Abstract
Background
More than 1 billion people are currently infected with soil-transmitted helminths and schistosomes. The global strategy to control helminthiases is the regular administration of anthelmintic drugs to at-risk populations. However, rapid re-infection occurs in areas where hygiene, access to clean water, and sanitation are inadequate.
Methodology
In July 2011, inhabitants from two villages and seven hamlets of the Taabo health demographic surveillance system in south-central Côte d’Ivoire provided stool and urine samples. Kato-Katz and ether-concentration methods were used for the diagnosis of Schistosoma mansoni, soil-transmitted helminths (Ascaris lumbricoides, Trichuris trichiura, and hookworm), and intestinal protozoa. Urine samples were subjected to a filtration method for the diagnosis of Schistosoma haematobium. A questionnaire was administered to households to obtain information on knowledge, attitude, practice, and beliefs in relation to hygiene, sanitation, and defecation behavior. Logistic regression models were employed to assess for associations between questionnaire data and parasitic infections.
Principal Findings
A total of 1,894 participants had complete data records. Parasitological examinations revealed prevalences of hookworm, S. haematobium, T. trichiura, S. mansoni, and A. lumbricoides of 33.5%, 7.0%, 1.6%, 1.3% and 0.8%, respectively. Giardia intestinalis and Entamoeba histolytica/E. dispar were detected in 15.0% and 14.4% of the participants, respectively. Only one out of five households reported the presence of a latrine, and hence, open defecation was common. Logistic regression analysis revealed that age, sex, socioeconomic status, hygiene, and defecation behavior are determinants for helminths and intestinal protozoa infections.
Conclusions/Significance
We found that inadequate sanitation and hygiene behavior are associated with soil-transmitted helminths and intestinal protozoa infections in the Taabo area of south-central Côte d’Ivoire. Our data will serve as a benchmark to monitor the effect of community-led total sanitation and hygiene education to reduce the transmission of helminthiases and intestinal protozoa infections.
Introduction
Hundreds of millions of people are still affected by neglected tropical diseases (NTDs), particularly in the developing world due to parasitic worm infections (helminthiases) [1], [2]. Taken together, soil-transmitted helminthiasis and schistosomiasis are responsible for 8.5 million disability-adjusted life years (DALYs) with more than 1 billion people infected [3]–[5]. Diseases caused by intestinal protozoa infections, such as giardiasis and amebiasis also cause considerable morbidity and mortality [6]–[9].
Current helminthiases control programs focus on preventive chemotherapy, that is the regular administration of anthelmintic drugs to at-risk populations, particularly school-aged children [10], [11]. However, preventive chemotherapy does not prevent re-infection, which might occur rapidly [12], [13]. Additionally, there is considerable concern about the development of drug resistance in the era of preventive chemotherapy, as experience has shown in livestock [14]. Although, the importance of integrated control approaches for the interruption of transmission of helminthiases is well established since almost a century [15], [16], current control efforts emphasize drug interventions, and do not give sufficient attention to hygiene behavior, clean water, and adequate sanitation [17]–[19]. Indeed, data from 2010 suggest that 2.6 billion people lacked access to some kind of improved sanitation [20]. To contribute to the achievement of several of the millennium development goals (MDGs), ongoing efforts to control NTDs have to be maintained and further intensified, including complementary approaches for prevention and control [21].
In July 2011, a project emphasizing an integrated control approach targeting intestinal parasites was launched in the Taabo health demographic surveillance system (HDSS) in south-central Côte d’Ivoire. The main objective is to assess the impact of community-led total sanitation (CLTS) and health education on the incidence of helminths and intestinal protozoa infections, implemented alongside preventive chemotherapy. CLTS not only focuses on the construction of latrines, but also on local knowledge, attitude, practice, and beliefs (KAPB) related to hygiene and defecation behavior, which play a key role for sustainability [22]. Through a participatory grassroots approach, CLTS aims to achieve and sustain an open defecation-free status of communities [23]. To our knowledge, the effect of CLTS on re-infection patterns with helminths and intestinal protozoa infections has yet to be determined. Here, we present helminth and intestinal protozoa infection profiles in a selection of villages and hamlets of the Taabo HDSS, including associations between infection and people’s KAPB related to hygiene and defecation behavior during the baseline cross-sectional survey. Our data will serve as a benchmark for monitoring the longer term impact of CLTS on people’s health and wellbeing.
Methods
Ethics Statement
This study received clearance from the ethics committees of Basel (Ethikkommission beider Basel; reference no. 177/11) and Côte d’Ivoire (Comité National de l’Ethique et de la Recherche; reference no. 13324 MSLS/CNER-P). Study participants were informed about the aims, procedures, and potential risks and benefits. Participants and parents/guardians of minors provided written informed consent (signature of a witness for illiterate participants). Participation was voluntary and people could withdraw from the study at any time without further obligation. To guarantee anonymity, each study participant was given a unique identification number.
At the end of the parasitological survey, anthelmintic treatment was administered to all people in the study villages and hamlets regardless of infection status and participation (single 400 mg oral dose of albendazole for individuals aged ≥2 years) [10], [11]. Additionally, participants aged ≥4 years who were diagnosed for Schistosoma spp. were given a single oral dose of praziquantel (40 mg/kg, using a “dose pole”) [10], [11]. Individuals who required other specific medical interventions were referred to the next health care facility. No treatments were given to participants identified with intestinal protozoa infections, as the results from the sodium acetate-acetic acid-formalin (SAF)-fixed stool samples subjected to an ether-concentration method were only available several weeks after completion of the field work and intestinal protozoa infection are often self-limiting.
Study Area and Population
The study was conducted in the Taabo HDSS, located in a primarily rural part of south-central Côte d’Ivoire [24]–[26]. General living standards are low. For instance, 71% of households lacked a toilet facility and two-third of the households used unprotected surface water (e.g., rivers and lakes) as drinking water according to the latest available data from the Taabo HDSS. Soil-transmitted helminthiasis, schistosomiasis, onchocerciasis, and lymphatic filariasis control activities have been implemented within the Taabo HDSS (preventive chemotherapy, using albendazole, praziquantel, and ivermectin) since 2008 and are on-going with yearly drug interventions. At the time of the execution of the current study, preventive chemotherapy was the main strategy implemented in the study area.
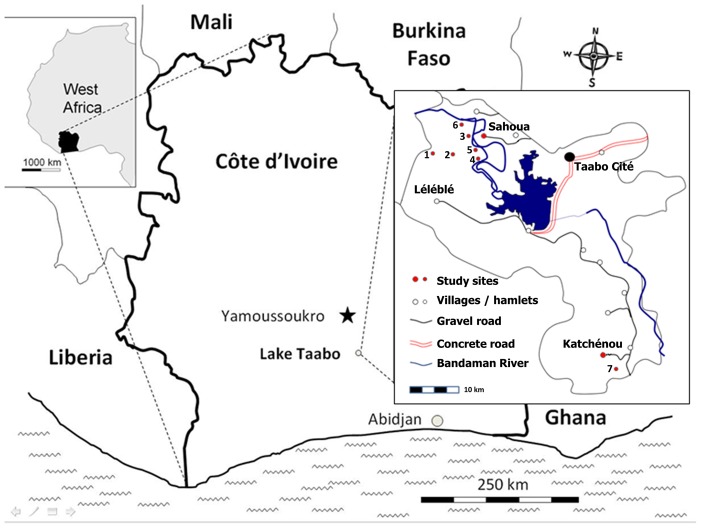
The study presented here was implemented in two villages (i.e., Katchénou and Sahoua), and seven hamlets of different villages, Yobouékro (belonging to Sahoua), Ouattafouékro and Kouadio Kouamékro (Ahondo), Boussoukro (Tokohiri), Amani Kouadiokro (Sokrogbo), and Beh N’Guessankro and Allah Thérèsekro (Léléblé) (Figure 1). These villages and hamlets were purposely selected because of their small population sizes, and the relatively homogeneous population structure. All inhabitants of the villages and hamlets were targeted as study population, using the readily available Taabo HDSS database.
Figure 1. Map of the study area in Taabo, situated in south-central Côte d’Ivoire.
The study was carried out in two villages (Sahoua, Katchénou) and seven hamlets (1 = Beh N’Guessankro, 2 = Allah Thérèsekro, 3 = Yobouékro, 4 = Ouattafouékro, 5 = Kouadio Kouamékro, 6 = Boussoukro, 7 = Amani Kouadiokro) that are part of the Taabo health demographic surveillance system. Results presented here pertain to the baseline cross-sectional parasitological and questionnaire surveys conducted in July 2011.
Study Design and Procedures
In July 2011, just shortly before the annual round of preventive chemotherapy, a cross-sectional survey was carried out to assess the baseline parasitological and KAPB situation. This cross-sectional survey was part of a larger, still ongoing project aiming to assess the effect of CLTS on helminths and intestinal protozoa re-infection patterns. This larger project consists of a baseline cross-sectional survey (design, field and laboratory procedures, questionnaire survey, and results are presented in this paper), implementation of CLTS combined with health education, and a 1-year follow-up survey.
Before commencement of the study, villages/hamlets were visited by the research team to get approval from the local authorities and to announce the exact date of the sampling day. The day before the sampling, participants were given empty plastic containers for stool and urine collection. Participants were invited to bring early morning stool and urine samples to a central place in the village/hamlet. For parasitological examinations, fecal and urine samples were transferred to our mobile field laboratories in Léléblé and Sokrogbo, or the laboratory of the hospital in Taabo-Cité.
Duplicate Kato-Katz thick smears were prepared on microscope slides from each stool sample, using standard templates holding 41.7 mg of feces. Slides were examined under a microscope and the eggs of Schistosoma mansoni, Ascaris lumbricoides, Trichuris trichiura, and hookworm were counted by experienced laboratory technicians the same day, and recorded for each species separately [27]. Ten milliliters of vigorously shaken urine were filtered, the filter placed on a microscope slide and a drop of Lugol’s iodine added. The slides were examined under a microscope and the number of S. haematobium eggs counted [28]. For quality control, all slides were read independently by different laboratory technicians. When inconsistencies were detected, the discordant slides were re-examined and the results discussed until agreement was reached.
Additionally, from each stool sample, 1–2 g was transferred into a small plastic tube filled with 10 ml of SAF [29]. The SAF-fixed stool samples were forwarded to a laboratory at the Centre Suisse de Recherches Scientifiques en Côte d’Ivoire (CSRS; Abidjan, Côte d’Ivoire) for further examination to detect intestinal protozoa infections. In brief, samples were processed using an ether-concentration method and the slides were analyzed by experienced laboratory technicians under a microscope [30]. Standard protocols were followed and intestinal protozoa (Blastocystis hominis, Chilomastix mesnili, Entamoeba coli, Entamoeba hartmanni, Entamoeba histolytica/E. dispar, Endolimax nana, Giardia intestinalis, and Iodamoeba bütschlii, recorded semi-quantitatively [31].
A questionnaire was administered to all households at the day of stool and urine sampling. The households were visited by a researcher accompanied by a trained field enumerator who speaks the local languages. Whenever the head of a household was present, he/she was interviewed; otherwise a present adult household member was interviewed. The questionnaire was designed in a structured manner with closed questions to obtain quantitative data for the analyses. The questionnaire consisted of basic questions on demographic factors (e.g., age, sex, ethnicity, and education), socioeconomic indicators (e.g., possession of a number of household assets), and KAPB. Topics covered by the KAPB were: (i) sanitation and defecation behavior (e.g., place of defecation, use of latrine); (ii) open defecation (e.g., reasons for open defecation, problems of open defecation); (iii) hygiene behavior (e.g., hand washing after defecation); (iv) opinions, taboos, and beliefs (e.g., preoccupations, gender-specific latrine use); and (v) intestinal parasitic infections (e.g., prevention, transmission, signs, and symptoms). The questionnaire was piloted in 10 households in a village not otherwise involved.
Statistical Analysis
Data were double-entered and cross-checked in EpiInfo version 3.5.1 (Centers for Disease Control and Prevention; Atlanta, United States of America) and analyzed in STATA version 10.0 (Stata Corp.; College Station, United States of America). Only participants with complete datasets (i.e., those with duplicate Kato-Katz thick smears, one SAF-fixed fecal sample, and one urine filtration) were included in the final analyses. For each participant, the arithmetic mean egg count was calculated and used to stratify the infection intensities (mean number of eggs per gram of stool (EPG)) into light, moderate, and heavy infections using cut-offs commonly employed by the World Health Organization (WHO) [32]. Participants were stratified into five age groups (i.e., <5; 5–14; 15–24; 25–40; and >40 years). For the variables and summary statistics of the KAPB questionnaire, frequency tables with indicators of central value and dispersion were calculated. Furthermore, the several categories of the KAPB questionnaire were coded for their importance with a value of 0 if the category was not mentioned at all, a value of 1 after a probed answer, and a value of 2 after a spontaneous answer [33]. The KAPB questionnaire data gathered at the unit of the household served as individual values for every participant living in a specific household, which might slightly distort our results for logistic regression. Participants with a particular helminth or intestinal protozoan infection were compared to participants not infected with that species. Test statistics included chi-square (χ2), Fisher’s exact test, Wilcoxon rank-sum, Kruskal-Wallis, two sample t-tests, and logistic regression models adjusted for participants’ socioeconomic status, age group, and sex. Hence, these characteristics were included wherever these parameters showed significant association with infection. Furthermore, all logistic regressions were corrected for potential clustering at the unit of the village/hamlet.
The socioeconomic status was calculated using a household asset-based approach [34]. Household asset weights were determined using principal component analysis (PCA). Missing values were replaced by the mean of the particular asset. Only binary variables were used for household assets. Household assets were excluded to make the first principal component (PC) stand for more than 30% of the variability. Greatest weight were given to the possession of a television (0.34), followed by the presence of a shower with cement floor (0.33), and the possession of a video recorder (0.33). The calculated scores were added up for each household and subsequently ranked according to the total score. The households were then separated into wealth quintiles: (i) poorest, (ii) very poor, (iii) poor, (iv) less poor, and (v) least poor. To estimate inequities in parasitic infection prevalence related to the participants’ socioeconomic status, the concentration index (CI) was used [35], that arises from the concentration curve. It quantifies the degree of socioeconomic-related inequality in a health variable and is twice the area between the concentration curve and the 45-degree line that is called the line of equality. The CI is 0 if there is no socioeconomic-related health variable. When the CI becomes negative then the curve lies above the line of equality indicating that there is a disproportionate concentration of the health variable among the poor and, vice versa, it takes a positive value if the concentration of the health variable is among the wealthier. Significance of the CI was assessed using standard deviations [36].
Results
Study Participation
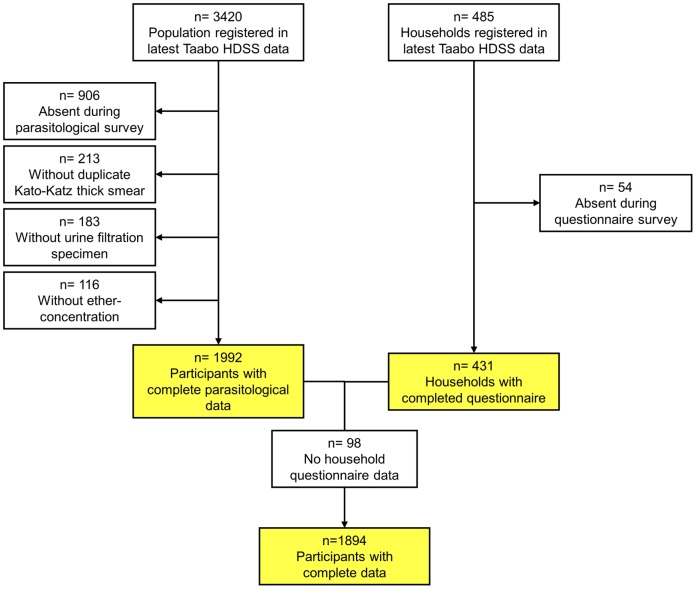
From 3,420 registered people in 485 households in the selected villages and hamlets in the Taabo HDSS, 2,514 individuals were present during the cross-sectional parasitological survey. As shown in Figure 2, 213 participants lacked duplicate Kato-Katz thick smears (no stool sample was provided), 183 had no urine filtration done (no urine sample was provided), and 116 had missing ether-concentration data (insufficient stool provided to perform the test). Complete parasitological data were available from 1,992 individuals (58.2% based on the registered population).
Figure 2. Flow chart showing the study cohort and compliance with emphasis on the three different samples considered in the analysis.
The study was carried out in the Taabo health demographic surveillance system in south-central Côte d’Ivoire in July 2011. The three sub-samples pertain to participants with complete parasitological data, households with complete questionnaire data, and participants with complete parasitological data from a household with complete questionnaire data.
In 54 households, adult members were either absent or refused to participate in the questionnaire survey. Interviews were conducted in the remaining 431 households (88.9%). For regression analysis, 98 participants dropped, due to missing questionnaire data leading to a final study sample of 1,894 people (55.4% of the registered population).
Parasitological Results
Among those 1,992 participants with complete parasitological data, we found prevalences for hookworm, S. haematobium, T. trichiura, S. mansoni, and A. lumbricoides of 33.5%, 7.0%, 1.6%, 1.3%, and 0.8%, respectively (Table 1). Only very few individuals were identified with moderate or heavy helminth infection intensities, with the exception of S. haematobium (25.9% of the infections were classified as heavy, i.e., ≥50 eggs/10 ml of urine). The prevalences of the pathogenic intestinal protozoa G. intestinalis and E. histolytica/E. dispar were 15.0% and 14.4%, respectively. The most common intestinal protozoa were E. coli and B. hominis with respective prevalences of 45.0% and 35.4%.
Table 1. Helminth infection prevalence and intensity among 1,992 participants in Taabo, south-central Côte d’Ivoire, in July 2011.
| Parasite species | Infected (%) | Minimum egg count | Maximumegg count | Infection intensitya | ||
| Light | Moderate | Heavy | ||||
| Hookworm | 667 (33.5) | 12 | 13,584 | 616 (96.7) | 14 (2.2) | 7 (1.1) |
| T. trichiura | 32 (1.6) | 12 | 2,316 | 25 (83.3) | 5 (16.7) | 0 (0.0) |
| S. mansoni | 26 (1.3) | 12 | 2,520 | 20 (87.0) | 2 (8.7) | 1 (4.3) |
| A. lumbricoides | 15 (0.8) | 24 | 5,832 | 8 (88.9) | 1 (11.1) | 0 (0.0) |
| S. haematobium | 139 (7.0) | 1 | 685 | 103 (74.1) | n.d. | 36 (25.9) |
Males were significantly more likely to be infected with hookworm than females (38.8% vs. 28.2%; χ2 = 25.49, p<0.001). The same patterns were found for E. coli (50.6% vs. 39.4%; χ2 = 25.08, p<0.001) and E. nana (31.8% vs. 25.2%; χ2 = 10.69, p = 0.001). In contrast, females were more likely to be infected with T. trichiura compared to males (2.2% vs. 1.0%; χ2 = 4.62, p = 0.032).
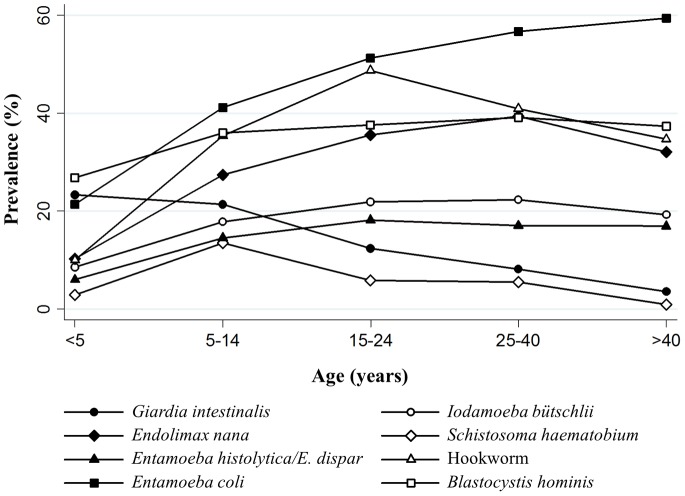
Several intestinal parasites were significantly associated with age group, including hookworm (χ2 = 123.35, degree of freedom (d.f.) = 4, p<0.001), S. mansoni (χ2 = 14.11, d.f. = 4, p = 0.007), S. haematobium (χ2 = 74.68, d.f. = 4, p<0.001) and six of the eight encountered intestinal protozoa (E. histolytica/E. dispar, E. coli, E. nana, I. bütschlii, G. intestinalis, and B. hominis). Age-prevalence curves are shown in Figure 3. Participants of poorer households were significantly more often infected with hookworm (CI = −0.0266, standard error (SE) = 0.0085), T. trichiura (CI = −0.2774, SE = 0.1230), E. histolytica/E. dispar (CI = −0.1072, SE = 0.0242), I. bütschlii (CI = −0.0414, SE = 0.0189), and G. intestinalis (CI = −0.0548, SE = 0.0162). However, the prevalence of S. haematobium was significantly higher in the richer participants compared to their poorer counterparts (CI = 0.2249, SE = 0.0382).
Figure 3. Age-prevalence curves of investigated parasites.
The results of the intestinal protozoa and helminth infections arise from the baseline cross-sectional survey carried out in July 2011 among community members of two villages and seven hamlets in the Taabo health demographic and surveillance system, south-central Côte d’Ivoire. Trichuris trichiura, Schistosoma mansoni and Ascaris lumbricoides are not displayed due to very low prevalence.
Results of the KAPB Survey
Table 2 shows the demographic and socioeconomic characteristics among the households, stratified by wealth quintiles. Muslims were more frequently part of the least poor quintile, compared to Christians and animists. Household size steadily increased from poorest to least poor. Household assets such as electricity, latrine, television, and a motorcycle were more often reported by the least poor quintile. The poorest more often obtained their drinking water from the nearby Bandama River or other unprotected open surface water bodies than their wealthier counterparts who were more likely to use a village pump as source of drinking water.
Table 2. Characteristics of the 431 households, participating in the knowledge, attitude, practice, and beliefs survey, stratified by wealth quintiles.
| Characteristics | Total (n = 431) | Wealth quintiles (%) | Ratio (poorest/least poor) | ||||
| Poorest(n = 85) | Very poor (n = 85) | Poor (n = 81) | Less poor (n = 91) | Least poor (n = 89) | |||
| Sex (%) | |||||||
| Male | 59.2 | 50.6 | 55.3 | 61.7 | 65.9 | 61.8 | 0.82 |
| Female | 40.8 | 49.4 | 44.7 | 38.3 | 34.1 | 38.2 | 1.29 |
| Age (years) | |||||||
| Mean (SD) | 40.3 (14.2) | 37.6 (12.3) | 39.4 (13.4) | 37.5 (11.8) | 43.3 (16.1) | 43.0 (15.8) | |
| Median (Q1–Q3) | 39 (30–48) | 36 (29–45) | 38 (30–47) | 37 (27–45) | 41 (31–53) | 40 (32–55) | |
| Status of the respondent (%) | |||||||
| Household chief | 57.3 | 55.3 | 56.5 | 59.3 | 60.4 | 55.1 | 1.00 |
| Wife | 28.5 | 37.7 | 31.8 | 24.7 | 26.4 | 22.5 | 1.68 |
| Son or daughter | 7.7 | 3.5 | 5.9 | 9.9 | 8.8 | 10.1 | 0.35 |
| Other | 4.4 | 3.5 | 4.7 | 2.5 | 4.4 | 7.9 | 0.44 |
| Brother or sister | 2.1 | 0.0 | 1.2 | 3.7 | 0.0 | 4.5 | 0.00 |
| Religion (%) | |||||||
| Christian | 44.1 | 47.6 | 45.8 | 46.9 | 51.1 | 29.6 | 1.61 |
| Muslim | 23.0 | 17.4 | 12.2 | 15.3 | 17.4 | 37.8 | 0.46 |
| Animist | 29.1 | 29.8 | 34.9 | 32.1 | 26.7 | 22.7 | 1.31 |
| Other religions | 3.8 | 5.2 | 7.1 | 5.7 | 4.8 | 9.9 | 0.53 |
| Educational attainment (%) | |||||||
| No education | 66.6 | 64.7 | 64.7 | 72.8 | 68.1 | 62.9 | 1.03 |
| Primary school | 18.3 | 25.9 | 22.4 | 17.3 | 13.2 | 13.5 | 1.92 |
| Secondary school | 9.7 | 7.1 | 9.4 | 8.6 | 11.0 | 12.4 | 0.57 |
| Koranic school | 4.4 | 2.4 | 3.5 | 1.2 | 5.5 | 9.0 | 0.27 |
| University | 0.9 | 0.0 | 0.0 | 0.0 | 2.2 | 2.2 | 0.00 |
| Reading-writing ability (%) | |||||||
| Reading | 27.8 | 31.8 | 32.9 | 24.7 | 24.2 | 25.8 | 1.23 |
| Writing | 27.6 | 31.8 | 32.9 | 23.5 | 24.2 | 25.8 | 1.23 |
| Household size | |||||||
| Mean (SD) | 7.3 (4.3) | 5.5 (2.8) | 6.5 (3.5) | 6.7 (3.8) | 8.5 (5.0) | 9.2 (4.9) | 0.60 |
| Median (Q1–Q3) | 6 (5–9) | 5 (4–7) | 6 (5–8) | 6 (4–9) | 8 (5–11) | 8 (6–11) | 0.75 |
| Household assets (%) | |||||||
| Shower | 88.4 | 88.2 | 92.9 | 70.0 | 91.2 | 97.7 | 0.90 |
| Bicycle | 79.3 | 64.7 | 80.0 | 77.5 | 82.4 | 91.0 | 0.71 |
| Radio | 72.8 | 65.9 | 77.7 | 70.0 | 72.5 | 77.5 | 0.85 |
| Motorcycle | 22.3 | 4.7 | 17.7 | 20.0 | 24.2 | 43.8 | 0.11 |
| Latrine | 20.7 | 10.6 | 9.4 | 12.5 | 33.0 | 38.2 | 0.28 |
| Television | 18.4 | 1.2 | 1.2 | 3.8 | 17.6 | 65.2 | 0.02 |
| Electricity | 14.2 | 1.2 | 1.2 | 4.9 | 11.0 | 51.1 | 0.02 |
| Drinking water source (%), multiple answers possible | |||||||
| Pond/river | 58.8 | 81.5 | 61.9 | 50.6 | 55.0 | 46.0 | 1.77 |
| Pump | 37.7 | 18.5 | 35.7 | 43.0 | 37.4 | 52.9 | 0.35 |
| River | 37.4 | 60.5 | 40.5 | 27.9 | 27.5 | 32.2 | 1.88 |
| Pond | 21.6 | 21.0 | 21.4 | 22.8 | 28.6 | 13.8 | 1.52 |
| Cistern | 19.4 | 16.1 | 17.9 | 17.7 | 30.8 | 13.8 | 1.17 |
| Well | 1.9 | 1.2 | 3.6 | 0.0 | 2.2 | 2.2 | 0.55 |
The study was carried out in the Taabo health demographic surveillance system in south-central Côte d’Ivoire in July 2011. Questionnaires were conducted with the household chief if present; otherwise the next higher household member was interviewed.
Q1–Q3 stands for first quartile to third quartile, defining the interquartile range.
Most interviewees (98.6%) said that they would wash their hands regularly. The most frequently mentioned occasions to wash hands were before a meal (99.8%), after a meal (92.5%), after defecation (85.3%), and when hands looked dirty (75.6%). Among these four categories, before eating was most often spontaneously mentioned (proportion 86%). Hand washing after defecation was only spontaneously mentioned by 27% of the interviewees (Table 3).
Table 3. Knowledge, attitude, practice, and beliefs related to hygiene behavior, latrine possession, and open defecation mentioned by the respondents.
| Total reported % | Proportion spontaneousa | Mean prominenceb | |
| Reasons to possess a latrine ( n = 89) | |||
| Safety | 75.3 | 0.42 | 1.07 |
| Clean environment | 68.5 | 0.51 | 1.03 |
| Comfort | 67.4 | 0.53 | 1.03 |
| Avoid diseases | 67.4 | 0.27 | 0.85 |
| Privacy | 65.2 | 0.29 | 0.84 |
| Visitors | 70.8 | 0.10 | 0.78 |
| Elderly people | 51.7 | 0.26 | 0.65 |
| Modern lifestyle | 18.1 | 0.12 | 0.31 |
| Time point of hand washing ( n = 427) | |||
| Before a meal | 99.8 | 0.86 | 1.85 |
| After a meal | 92.5 | 0.53 | 1.42 |
| Always when dirty | 75.6 | 0.64 | 1.24 |
| After defecation | 85.3 | 0.27 | 1.08 |
| Before preparing a meal | 50.8 | 0.31 | 0.67 |
| Before nourishing a child | 42.2 | 0.13 | 0.48 |
| After cleaning a child | 38.9 | 0.10 | 0.43 |
| Problems associated with open defecation ( n = 384) | |||
| Safety | 85.2 | 0.65 | 1.40 |
| Clean environment | 71.1 | 0.28 | 0.91 |
| Hygiene | 63.5 | 0.36 | 0.86 |
| No comfort | 62.2 | 0.37 | 0.85 |
| Privacy | 58.3 | 0.25 | 0.73 |
| Elderly people | 56.8 | 0.11 | 0.63 |
| Visitors | 58.6 | 0.05 | 0.62 |
| Drinking water | 52.1 | 0.04 | 0.54 |
| Reason not to possess a latrine ( n = 334) | |||
| No technical comprehension | 51.1 | 0.91 | 0.98 |
| Traditional habit | 24 | 0.70 | 0.41 |
| Soil not stable | 9.9 | 1.00 | 0.20 |
| No material | 12 | 0.60 | 0.19 |
| Low priority | 6.9 | 0.52 | 0.10 |
| Reason to practice open defecation ( n = 320) | |||
| No latrine in the household | 88.8 | 0.95 | 1.73 |
| Traditional habit | 43.4 | 0.66 | 0.56 |
The study was carried out in the Taabo health demographic surveillance system in south-central Côte d’Ivoire in July 2011.
Proportion of categories reported spontaneously.
Mean prominence based on values assigned to each category (0 = not mentioned, 1 = probed, 2 = spontaneous).
Place and defecation frequency index assessed on a semi-quantitative scale, stratified by possession of a latrine, are summarized in Table 4. Study participants frequently reported to defecate into the nearby bush or open plantations. People living in households with latrines mostly used them, but they also practiced open defecation. Members of households without a toilet most of the time defecated in the open. A significant difference of defecation frequency could only be found between households possessing a latrine and households without a latrine for the nearby bush (0.73 vs. 3.28, p<0.001) and latrine (3.38 vs. 0.05, p<0.001), while no significant difference was found for the plantation (1.64 vs. 1.68, p = 0.969).
Table 4. Defecation behavior assessed with the parameters place and frequency, stratified by the abundance of household-owned latrines.
| Place and defecation frequency index | Total householdsa | With latrinea | Without latrinea | P-valueb |
| Near bush | 2.75 (0.09) | 0.73 (0.14) | 3.28 (0.08) | <0.001 |
| Plantation | 1.67 (0.08) | 1.64 (0.15) | 1.68 (0.10) | 0.969 |
| Latrine | 0.74 (0.07) | 3.38 (0.12) | 0.05 (0.02) | <0.001 |
| Shared latrine | 0.28 (0.04) | 0.17 (0.08) | 0.30 (0.05) | 0.069 |
| River/pond | 0.06 (0.02) | 0.03 (0.03) | 0.07 (0.02) | 0.207 |
| Behind the house | 0.05 (0.02) | 0 | 0.07 (0.03) | 0.173 |
The study was carried out among 431 households in the Taabo health demographic surveillance system in south-central Côte d’Ivoire in July 2011.
Frequency of defecation (defecation frequency index) assessed on a semi-quantitative scale (0 = never, 1 = irregular, 2 = regular, 3 = often, 4 = always) for each place of defecation. Frequency is indicated as means (standard error in brackets).
P-value assessed with Wilcoxon rank-sum test.
Most household members said that they need a latrine (98.5%) and nine out of 10 interviewees perceived open defecation as a problem. The most frequently stated reasons why a household does not have a latrine were the high cost (51.1%), traditional habit of open defecation (24.0%), not all of the required material locally available (12.0%), and soil not stable enough or the groundwater table too high for a durable construction (9.9%).
There was poor knowledge of schistosomiasis and parasitic worms in general (Table 5). Only 64.0% and 49.3% stated that prevention of schistosomiasis and parasitic worms, respectively, is possible. Open defecation was most frequently practiced because households simply did not have a latrine (88.8%), or because a deeply rooted tradition of open defecation (43.4%). Perceived problems with open defecation were safety issues with regard to different dangers lurking in the bush such as snakes (85.2%), pollution of the environment (71.1%), lack of hygiene (63.5%), lack of comfort (62.2%), and lack of privacy (58.3%). The only category with more than the half spontaneous answers of all reports was safety (65.0%). The top reasons to own a latrine were safety (75.3%), provide a decent place to defecate for visitors (70.8%), keep the environment clean (68.5%), preventing the spread of diseases (67.4%), enhanced comfort (67.4%), and higher level of privacy (65.2%).
Table 5. Knowledge of prevention of urogenital schistosomiasis and intestinal helminths.
| Schistosomiasis | ||
| Can you prevent yourself from getting schistosomiasis? | ||
| ( n = 253) | Yes | 64.0% |
| Don’t know | 32.0% | |
| No | 4.0% | |
| If yes, how? (multiple answers allowed) | ||
| ( n = 162) | Do not bath | 58.0% |
| Do not drink dirty water | 53.7% | |
| Do not defecate in the water | 22.2% | |
| Do not eat overripe fruits | 4.9% | |
| Do not eat washed fruits | 1.2% | |
| Soil-transmitted helminthiasis | ||
| Can you prevent yourself from getting parasitic worms? | ||
| ( n = 424) | Yes | 49.3% |
| Don’t know | 45.3% | |
| No | 5.4% | |
| If yes, how? (multiple answers allowed) | ||
| ( n = 209) | Do not eat overripe fruits | 38.8% |
| Wash hands before eating | 38.3% | |
| Eat candies | 34.0% | |
| Wash hands after defecation | 33.5% | |
| Taking medication | 27.8% | |
| Wash fruits | 27.3% | |
| Do not eat meat | 13.9% | |
| Wearing shoes | 4.3% | |
The study was carried out among 431 households in the Taabo health demographic surveillance system in south-central Côte d’Ivoire in July 2011. Questionnaires were applied on a household level and the question only asked if the participant stated to know the disease.
Association of Parasitic Infection with Hygiene and Defecation Behavior
All significant associations between a specific parasite infection and hygiene and defecation behavior and demographic factors are summarized in Table 6. For several different parasitic infections (A. lumbricoides, E. coli, E. nana, I. mesnili, and C. bütschlii) Muslims had lower odds of an infection than their counterparts with other religious beliefs. Besides demographic characteristics, place of defecation and hand washing behavior showed statistically significant associations with intestinal parasitic infections, including hookworm, T. trichiura, E. hartmanni, E. nana, and B. hominis.
Table 6. Significant associations between parasitic infections and household assets, hygiene, and defecation behavior.
| Parasite | Association | Adjusted odds ratio (95% CI) | P-valuea |
| S. haematobium | Christian | 1.00 | |
| Muslim | 7.18 (2.60–19.80) | <0.001 | |
| Animist | 2.10 (1.47–3.00) | <0.001 | |
| Hand washing for personal hygiene | 3.50 (1.68–7.28) | 0.001 | |
| Use of pond water for hand washing | 3.76 (1.75–8.08) | 0.001 | |
| Hand washing time points spontaneously correct answered | 2.61 (1.52–4.49) | <0.001 | |
| Soil–transmitted helminths | |||
| Hookworm | Latrine | 0.63 (0.40–1.00) | 0.050 |
| Hand washing to prevent diseases | 0.75 (0.58–0.98) | 0.037 | |
| Defecation in the bush | 1.70 (1.07–2.69) | 0.025 | |
| Children defecating in latrine | 0.53 (0.34–0.83) | 0.006 | |
| Children defecating in the bush | 1.64 (1.06–2.54) | 0.027 | |
| A. lumbricoides | Christian | 1.00 | |
| Muslim | 0.27 (0.09–0.87) | 0.028 | |
| Knowledge of parasitic worms | 0.39 (0.20–0.78) | 0.008 | |
| T. trichiura | Hand washing to prevent diseases | 0.37 (0.16–0.86) | 0.020 |
| Children defecating in latrine | 0.50 (0.25–1.00) | 0.048 | |
| Intestinal protozoa | |||
| E. hartmanni | Farmer | 0.54 (0.33–0.90) | 0.019 |
| Drinking water from pump | 0.52 (0.29–0.94) | 0.031 | |
| Defecation in latrine | 0.27 (0.11–0.67) | 0.005 | |
| E. coli | Christian | 1.00 | |
| Muslim | 0.75 (0.64–0.88) | <0.001 | |
| Fisher | 1.57 (1.30–1.91) | <0.001 | |
| E. nana | Christian | 1.00 | |
| Muslim | 0.81 (0.67–0.99) | 0.039 | |
| Latrine | 0.80 (0.66–0.97) | 0.027 | |
| I. mesnili | Christian | 1.00 | |
| Muslim | 0.58 (0.40–0.85) | 0.004 | |
| Animist | 0.80 (0.65–1.00) | 0.047 | |
| Other religion | 0.57 (0.44–0.75) | <0.001 | |
| C. bütschlii | Christian | 1.00 | |
| Muslim | 0.51 (0.29–0.91) | 0.023 | |
| Animist | 1.53 (1.09–2.15) | 0.014 | |
| B. hominis | Fisher | 0.61 (0.37–0.98) | 0.041 |
| Hand washing to prevent diseases | 0.71 (0.51–0.97) | 0.030 | |
The study was carried out among 431 households in the Taabo health demographic surveillance system in south-central Côte d’Ivoire in July 2011. Logistic regression analysis was used with village level exchangeable random effects. Variables included as potential confounders were age groups (<5, 5–14, 15–24, 25–40, and >40 years), wealth quintiles and sex whenever age, sex, and socioeconomic status were significantly associated with a given parasitic infection.
No significant associations for E. histolytica/E. dispar and G. intestinalis with household assets, hygiene, and defecation behavior have been found after correction for potential confounders (sex, age group, or wealth quintile).
P-value based on Wald test.
Discussion
The global strategy for the control of helminthiases emphasizes preventive chemotherapy [4], [10], [11], [37]. The impact of this strategy on morbidity control is undeniable [38]. However, there is rapid re-infection after deworming, and hence the importance of improved sanitation is widely acknowledged in the literature dating back almost 100 years [13], [15], [16], [19]. Yet, compared to preventive chemotherapy, relatively little attention is paid on improving sanitation and clean water in contemporary helminthiases control programs [17], [18], [39]. In the present study we assessed the prevalence (and intensity) of helminths and intestinal protozoa infections and associated these findings with the local KAPB in nine purposely selected villages/hamlets of the Taabo HDSS in south-central Côte d’Ivoire, where annual preventive chemotherapy against helminth infections is administered to the entire population. The most prevalent helminth infection was hookworm (33.5%), followed by S. haematobium (7.0%). Other helminths were encountered only rarely.
The investigated parasitic infection prevalences and intensities were much lower than some 10 years ago; initial hookworm infections in the Taabo area in the late 1990s/early 2000s were high (34.4–54.0%), while initial prevalences for A. lumbricoides and T. trichiura infections were low; 0–1.3% and 3.3–7.5%, respectively [40]. The reduction of the highly prevalent infections can partly be explained by the interventions carried out within the Taabo HDSS as well as preceding research and control activities against schistosomiasis [24]–[26], [41]–[43]. Indeed, our continuous research-cum-action activities pertaining to helminthiases in selected localities in the study area might have had a positive influence by reducing the incidence through improved knowledge about these otherwise neglected disease in the population. In previous work on schistosomiasis in western Côte d'Ivoire we found that our research activities considerably improved knowledge in the community [22]. Furthermore, while S. haematobium and S. mansoni infections are a problem for only certain localities due to the focal distribution of the disease, it can be tackled comparably easy once these foci are identified. In contrast, hookworm infections are more homogeneously distributed throughout the Taabo HDSS and considerable in- and out-migration and the challenge to reach high coverage with preventive chemotherapy are important underlying issues. It should be noted that, despite continuous control efforts through annual deworming, re-infection with hookworm occurs rapidly. Hence, there is a need to continue preventive chemotherapy, coupled with additional control measures to prevent rapid re-infection [13], [44], [45].
Two limitations of our study are offered for discussion. First, although duplicate Kato-Katz thick smears were performed on single stool samples in order to increase sensitivity of the technique [46] it is conceivable that the reported helminth infection prevalences are an underestimation of the “true” situation in the study area. The issue of missing low infection intensities based on microscopic examination of single specimens has been discussed before [47], partially explained by considerable day-to-day variation of helminth egg output [48], [49]. Other new diagnostic tools such as the FLOTAC technique [50], molecular approaches (i.e., polymerase chain reaction (PCR) [51]), or the collection of samples over several days should be considered in future studies to increase sensitivity [52]. Second, the low prevalence of infections with T. trichiura and A. lumbricoides made it difficult to draw conclusive evidence about the direction and strength of association between these helminth species and risk factors.
Several intestinal parasite infections showed significant association with socioeconomic status, confirming observations from western Côte d’Ivoire of significant disparities of parasitic infection status among study participants [53]. Hookworm, T. trichiura, E. histolytica/E. dispar, G. intestinalis, and I. bütschlii were more prevalent among the poorer wealth quintiles. Surprisingly, S. haematobium was positively associated with higher socioeconomic status and Muslim showed a higher risk than people with other religious beliefs. However, these observations might be explained by the focal distribution of urogenital schistosomiasis; 97% of all S. haematobium cases were found in Sahoua, situated in close proximity to the Bandama River. In this village, the majority of inhabitants are Muslims. Moreover, the average socioeconomic status of Sahoua is considerably higher than other study village and hamlets.
A generally good hygiene behavior (e.g., not drinking dirty water, hand washing after defection) was recorded, which undoubtedly impacts on parasitic worms. Knowledge of schistosomiasis transmission (e.g., swimming and bathing in Lake Taabo) is widely known (58%), while wearing shoes to prevent hookworm infections was rarely mentioned (4.3%). This lack of knowledge about hookworm transmission might explain the relatively high prevalence of this helminth species despite several rounds of deworming.
Open defecation was commonly reported by the study participants. Indeed, the habit of open defecation is so deeply rooted that it was also reported (at least partially) among households having a latrine. As expected, we found a significant negative association between hookworm infection and the use of a latrine, confirming results from a systematic review and meta-analysis [19]. Literally all variables related with latrine availability or use were associated with a significantly lower odds of certain helminth infections (most importantly hookworm), but also some intestinal protozoa infections (e.g., E. hartmanni and E. nana) [54], [55].
Sanitation and hygiene behavior have proven to be substantial contributors to a sustainable control of soil-transmitted helminthiasis, schistosomiasis, diarrhea, and other fecal-orally transmitted diseases [56]. However, the promotion of sanitary solutions and the improvement of hygiene behavior are of a higher complexity than the regular administration of anthelmintic drugs to at-risk populations, as the former entail many locally rooted socio-cultural idiosyncrasies. For example, the possession of a latrine does not necessarily mean that it is being used [19], [57]. In the current study, however, the participants living in a household with a latrine reported its use, but we did not further verify these self-reports through direct observations. Open defecation is still commonly practiced among households possessing a latrine, particularly when people pursue agricultural activities, often several kilometers away from home. Importantly though, open defecation while pursuing agricultural activities was not associated with a higher odds of helminths and intestinal protozoa infections, which is in contrast to open defecation within a human settlement (village or hamlet) and in close proximity to open water sources. Population density in human settlements is higher than on plantations, and hence contaminated feces in the village/hamlet are a source of infection for villagers. Nevertheless, open defecation is not desirable in any case and should be stopped for the reason that plantations and agricultural fields in close proximity to open water bodies could contaminate the environment.
The main reasons advanced by interviewees regarding the possession of a latrine were issues of safety, privacy, enhanced comfort, clean environment, and hygiene. Indeed, households that attributed importance to these issues were more likely to have a latrine. Health-related reasons such as hygiene or prevention of disease were frequently reported, but only a small number of interviewees mentioned such reasons spontaneously, indicating that health-related issues were perceived as less important. Although, everyone stated the need for a latrine, not everyone was motivated to build one, mainly because the construction of latrines was perceived as an expensive undertaking. Furthermore, open defecation was seen as a traditional behavior that the whole village/hamlet is practicing. Overall, we found that health-related reasons played a minor role in the decision-making process. Therefore, health education interventions are necessary to increase the motivation of change or sanitation promotion focusing on these socio-cultural and socioeconomic reasoning and taking the whole spectrum of the villagers’ concerns into account [23], [33], [58].
Most people reported that they regularly wash their hands, but myriad reasons for hand washing were given. However, villagers seemed not to associate disease prevention with general cleanliness as the two elements were mentioned separately. Our analyses revealed that the most important factor for regular hand washing was the type of preceding (e.g., field work or defecation) or subsequent activity (e.g., food consumption). “Before a meal” was mentioned by almost all interviewees and indeed with a high proportion of spontaneous responses, which is important for the prevention of diarrheal diseases [59]. Although hand washing after defecation was reported by 85.3% of the interviewees, it was reported spontaneously only by a small proportion of study participants. This could indicate that people only answered yes to please the interviewer, but in reality, they do not wash their hands regularly after defecation. Failing to wash hands after defecation favors the transmission of fecal-orally transmitted diseases [60].
In conclusion, our results show that the use of latrines is associated with lower odds of hookworm infection. The study also indicates that morbidity due to soil-transmitted helminthiasis and schistosomiasis has been greatly reduced in the Taabo HDSS and preventive chemotherapy certainly played a key role [24], [43]. However, there is rapid re-infection after deworming, and hence integrated control approaches are necessary to keep the prevalence and intensity of infection – and thus morbidity – low. The parasitological and questionnaire data reported here will serve as a benchmark to monitor the effect of CLTS and hygiene education with the goal to reduce and interrupt the transmission of helminth and intestinal protozoa infections.
Acknowledgments
Many thanks are addressed to Dr. Lukas G. Adiossan, Mr. Yao N’Guessan, Ms. Sarah E. Mara, and all the technicians and drivers for their skillful work in the field and the laboratory. Furthermore, we are indebted to the entire Taabo health demographic surveillance system team for their excellent cooperation. Finally, we are deeply grateful for the inhabitants of the selected villages and hamlets for their enthusiastic participation.
Funding Statement
This study received financial support from the UBS Optimus Foundation. Jürg Utzinger and Giovanna Raso acknowledge financial support from the Swiss National Science Foundation (project no. IZ70Z0_123900 and project no. 32003B_132949/1). Thomas Schmidlin acknowledges financial support from the Swiss Tropical and Public Health Institute (Teaching & Training). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1. Hotez PJ, Molyneux DH, Fenwick A, Ottesen E, Ehrlich Sachs S, et al. (2006) Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS Med 3: e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Utzinger J, Becker SL, Knopp S, Blum J, Neumayr AL, et al. (2012) Neglected tropical diseases: diagnosis, clinical management, treatment and control. Swiss Med Wkly 142: w13727. [DOI] [PubMed] [Google Scholar]
- 3. Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J (2006) Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 6: 411–425. [DOI] [PubMed] [Google Scholar]
- 4. Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, et al. (2008) Helminth infections: the great neglected tropical diseases. J Clin Invest 118: 1311–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, et al. (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2197–2223. [DOI] [PubMed] [Google Scholar]
- 6. Walsh JA (1986) Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev Infect Dis 8: 228–238. [DOI] [PubMed] [Google Scholar]
- 7. Minenoa T, Avery MA (2003) Giardiasis: recent progress in chemotherapy and drug development. Curr Pharm Des 9: 841–855. [DOI] [PubMed] [Google Scholar]
- 8. Savioli L, Smith H, Thompson A (2006) Giardia and Cryptosporidium join the “neglected diseases initiative.”. Trends Parasitol 22: 203–208. [DOI] [PubMed] [Google Scholar]
- 9. Becker SL, Vogt J, Knopp S, Panning M, Warhurst DC, et al. (2013) Persistent digestive disorders in the tropics: causative infectious pathogens and reference diagnostic tests. BMC Infect Dis 13: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. WHO (2002) Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. WHO Tech Rep Ser 912: 1–57. [PubMed] [Google Scholar]
- 11.WHO (2006) Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Geneva: World Health Organization. [Google Scholar]
- 12. Quinnell RJ, Slater AF, Tighe P, Walsh EA, Keymer AE, et al. (1993) Reinfection with hookworm after chemotherapy in Papua New Guinea. Parasitology 106: 379–385. [DOI] [PubMed] [Google Scholar]
- 13. Jia TW, Melville S, Utzinger J, King CH, Zhou XN (2012) Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta-analysis. PLoS Negl Trop Dis 6: e1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geerts S, Gryseels B (2000) Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev 13: 207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cort WW, Schapiro L, Stoll NR (1929) A study of reinfection after treatment with hookworm and Ascaris in two villages in Panama. Am J Epidemiol 10: 614–625. [Google Scholar]
- 16. Stiles CW (1939) Early history, in part esoteric, of the hookworm (Uncinariasis) campaign in our southern United States. J Parasitol 25: 283–308. [Google Scholar]
- 17. Utzinger J, Bergquist R, Xiao SH, Singer BH, Tanner M (2003) Sustainable schistosomiasis control – the way forward. Lancet 362: 1932–1934. [DOI] [PubMed] [Google Scholar]
- 18. Singer BH, Castro MC (2007) Bridges to sustainable tropical health. Proc Natl Acad Sci U S A 104: 16038–16043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, et al. (2012) Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med 9: e1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO/UNICEF (2010) Progress on sanitation and drinking-water –2010 update. Geneva: World Health Organization and UNICEF, 55 pp. [Google Scholar]
- 21. Utzinger J, N’Goran EK, Caffrey CR, Keiser J (2011) From innovation to application: social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop 120 (Suppl 1)121–137. [DOI] [PubMed] [Google Scholar]
- 22. Acka CA, Raso G, N’Goran EK, Tschannen AB, Bogoch II, et al. (2010) Parasitic worms: knowledge, attitudes, and practices in western Côte d’Ivoire with implications for integrated control. PLoS Negl Trop Dis 4: e910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambers R, Kar K (2008) Handbook on community-led total sanitation. London: Plan International. [Google Scholar]
- 24. Fürst T, Silué KD, Ouattara M, N’Goran DN, Adiossan LG, et al. (2012) Schistosomiasis, soil-transmitted helminthiasis, and sociodemographic factors influence quality of life of adults in Côte d’Ivoire. PLoS Negl Trop Dis 6: e1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Righetti AA, Koua AYG, Adiossan LG, Glinz D, Hurrell RF, et al. (2012) Etiology of anemia among infants, school-aged children, and young non-pregnant women in different settings of south-central Côte d’Ivoire. Am J Trop Med Hyg 87: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Righetti AA, Adiossan LG, Ouattara M, Glinz D, Hurrell RF, et al. (2013) Dynamics of anemia in relation to parasitic infections, micronutrient status, and increasing age in south-central Côte d’Ivoire. J Infect Dis 207: 1604–1615. [DOI] [PubMed] [Google Scholar]
- 27. Katz N, Chaves A, Pellegrino J (1972) A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop São Paulo 14: 397–400. [PubMed] [Google Scholar]
- 28. Savioli L, Hatz C, Dixon H, Kisumku UM, Mott KE (1990) Control of morbidity due to Schistosoma haematobium on Pemba Island: egg excretion and hematuria as indicators of infection. Am J Trop Med Hyg 43: 289–295. [DOI] [PubMed] [Google Scholar]
- 29. Marti H, Escher E (1990) SAF - Eine alternative Fixierlösung für parasitologische Stuhluntersuchungen. Schweiz Med Wochenschr 120: 1473–1476. [PubMed] [Google Scholar]
- 30. Allen AVH, Ridley DS (1970) Further observations on the formol-ether concentration technique for faecal parasites. J Clin Pathol 23: 545–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Utzinger J, Botero-Kleiven S, Castelli F, Chiodini P, Edwards H, et al. (2010) Microscopic diagnosis of sodium acetate-acetic acid-formalin-fixed stool samples for helminths and intestinal protozoa: a comparison among European reference laboratories. Clin Microbiol Infect 16: 267–273. [DOI] [PubMed] [Google Scholar]
- 32.Montresor A, Crompton DWT, Hall A, Bundy DAP, Savioli L (1998) Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level. Geneva: World Health Organization, 49 pp. [Google Scholar]
- 33. Kouadio MKD, Righetti AA, Abé NN, Wegmüller R, Weiss MG, et al. (2013) Local concepts of anemia-related illnesses and public health implications in the Taabo health demographic surveillance system, Côte d’Ivoire. BMC Hematol 13: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gwatkin DR (2000) Health inequalities and the health of the poor: what do we know? What can we do? Bull World Health Organ 78: 3–18. [PMC free article] [PubMed] [Google Scholar]
- 35. Wagstaff A, Paci P, van Doorslaer E (1991) On the measurement of inequalities in health. Soc Sci Med 33: 545–557. [DOI] [PubMed] [Google Scholar]
- 36. Kakwani N, Wagstaff A, van Doorslaer E (1997) Socioeconomic inequalities in health: measurement, computation, and statistical inference. J Econ 77: 87–103. [Google Scholar]
- 37. Savioli L, Gabrielli AF, Montresor A, Chitsulo L, Engels D (2009) Schistosomiasis control in Africa: 8 years after World Health Assembly Resolution 54.19. Parasitology 136: 1677–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Touré S, Zhang Y, Bosqué-Oliva E, Ky C, Ouedraogo A, et al. (2008) Two-year impact of single praziquantel treatment on infection in the national control programme on schistosomiasis in Burkina Faso. Bull World Health Organ 86: 780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Utzinger J, Raso G, Brooker S, de Savigny D, Tanner M, et al. (2009) Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology 136: 1859–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. N’Goran EK, Utzinger J, Gnaka HN, Yapi A, N’Guessan NA, et al. (2003) Randomized, double-blind, placebo-controlled trial of oral artemether for the prevention of patent Schistosoma haematobium infections. Am J Trop Med Hyg 68: 24–32. [PubMed] [Google Scholar]
- 41. N’Goran EK, Utzinger J, N’Guessan AN, Müller I, Zamblé K, et al. (2001) Reinfection with Schistosoma haematobium following school-based chemotherapy with praziquantel in four highly endemic villages in Côte d’Ivoire. Trop Med Int Health 6: 817–825. [DOI] [PubMed] [Google Scholar]
- 42. Becker SL, Sieto B, Silué KD, Adjossan L, Koné S, et al. (2011) Diagnosis, clinical features, and self-reported morbidity of Strongyloides stercoralis and hookworm infection in a co-endemic setting. PLoS Negl Trop Dis 5: e1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fürst T, Ouattara M, Silué KD, N’Goran DN, Adiossan LG, et al. (2013) Scope and limits of an anamnestic questionnaire in a control-induced low-endemicity helminthiasis setting in south-central Côte d’Ivoire. PLoS One 8: e64380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Knopp S, Mohammed KA, Speich B, Hattendorf J, Khamis IS, et al. (2010) Albendazole and mebendazole administered alone or in combination with ivermectin against Trichuris trichiura: a randomized controlled trial. Clin Infect Dis 51: 1420–1428. [DOI] [PubMed] [Google Scholar]
- 45. Scherrer AU, Sjöberg MK, Allangba A, Traoré M, Lohourignon LK, et al. (2009) Sequential analysis of helminth egg output in human stool samples following albendazole and praziquantel administration. Acta Trop 109: 226–231. [DOI] [PubMed] [Google Scholar]
- 46. Booth M, Vounatsou P, N’Goran EK, Tanner M, Utzinger J (2003) The influence of sampling effort and the performance of the Kato-Katz technique in diagnosing Schistosoma mansoni and hookworm co-infections in rural Côte d’Ivoire. Parasitology 127: 525–531. [DOI] [PubMed] [Google Scholar]
- 47. Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, et al. (2008) Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis 2: e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Utzinger J, Booth M, N’Goran EK, Müller I, Tanner M, et al. (2001) Relative contribution of day-to-day and intra-specimen variation in faecal egg counts of Schistosoma mansoni before and after treatment with praziquantel. Parasitology 122: 537–544. [DOI] [PubMed] [Google Scholar]
- 49. Coulibaly J, Fürst T, Silué K, Knopp S, Hauri D, et al. (2012) Intestinal parasitic infections in schoolchildren in different settings of Côte d’Ivoire: effect of diagnostic approach and implications for control. Parasit Vectors 5: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cringoli G, Rinaldi L, Maurelli MP, Utzinger J (2010) FLOTAC: new multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat Protoc 5: 503–515. [DOI] [PubMed] [Google Scholar]
- 51. Verweij JJ, Brienen EA, Ziem J, Yelifari L, Polderman AM, et al. (2007) Simultaneous detection and quantification of Ancylostoma duodenale, Necator americanus, and Oesophagostomum bifurcum in fecal samples using multiplex real-time PCR. Am J Trop Med Hyg 77: 685–690. [PubMed] [Google Scholar]
- 52. Glinz D, Silué KD, Knopp S, Lohourignon LK, Yao KP, et al. (2010) Comparing diagnostic accuracy of Kato-Katz, Koga agar plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soil-transmitted helminths. PLoS Negl Trop Dis 4: e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Raso G, Utzinger J, Silué KD, Ouattara M, Yapi A, et al. (2005) Disparities in parasitic infections, perceived ill health and access to health care among poorer and less poor schoolchildren of rural Côte d’Ivoire. Trop Med Int Health 10: 42–57. [DOI] [PubMed] [Google Scholar]
- 54. Esrey SA, Potash JB, Roberts L, Shiff C (1991) Effects of improved water supply and sanitation on ascariasis, diarrhoea, dracunculiasis, hookworm infection, schistosomiasis, and trachoma. Bull World Health Organ 69: 609–621. [PMC free article] [PubMed] [Google Scholar]
- 55. Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, et al. (2005) Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis 5: 42–52. [DOI] [PubMed] [Google Scholar]
- 56. Bartram J, Cairncross S (2010) Hygiene, sanitation, and water: forgotten foundations of health. PLoS Med. 7: e1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mara D, Lane J, Scott B, Trouba D (2010) Sanitation and health. PLoS Med 7: e1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jenkins MW, Scott B (2007) Behavioral indicators of household decision-making and demand for sanitation and potential gains from social marketing in Ghana. Soc Sci Med 64: 2427–2442. [DOI] [PubMed] [Google Scholar]
- 59.Ejemot RI, Ehiri JE, Meremikwu MM, Critchley JA (2008) Hand washing for preventing diarrhoea. Cochrane Database Syst Rev: CD004265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Curtis V, Cairncross S (2003) Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. Lancet Infect Dis 3: 275–281. [DOI] [PubMed] [Google Scholar]