed] [Google Scholar]
- Schiedt RE, Kale S, Heinz MG. Noise-induced hearing loss alters the temporal dynamics of auditory-nerve responses. Hear Res. 2010;269:23–33. doi: 10.1016/j.heares.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA, Lang HN, Okamura H, Schulte BA. Effects of furosemide applied chronically to the round window: A model of metabolic presbycusis. J Neurosci. 2002;22:9643–9650. doi: 10.1523/JNEUROSCI.22-21-09643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Oto Rhinol Laryn. 1993;102:1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- Shamma SA. Speech processing in the auditory system 1: the representation of speech sounds in the responses of the auditory nerve. J Acoust Soc Am. 1985;78:1612–1621. doi: 10.1121/1.392799. [DOI] [PubMed] [Google Scholar]
- Swaminathan J, Heinz MG. Psychophysiological analyses demonstrate the importance of neural envelope coding for speech perception in noise. J Neurosci. 2012;32:1747–1756. doi: 10.1523/JNEUROSCI.4493-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GI, Delgutte B. Sensitivity of cochlear nucleus neurons to spatio-temporal changes in auditory nerve activity. J Neurophysiol. 2012 doi: 10.1152/jn.00160.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman LA, Smith RL. Rapid and short-term adaptation in auditory nerve responses. Hear Res. 1984;15:249–260. doi: 10.1016/0378-5955(84)90032-7. [DOI] [PubMed] [Google Scholar]
- Woods DL, Yund EW, Herron TJ. Measuring consonant identification in nonsense syllables, words, and sentences. J Rehabil Res Dev. 2010;47:243–260. doi: 10.1682/jrrd.2009.04.0040. [DOI] [PubMed] [Google Scholar]
- Woolf NK, Ryan AF, Bone RC. Neural phase-locking properties in the absence of cochlear outer hair cells. Hear Res. 1981;4:335–346. doi: 10.1016/0378-5955(81)90017-4. [DOI] [PubMed] [Google Scholar]
- Woolley SMN, Fremouw TE, Hsu A, Theunissen FE. Tuning for spectro-temporal modulations as a mechanism for auditory discrimination of natural sounds. Nat Neurosci. 2005;8:1371–1379. doi: 10.1038/nn1536. [DOI] [PubMed] [Google Scholar]
- Young ED. Neural Coding of Sound with Cochlear Damage. In: Henderson D, LePrell CG, editors. Noise-Induced Hearing Loss: Scientific Advances. Springer; New York: 2012. pp. 87–135. [Google Scholar]
- Young ED, Sachs MB. Representation of steady-state vowels in the temporal aspects of the discharge patterns of populations of auditory-nerve fibers. J Acoust Soc Am. 1979;66:1381–1403. doi: 10.1121/1.383532. [DOI] [PubMed] [Google Scholar]