Abstract
Rac1, a member of the Rho family of GTPases, regulates diverse cellular functions, including cytoskeleton reorganization and cell migration. F-box proteins are major subunits within the Skp1-Cul1-F-box (SCF) E3 ubiquitin ligases that recognize specific substrates for ubiquitination. The role of F-box proteins in regulating Rac1 stability has not been studied. Mouse lung epithelial (MLE12) cells were used to investigate Rac1 stability and cell migration. Screening of an F-box protein library and in vitro ubiquitination assays identified FBXL19, a relatively new member of the F-box protein family that targets Rac1 for its polyubiquitination and proteasomal degradation. Overexpression of FBXL19 decreased both Rac1 active and inactive forms and significantly reduced cellular migration. Protein kinase AKT-mediated phosphorylation of Rac1 at serine71 was essential for FBXL19-mediated Rac1 ubiquitination and depletion. Lysine166 within Rac1 was identified as a polyubiquitination acceptor site. Rac1S71A and Rac1K166R mutant proteins were resistant to FBXL19-mediated ubiquitination and degradation. Further, ectopically expressed FBXL19 reduced cell migration in Rac1-overexpressing cells (P<0.01, Rac1 cells vs. FBXL19+Rac1 cells), but not in Rac1 lysine166 mutant-overexpressing cells. FBXL19 diminished formation of the migratory leading edge. Thus, SCFFBXL19 targets Rac1 for its disposal, a process regulated by AKT. These findings provide the first evidence of an F-box protein targeting a small G protein for ubiquitination and degradation to modulate cell migration.—Zhao, J., Mialki, R. K., Wei, J., Coon, T. A., Zou, C., Chen, B. B., Mallampalli, R. K., Zhao, Y. SCF E3 ligase F-box protein complex SCFFBXL19 regulates cell migration by mediating Rac1 ubiquitination and degradation.
Keywords: small GTPase protein, protein stability, phosphorylation, cell motility
Protein stability is regulated by many post-translational modifications, including ubiquitination (1–5). The ubiquitin-proteasome pathway is the major system for protein degradation (1, 4). The Skp1-Cul1-F-box (SCF) protein ligase complex is one of the largest E3 ubiquitin ligase families (6, 7). The F-box protein in the E3 ligase complex functions as the bridge linking the ligase complex and specific substrates via its F-box domain and substrate binding motif. The FBXL family contains leucine-rich repeats (LRRs); the FBXW family contains Trp-Asp (WD) repeats; and the FBXO family contains other protein-protein interaction domains, such as zinc-finger and proline-rich domains (8, 9). Intracellular protein degradation plays an important role in the regulation of the cell cycle, signal transduction, and disposal of improperly folded proteins. Skp2 (also termed FBXL1) was the first identified F-box protein known to regulate cell cycle signaling by targeting Cdk inhibitor p27 during cell cycle (10). The role of the F-box protein-mediated protein ubiquitination in regulation of NF-κB activation has been well studied. β-Trcp1 and β-Trcp (also termed FBXW1a and FBXW1b; refs. 11, 12) and homologous to Slimb (HOS; refs. 13, 14) target phosphorylated-I-κB and trigger I-κB ubiquitination and degradation in the proteasome, thus inducing NF-κB nuclear translocation and increasing transcriptional activity. In addition to I-κB as a substrate, we have shown that β-Trcp targets cortactin for its ubiquitination and degradation (15). Recently, we demonstrated that an orphan F-box protein, FBXL19, regulates interleukin (IL)-33 signaling by targeting its cognate receptor, ST2L, for ubiquitination, which, in turn, triggers its proteasomal degradation to alter the innate immune response (16).
Rac1 is a member of the RhoGTPase family that regulates numerous cellular functions, including cell migration. Rac1 is activated in a GTP-bound state, but is inactivated when bound to GDP. Rac1 stability has been known to be regulated by 2 different E3 ligases: inhibitors of apoptosis proteins (IAPs) and HACE1. IAPs bind to Rac1 in a guanine nucleotide-independent manner; however, an increased susceptibility of active Rac1 for degradation was observed (17). HACE1 specifically catalyzes the ubiquitination of active Rac1 (18). The role of the SCF E3 ligase in the regulation of Rac1 stability has not yet been revealed. Because of the diverse actions of Rho family GTPases in orchestrating many complex cellular processes within different subcellular compartments, it is likely that Rac1 concentrations are controlled by actions of additional ubiquitin E3 ligase components. Here we show that SCFFBXL19 uniquely targets both the active and inactive forms of Rac1 for ubiquitination and degradation, a process facilitated by AKT that phosphorylates the GTPase. Further, we demonstrate that ectopically expressed FBXL19 reduces Rac1-mediated cell migration. These data suggest a new biological function for FBXL19 in regulating cell motility.
MATERIALS AND METHODS
Cells and reagents
Murine lung epithelial (MLE12) cells [American Type Culture Collection (ATCC), Manassas, VA, USA] were cultured with HITES medium containing 10% FBS and antibiotics at 37°C in 5% CO2. V5 antibody, mammalian expressional plasmid pcDNA3.1D/His-V5-TOPO, and Escherichia coli Top10 competent cells were from Invitrogen (Carlsbad, CA, USA). AKT (11E7), HA tag (29F4), myc tag (9B11), and ubiquitin (P4D1) antibodies were from Cell Signaling Technology (Danvers, MA, USA). Cycloheximide, leupeptin, β-actin antibody, individual FBXL19 shRNAs, and scrambled shRNA were from Sigma-Aldrich (St. Louis, MO, USA). MG-132 was from Calbiochem (La Jolla, CA, USA). Rac1 (C-11) and Rho GDP-dissociation inhibitor (RhoGDI) antibodies, immunobilized protein A/G beads, and control IgG were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). FBXL19 antibody was from Abgent (San Diego, CA, USA). All materials in highest grades used in the experiments are commercially available.
Construction of FBXL19 and Rac1 plasmids
A series of F-box cDNA was cloned using a cDNA library as a template for PCR amplification. The forward primer 5′-CACCATGGGTATGAAAGTCCCCGG-3′ and the reverse primer 5′-GCTGTCCTTGAGAAGCAGCTTC-3′ were used to generate the FBXL19-V5. The resulting PCR products were purified, followed by 1-step cloning into a pcDNA3.1D/V5-His vector. The PCR conditions were as follows: 98°C for 15 s and 35 cycles of 98°C for 15 s, 58°C for 15 s, and 72°C for 30 s. Human FBXL19 cDNAs were also subcloned into a pAcGFP1-C1 vector (Clontech, Mountain View, CA, USA). Site-directed mutagenesis was performed to clone Rac1 lysine or serine mutants according the manufacturer's instructions (Agilent Technologies, Santa Clara, CA, USA).
Immunoblotting and immunoprecipitation
Cells were washed with PBS and collected in cell lysis buffer containing 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2 mM EGTA, 5 mM β-glycerophosphate, 1 mM MgCl2, 1% Triton X-100, 1 mM sodium orthovanadate, 10 μg/ml protease inhibitors, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin. An equal amount of cell lysates (20 μg) was subjected to SDS-PAGE, electrotransferred to membranes, and immunoblotted as described previously (19). For immunoprecipitation, equal amounts of cell lysates (1 mg) were incubated with specific primary antibodies overnight at 4°C, followed by the addition of 40 μl of protein A/G-agarose and incubation for 2 h at 4°C. The immunoprecipitated complex was washed 3 times with 1% Triton X-100 in ice-cold phosphate-buffered saline and analyzed by Western blotting with an enhanced ECL system.
Immunostaining
Cells were plated on 35-mm glass-bottom culture dishes. After treatment, cells were fixed in 3.7% formaldehyde for 20 min, followed by permeabilization with 0.1% Triton X-100 for 2 min. Cells were incubated with a 1:200 dilution of antibodies to Rac1 or V5 tag, followed by a 1:200 dilution of fluorescence-conjugated secondary antibody sequentially for immunostaining. The actin cytoskeleton was stained with fluorescence-conjugated phalloidin. Immunofluorescent cell imaging was performed on a Nikon confocal microscope.
Plasmid transfection by electroporation
MLE12 cells were nucleofected with plasmids. MLE cells (1×106) were suspended in 120 μl of nucleofection buffer and well mixed with 3 μg of plasmid DNA in an electroporation cuvette. Electroporation was performed in the Nucleofection II System (Lonza, Gaithersburg, MD, USA), and the cells were cultured in 2 ml of complete HITES medium for 48 h. shRNA plasmids were also delivered into cells by using nucleofection with the same protocol, and the cells were cultured for 72 h.
Rac1 activity assay
MLE12 cells were cultured in 100-mm dishes, exposed to lysophosphatidic acid (LPA; 10 min), and Rac1 activation was evaluated using the Rac1 Activation Assay Kit (Millipore, Billerica, MA, USA) as per the manufacturer's protocol. Briefly, cell lysates (0.5–1 mg/ml) were loaded with 10 μg of PAK-1 p21-binding domain fusion-protein conjugated to agarose for 1 h to bind Rac1-GTP, centrifuged, and washed 3 times with lysis buffer. The proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with antibodies as indicated. Total cell lysates were also probed separately with anti-Rac1 and anti-β-actin antibodies to confirm equal loading.
In vitro translation of cDNA of Rac1 and FBXL19
In vitro transcription and translation (TnT) was performed using an in vitro translation system from Promega (Madison, WI, USA) according to the manufacturer's instructions. Translated Rac1 and FBXL19 were confirmed by immunoblotting.
In vitro ubiquitin conjugation assay
The ubiquitination of Rac1 and FBXL19 was performed in a reaction mixture containing synthesized substrates, 50 mM Tris (pH 7.6), 5 mM MgCl2, 0.6 mM DTT, 2 mM ATP, 1.5 ng/μl E1, 10 ng/μl Ubc5, 10 ng/μl Ubc7, 1 μg/μl ubiquitin, 1 μM ubiquitin aldehyde, and His-purified recombinant Cullin 1, Skp1, Rbx1 (Boston Biochem, Cambridge, MA, USA), and synthesized F-box proteins from the TnT system. The mixtures were subjected to immunoblotting.
Transwell migration assay
Cell migration was measured by using the transwell invasion assay kit from Trevigen (Gaithersburg, MD, USA). Briefly, 200 μl of MLE12 cells (1×105) in serum-free medium was added to the top chamber, and 500 μl of serum-free medium was added to the bottom chamber. LPA (5 μM) or FBS (1%) was added to top chambers. After 18 h of incubation at 37°C, medium from the top and bottom chambers was aspirated. The cells that had migrated inside the chamber were dissociated with cell dissociation/calcein-AM (calcein acetoxymethyl ester), and the degree of cell invasion was examined using a fluorescence microplate reader with a 488-nm excitation and 520-nm emission wavelength.
Statistics
All results were subjected to statistical analysis using 2-way analysis of variance and, wherever appropriate, analyzed by Student-Newman-Keuls test. Data are expressed as means ± sd of triplicate samples from ≥3 independent experiments; values of P < 0.05 were considered statistically significant.
RESULTS
FBXL19 targets Rac1 for its degradation
The small G protein, Rac1, is a key protein in regulation of cell migration in various cell types, including alveolar epithelial cells (20, 21). Rac1 stability is regulated by polyubiquitination via ubiquitin E3 ligases, such as IAPs and HACE1 (17, 18). Consistently, we were able to confirm that Rac1 is polyubiquitinated in MLE12 cells (Fig. 1A). To test whether an SCF E3 ligase complex contributes to Rac1 stability, we screened F-box E3 ligase subunits from an overexpression library (16, 22). Supplemental Fig. S1A shows that among 6 such F-box proteins, ectopically expressed V5-tagged FBXL19 (FBXL19-V5) decreased immunoreactive Rac1 content. The effect of overexpressed FBXL19-V5 on Rac1 degradation was similar to the effects of overexpressed HACE1 and XIAP (Supplemental Fig. S1B). Further, overexpression of FBXL19 with a V5 tag (FBXL19-V5) degraded Rac1 in a dose-dependent manner (Fig. 1B). FBXL19 shRNA transfection increased endogenous Rac1 protein levels with significant knockdown efficiency (Fig. 1C). FBXL19 expression was detected in both MLE12 cells and human bronchial epithelial cells (Beas2B line; Supplemental Fig. S1C). Overexpression of FBXL19 reduced Rac1 expression in Beas2B cells (Supplemental Fig. S1D), consistent with effects seen in MLE12 cells. Interestingly, overexpression of FBXL19 also reduced levels of Rac2 and Rac3, two related species that have previously not been studied in lung epithelia (Supplemental Fig. S2). To examine the importance of the F-box domain for FBXL19-mediated Rac1 degradation, we overexpressed the FBXL19-V5 and F-box deletion mutant (FBXL19N305-V5) in MLE12 cells. FBXL19-V5, but not FBXL19N305-V5, reduced endogenous Rac1 protein levels (Fig. 1D). To investigate whether FBXL19 regulates endogenous Rac1 stability, cycloheximide (CHX; 20 μg/ml) was used to inhibit new protein synthesis and track protein degradation. Knockdown of FBXL19 attenuated CHX-induced Rac1 degradation; this effect was partly reversed by overexpression of FBXL19-V5 in FBXL19 shRNA-transfected cells (Fig. 1E). To investigate whether FBXL19-mediated Rac1 degradation is associated with its activation state, we overexpressed FBXL19-V5 with Rac1 wild type (WT; Rac1-V5), an inactive form (Rac1N17-V5), or an active form (Rac1V12-V5) in MLE12 cells. Figure 1F shows that FBXL19 reduced all forms of immunoreactive Rac1 regardless of their activity. To further examine whether FBXL19 reduces Rac1 protein levels in an activity-independent manner, Rac1 activity was inhibited by treatment of cells with Rac1 inhibitor or after cellular overexpression of V5-tagged RhoGDIα (RhoGDIα-V5), approaches that increase amounts of inactive Rac1. Cells were also exposed to LPA or transfected with shRhoGDIα plasmid that activates Rac1. Figure 1G, H shows that overexpression of FBXL19-V5 induced degradation of the inactive form of Rac1 in the inhibitor-treated or RhoGDIα-transfected cells. Knockdown of RhoGDIα activates Rac1 and reduces its protein levels (17, 23). Here we observed that overexpression of FBXL19-V5 further degraded the active from of Rac1 in RhoGDIα-knockdown cells (Fig. 1I), as well as in LPA-treated cells (Supplemental Fig. S3A). Further, incubation of Rac1 WT, Rac1-V12, or Rac1-N17 with purified E1, E2, Cullin1, skp1, ubiquitin, ATP, and FBXL19-V5 in vitro showed that FBXL19 induced polyubiquitination of WT, active, and inactive Rac1 (Fig. 1I). Hence, SCFFBXL19 targets Rac1 regardless of its activation state.
Figure 1.
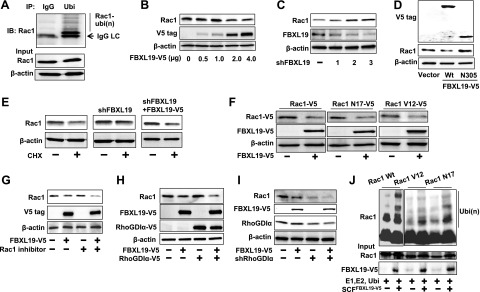
FBXL19 induces Rac1 degradation. A) MLE12 cell lysates (1 mg) were subjected to immunoprecipitation with antibodies to IgG or ubiquitin, followed by immunoblotting with Rac1. Input cell lysates were analyzed for Rac1 and β-actin by immunoblotting. B) MLE12 cells were overexpressed with different amounts of V5-tagged FBXL19 (FBXL19-V5) plasmid. Cell lysates were analyzed for Rac1, V5 tag, and β-actin by immunoblotting. C) MLE12 cells were transfected with 3 distinct FBXL19 shRNA plasmids (lanes 1–3) for 72 h. Rac1, FBXL19, and β-actin expression were analyzed by immunoblotting. D) MLE12 cells were transfected with FBXL19-V5 wild-type (Wt) and FBXL19N305-V5 plasmids. Cell lysates were analyzed for V5 tag, Rac1, and β-actin by immunoblotting. E) MLE12 cells transfected with vector alone, shFBXL19, or shFBXL19 + FBXL19-V5 plasmids were treated with cycloheximide (CHX; 20 μg/ml) for 18 h. Rac1 and β-actin expression were analyzed by immunoblotting. F) MLE12 cells were cotransfected with FBXL19-V5 plasmid and Rac1-V5 or Rac1N17-V5, or Rac1V12-V5 plasmids. Cell lysates were analyzed for V5 tag and β-actin by immunoblotting. G) MLE12 cells were transfected with FBXL19-V5 plasmid, following by adding Rac1 inhibitor (50 nM, 16 h). Cell lysates were analyzed for Rac1, V5 tag, and β–actin by immunoblotting. H) MLE12 cells were transfected with FBXL19-V5 with or without RhoGDIα-V5 plasmids. Cell lysates were analyzed for Rac1, V5 tag, and β-actin by immunoblotting. I) MLE12 cells were transfected with FBXL19-V5 with or without shRhoGDIα plasmids. Cell lysates were analyzed for Rac1, V5 tag, RhoGDIα, and β-actin by immunoblotting. J) FBXL19, Rac1Wt, Rac1V12, and Rac1N17 were synthesized by the TnT system, and in vitro ubiquinations were measured by incubation with E1, E2, ubiquitin, Cullin1, Skp1, and ATP, followed by Rac1 immunoblotting. Input lysates were analyzed for Rac1 and FBXL19 by immunoblotting. Blots are representative from 3 independent experiments.
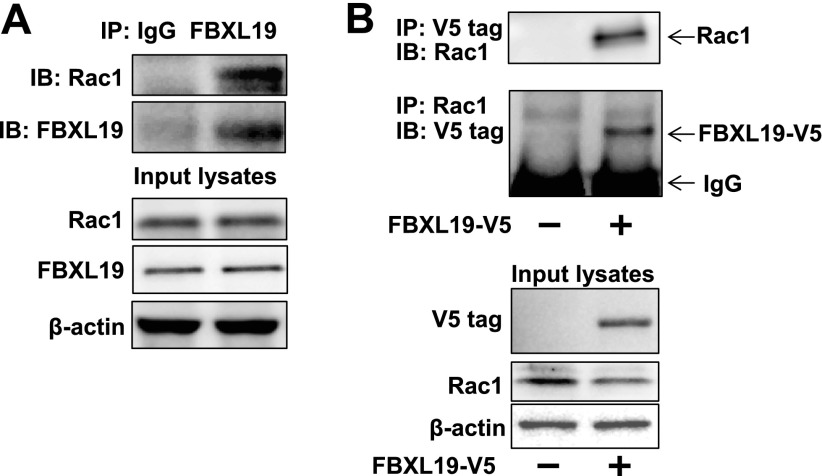
Coimmunoprecipitation (co-IP) with an antibody to Rac1 or FBXL19 revealed that endogenous FBXL19 associates with Rac1 (Fig. 2A). The association between overexpressed FBXL19 and Rac1 was also confirmed by co-IP (Fig. 2B). These results indicate that FBXL19 targets Rac1 to regulate its protein stability.
Figure 2.
FBXL19 interacts with Rac1. A) MLE12 cells were treated with MG-132 (20 μM) for 8 h. Cell lysates (1 mg) were subjected to immunoprecipitation with FBXL19 antibody, followed by Rac1 immunoblotting. Input cell lysates were analyzed for Rac1, FBXL19, and β-actin by immunoblotting. B) FBXL19-V5-overexpressing MLE12 cells were treated with MG-132 (20 μM) for 8 h. Cell lysates were subjected to immunoprecipitation with V5 tag or Rac1 antibody, followed by Rac1 or V5 tag immunoblotting. Input lysates were analyzed by immunoblotting with antibodies to V5 tag, Rac1, and β-actin. Blots are representative from 3 independent experiments.
AKT regulates FBXL-19-mediated Rac1 ubiquitination and degradation
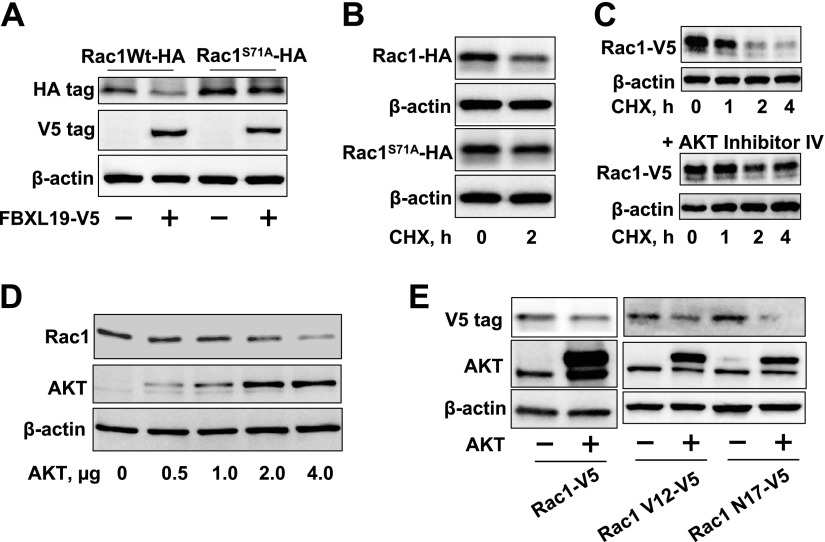
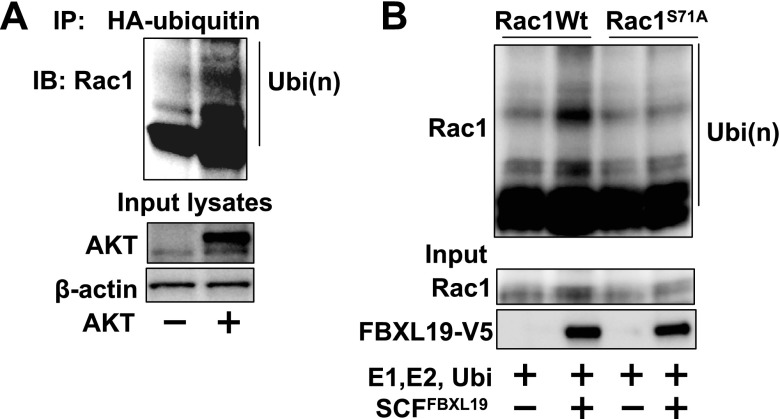
F-box proteins target phosphorylated substrates for their ubiquitination. Rac1 has been known to be phosphorylated at serine71 by AKT (24). To investigate the role of AKT in FBXL19-mediated Rac1 degradation, we cooverexpressed a HA-tagged Rac1 serine71 mutant (Rac1S71A-HA) with FBXL19-V5. Figure 3A shows that unlike Rac1 WT, Rac1S71A-HA was resistant to FBXL19-mediated degradation. Rac1 WT degraded in the presence of CHX, while Rac1S71A-HA (Fig. 3B) or pretreatment with an AKT inhibitor IV (5 μM; Fig. 3C) reduced the rate of Rac1 protein degradation. Further, overexpression of AKT reduced endogenous Rac1 levels in a dose-dependent manner (Fig. 3D). Cotransfection of AKT plasmid with V5-tagged Rac1 WT, or Rac1-V12, or Rac1-N17 plasmids revealed that AKT diminished Rac1 levels in an activity-independent manner (Fig. 3E). To examine whether AKT promotes Rac1 ubiquitination, MLE12 cells were overexpressed with AKT- and HA-tagged ubiquitin, followed by incubation with the proteasome inhibitor, MG-132. Immunoblot analysis of HA-ubiquitin immunoprecipitated proteins with a Rac1 antibody revealed that polyubiquitination of Rac1 was enhanced by overexpression of AKT (Fig. 4A). Incubation of Rac1 WT or Rac1S71A with purified E1, E2, Cullin1, skp1, ubiquitin, ATP, and FBXL19-V5 in vitro showed that FBXL19 induced Rac1 WT, but not Rac1S71A ubiquitination (Fig. 4B); these data suggest that Rac1 is a direct substrate for FBXL19 and that a known consensus AKT phosphorylation site might serve as a molecular signal to regulate FBXL19-mediated Rac1 ubiquitination and degradation.
Figure 3.
Rac1 phosphorylation by AKT regulates FBXL19-mediated Rac1 degradation. A) MLE12 cells were cotransfected with FBXL19-V5 and Rac1Wt-HA or Rac1S71A-HA plasmids. Cell lysates were analyzed for V5 tag, HA tag, and β-actin by immunoblotting. B) MLE12 cells were transfected with Rac1Wt-HA or Rac1S71A-HA plasmid prior to CHX treatment (20 μg/ml, 2 h). Cell lysates were analyzed for HA tag and β-actin immunoblotting. C) MLE12 cells were transfected with Rac1-V5 plasmid and then treated with AKT inhibitor IV (5 μM) prior to CHX treatment (20 μg/ml, 0–4 h). Cell lysates were analyzed for V5 tag and β-actin by immunoblotting. D) MLE12 cells were transfected with AKT plasmid (0–4 μg) for 48 h. Cell lysates were analyzed for Rac1, AKT, and β-actin by immunoblotting. E) MLE12 cells were transfected with Rac1-V5, Rac1V12-V5, Rac1N17-V5 with or without AKT plasmids. Cell lysates were analyzed for V5 tag, AKT, and β-actin by immunoblotting. Blots are representative from 3 independent experiments.
Figure 4.
AKT regulates FBXL19-mediated Rac1 ubiquitination. A) MLE12 cells were cotransfected with HA-ubiquitin and AKT plasmids. Cell lysates were subjected to immunoprecipitation with HA-tag antibody, followed by Rac1 immunoblotting. Input lysates were analyzed for AKT and β-actin by immunoblotting. B) FBXL19, Rac1Wt, and Rac1S71A were synthesized by TnT system, and in vitro ubiquination was measured by incubation with E1, E2, ubiquitin, Cullin1, Skp1, and ATP, followed by Rac1 immunoblotting. Input lysates were analyzed with Rac1 and FBXL19 immunoblotting. Blots are representative blots from 3 independent experiments.
Rac1 lysine166 is the ubiquitin acceptor site for FBXL19
Lysine (K) residues within a target protein are ubiquitin acceptor sites for linking mono- or polyubiquitin. To identify the putative ubiquitin acceptor site within Rac1 in the SCFFBXL19 complex, we substituted several candidate K residues of Rac1 with Arg (R), including K147, which has been reported to be ubiquitinated by the E3 ligase complex IAP (17). Of several mutants tested, only Rac1K166R, not Rac1 WT, Rac1K123R, or Rac1K147R, was resistant to FBXL19-mediated degradation (Fig. 5A). Rac1K166R displayed greater protein stability in response to CHX treatment (Fig. 5B). In vitro ubiquitination assays showed that FBXL19-induced polyubiquitination was reduced using Rac1K166R as a substrate vs. WT Rac1 (Fig. 5C).
Figure 5.
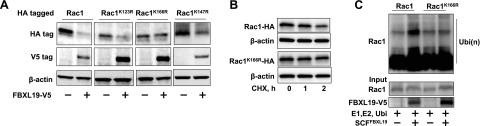
Lysine 166 within Rac1 is the ubiquitin acceptor site for FBXL19. A) MLE12 cells were cotransfected with FBXL19 and Rac1-HA or lysine mutant plasmids. Cell lysates were analyzed for HA tag, V5 tag, and β-actin by immunoblotting. B) MLE12 cells were transfected with Rac1-HA or Rac1K166R-HA plasmid prior to CHX treatment. Cell lysates were analyzed for HA tag and β-actin by immunoblotting. C) FBXL19, Rac1Wt-HA, and Rac1K166R-HA were synthesized by TnT system, and in vitro ubiquitination was measured by incubation with E1, E2, ubiquitin, Cullin1, Skp1, and ATP, followed by HA-tag immunoblotting. Blots are representative from 3 independent experiments.
FBXL19 regulates cell migration
Rac1 plays a critical role in regulating cell migration, in part, by modulating cytoskeletal reorganization. LPA is a potent cell migration stimulator in serum (25, 26). LPA treatment induced Rac1 activity (Supplemental Fig. S3), migratory leading edge formation (Fig. 6D and Supplemental Fig. S4), and Rac1 relocalization to the leading edge (Supplemental Fig. S4) in MLE12 cells. Down-regulation of Rac1 by transfection with Rac1 shRNA attenuated LPA-induced leading edge formation and MLE12 cell migration (Supplemental Fig. S4). Overexpression of FBXL19 reduced both total Rac1 and active Rac1 levels, while down-regulation of FBXL19 exhibited opposite effects (Supplemental Fig. S3). We hypothesized that FBXL19 reduces the Rac1 level, thus inhibiting cell migration. To investigate this, we examined cell migration using a transwell cell migration assay. Ectopically expressed FBXL19 attenuated cell migration both under baseline conditions and after the LPA treatment (Fig. 6A), while LPA-induced cell migration was enhanced in FBXL19 shRNA-transfected cells (Fig. 6B). Overexpression of FBXL19 reduced LPA-mediated cell migration in Rac1 WT-overexpressing cells, but it had a limited effect on LPA-mediated cell migration in Rac1K166R-overexpressing cells (Fig. 6C). Further, overexpression of FBXL19-V5 abolished LPA-induced migratory leading edge formation and Rac1 relocalization to the leading edge, a process central to epithelial cell mobility (Fig. 6D and Supplemental Fig. S4). Consistent with these findings, FBS (1%)-induced migration-related leading edge formation was attenuated in FBXL19-V5-overexpressing cells (Fig. 6D). These results suggest that FBXL19 regulates cell migration via degradation of Rac1.
Figure 6.
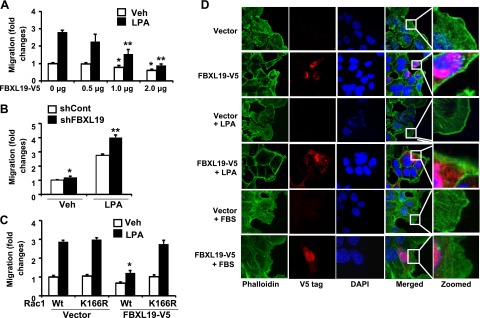
FBXL19 regulates cell migration. A) MLE12 cells transfected with FBXL19-V5 plasmids (0.5–2 μg) for 24 h were treated with LPA (5 μM). Cell migration was measured at 18 h by a transwell migration assay. *P < 0.05 vs. FBXL19-V5 (0 μg)-transfected cells; **P < 0.01 for ratio between vehicle (veh) and LPA vs. FBXL19 (0 μg)-transfected cells. B) FBXL19 shRNA-transfected MLE12 cell migration in presence of LPA (5 μM) was measured by a transwell migration assay. *P < 0.01 vs. veh in vector-transfected cells; **P < 0.01 for ratio between shCont and shFBXL19 vs. veh cells. C) MLE12 cells were cotransfected with FBXL19-V5 and Rac1Wt-HA or Rac1K166R-HA plasmid. Cell migration was measured by a transwell migration assay in presence of LPA (5 μM). *P < 0.01 vs. veh in vector transfected cells. D) MLE12 cells transfected with FBXL19-V5 or vector plasmid were subjected to a scratch and then incubated with LPA (5 μM) or FBS (1%) for 1 h. Cells were fixed, and actin filaments were stained with phalloidin (green), FBXL19-V5 were stained with V5 tag (red), and nuclei were stained with DAPI (blue). Images are representative from 3 independent experiments.
DISCUSSION
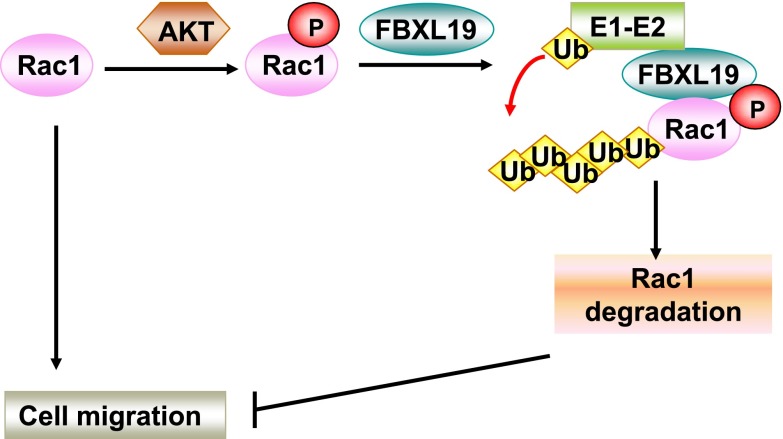
Rac1, a member of the small G-protein Rho family, triggers intracellular signaling and regulates important biological functions, such as cytoskeletal reorganization and cell motility. The elucidation of mechanisms for control of Rac1 protein stability therefore might have important implications for devising interventions that affect neoplasia, injury and repair, and cellular homeostasis. Here Rac1 life span is regulated by the SCFFBXL19 E3 ligase complex, and Rac1 represents the second substrate for FBXL19. While the ability of FBXL19 to target ST2L for its ubiquitination and degradation is mediated by glycogen synthase kinase β (16), Rac1 ubiquitination and degradation by the F-box protein was achieved by an AKT-mediated phosphorylation-dependent mechanism that effectively reduced cell migration (Fig. 7). A unique finding here is that unlike other ubiquitin E3 ligase complexes that selectively target Rac1 active species, FBXL19 displayed activities against both active and inactive forms of the GTPase to modulate cell motility. Thus, FBXL19 might serve as an endogenous feedback inhibitor or in a redundant capacity to restrict global cell migratory activity.
Figure 7.
FBXL19 regulates Rac1 stability and cell migration. Rac1 ubiquitination and stability are regulated by SCFFBXL19. Phosphorylation of Rac1 by AKT serves as a phospho-signal for FBXL19 associating with Rac1. Overexpression of FBXL19 reduces Rac1 level, thereby inhibiting cell migration.
Ubiquitination is an essential step for protein degradation in the proteasome system (4, 27). Linking ubiquitin to its target protein requires the ubiquitin ligase complex. As the major component of the complex, F-box proteins target intracellular signaling proteins to mediate its stability, thereby regulating biological functions, such as cell cycle regulation (9, 28), transcriptional activities (12, 29, 30), and lipid synthesis (31, 32). We have shown that FBXL19 targets IL-33 receptor ST2L for its polyubiquitination and turnover within the proteasome system (16). Here we reveal that Rac1 is a new substrate for FBXL19. Rac1-mediated cell polarization through lamellipodia formation is the initial step for cell migration. Inactivation or knockdown of Rac1 significantly reduces the cell migration rate in various cell types (33, 34), and these results indicate that FBXL19 recapitulates the effect of Rac1 silencing. Consistent with the behavior of the E3 ligases IAPs (17) and HACE1 (18) to trigger Rac1 ubiquitination, SCFFBXL19 also targets Rac1 for its ubiquitination and degradation. Interestingly, unlike IAPs and HACE1, SCFFBXL19 induces ubiquitination and degradation of both active and inactive forms of Rac1. Future studies will determine the FBXL19 docking site within Rac1. IAPs (17) and HACE1 (18) catalyze ubiquitination of the Rac1 at lysine147, while here SCFFBXL19 induces ubiquitination of Rac1 at lysine166. This acceptor site is distant from either the GTP binding or NADPH oxidase formation sites and resides within a c-terminal α-helix distinct from the polybasic tail (35). It is possible that polyubiquitination within this α-helix affects membrane association; however, this requires additional investigation. Further, the observation that Rac1K147R mutant is still degraded by FBXL19 suggests that different E3 ligases transfer ubiquitin to Rac1 at different lysine sites. Future studies will focus on the balance of the role of these E3 ligases on Rac1 degradation under various stimulatory conditions.
The F-box proteins target phosphorylated substrates (36). For example, SCFβ-Trcp increases ubiquitination of I-κB dependent on I-κB phosphorylation by I-κB kinases (11, 29). AKT is a serine/threonine protein kinase that plays a key role in cellular responses, such as proliferation (37) and cell motility (38). Rac1 is phosphorylated by AKT (24). The role of phosphorylation of Rac1 by AKT in IAP- or HACE1-mediated Rac1 degradation has not yet been studied. Here Rac1 phosphorylation by AKT promotes FBXL19-mediated Rac1 degradation. The AKT phosphorylation site serine71 within Rac1 also serves as a key molecular phospho-degron signal for FBXL19 targeting Rac1, as the Rac1 point mutant (S71A) exhibited greater stability and reduced polyubiquitination compared to WT Rac1. These data are consistent with the previous findings that AKT regulates several proteins for ubiquitination and degradation, including tumor-suppressor merlin (39), androgen receptor (40), and p53 (41).
The role of SCF-based components in regulation of cell motility has not been well studied. We have shown that SCFβ-Trcp reduces cell migration by targeting cortactin for degradation (15). Both Rac1 inhibitors, IAP and HACE1, reduce cell migration by degrading the active form of Rac1 (17, 18). Here FBXL19 inhibits cell migration by inducing Rac1 ubiquitination and degradation independent on Rac1 activity. The interaction between HACE1 and Rac1 is enhanced by hepatocyte growth factor or cytotoxic necrotizing factor-1 (CNF-1), while the association between FBXL19 and Rac1 is not affected by LPA treatment. The Rac1K166R mutant, a nonubiquitylatable Rac1, is resistant to SCFFBXL19-mediated degradation and resists inhibitory effects of the F-box protein on cell migration. Thus, SCFFBXL19 inhibition on cell migratory behavior is partly dependent on Rac1 degradation. The results indicate that in addition to inhibition of cytokine receptor signaling, FBXL19 functions as an antagonist of cell migration through its ability to reduce Rac1 levels. IAP regulation of cell migration involves not only targeting Rac1, but also regulating C-RAF kinase stability (42). The ability of an F-box protein to target multiple substrates is not unusual, given that other components, such as SCFFBXW7, induce polyubiquitination and degradation of Notch (30), cyclin E (43), c-Myc (44, 45), and c-Jun (30). The identification of additional substrates for FBXL19 will be important to understand the mechanistic behavior of this protein comprehensively in biologically relevant cellular responses.
The current study unveils a new molecular target for FBXL19 that may affect cell motility. Rac1 is a major intracellular signaling protein contributing to various cellular responses, such as cytokine release, oxidant generation, and cell motility. Thus, it is likely that SCF components may exert divergent actions on several fundamental processes that regulate cell and tissue homeostasis. Future studies will examine the role of FBXL19 in Rac1-mediated immune responses and will focus on high-throughput small molecule screening of novel FBXL19 peptide mimics to manipulate cell motility.
Supplementary Material
Acknowledgments
This study was supported by U.S. National Institutes of Health grants RO1 HL01916 and HL112791 (to Y.Z.), HL097376 and HL098174 (to R.K.M.), and HL116472 (to B.B.C.); American Heart Association awards 12SDG9050005 (to J.Z.) and 12SDG12040330 (to C.Z.); and a Merit Review Award from the United States (to R.K.M.).
The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- CHX
- cycloheximide
- co-IP
- coimmunoprecipitation
- IAP
- inhibitor of apoptosis proteins
- IL
- interleukin
- LPA
- lysophosphatidic acid
- LRR
- leucine-rich repeat
- MLE
- mouse lung epithelial
- RhoGDI
- Rho GDP-dissociation inhibitor
- SCF
- Skp1-Cul1-F-box
- TnT
- transcription and translation
- WT
- wild type
REFERENCES
- 1. Bhoj V. G., Chen Z. J. (2009) Ubiquitylation in innate and adaptive immunity. Nature 458, 430–437 [DOI] [PubMed] [Google Scholar]
- 2. Brooks C. L., Gu W. (2003) Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr. Opin. Cell Biol. 15, 164–171 [DOI] [PubMed] [Google Scholar]
- 3. Gronroos E., Hellman U., Heldin C. H., Ericsson J. (2002) Control of Smad7 stability by competition between acetylation and ubiquitination. Mol. Cell 10, 483–493 [DOI] [PubMed] [Google Scholar]
- 4. Pickart C. M., Eddins M. J. (2004) Ubiquitin: structures, functions, mechanisms. Biochim. Biophys. Acta 1695, 55–72 [DOI] [PubMed] [Google Scholar]
- 5. Li M., Luo J., Brooks C. L., Gu W. (2002) Acetylation of p53 inhibits its ubiquitination by Mdm2. J. Biol. Chem. 277, 50607–50611 [DOI] [PubMed] [Google Scholar]
- 6. Willems A. R., Goh T., Taylor L., Chernushevich I., Shevchenko A., Tyers M. (1999) SCF ubiquitin protein ligases and phosphorylation-dependent proteolysis. Philos. Trans. R. Soc. Lond. 354, 1533–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zheng N., Schulman B. A., Song L., Miller J. J., Jeffrey P. D., Wang P., Chu C., Koepp D. M., Elledge S. J., Pagano M., Conaway R. C., Conaway J. W., Harper J. W., Pavletich N. P. (2002) Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature 416, 703–709 [DOI] [PubMed] [Google Scholar]
- 8. Ho M. S., Tsai P. I., Chien C. T. (2006) F-box proteins: the key to protein degradation. J. Biomed. Sci. 13, 181–191 [DOI] [PubMed] [Google Scholar]
- 9. Craig K. L., Tyers M. (1999) The F-box: a new motif for ubiquitin dependent proteolysis in cell cycle regulation and signal transduction. Prog. Biophys. Mol. Biol. 72, 299–328 [DOI] [PubMed] [Google Scholar]
- 10. Tsvetkov L. M., Yeh K. H., Lee S. J., Sun H., Zhang H. (1999) p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr. Biol. 9, 661–664 [DOI] [PubMed] [Google Scholar]
- 11. Spencer E., Jiang J., Chen Z. J. (1999) Signal-induced ubiquitination of IkappaBalpha by the F-box protein Slimb/beta-TrCP. Genes Dev. 13, 284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu C., Ghosh S. (1999) beta-TrCP mediates the signal-induced ubiquitination of IkappaBbeta. J. Biol. Chem. 274, 29591–29594 [DOI] [PubMed] [Google Scholar]
- 13. Tan P., Fuchs S. Y., Chen A., Wu K., Gomez C., Ronai Z., Pan Z. Q. (1999) Recruitment of a ROC1-CUL1 ubiquitin ligase by Skp1 and HOS to catalyze the ubiquitination of I kappa B alpha. Mol. Cell 3, 527–533 [DOI] [PubMed] [Google Scholar]
- 14. Fuchs S. Y., Chen A., Xiong Y., Pan Z. Q., Ronai Z. (1999) HOS, a human homolog of Slimb, forms an SCF complex with Skp1 and Cullin1 and targets the phosphorylation-dependent degradation of IkappaB and beta-catenin. Oncogene 18, 2039–2046 [DOI] [PubMed] [Google Scholar]
- 15. Zhao J., Wei J., Mialki R., Zou C., Mallampalli R. K., Zhao Y. (2012) Extracellular signal-regulated kinase (ERK) regulates cortactin ubiquitination and degradation in lung epithelial cells. J. Biol. Chem. 287, 19105–19114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao J., Wei J., Mialki R. K., Mallampalli D. F., Chen B. B., Coon T., Zou C., Mallampalli R. K., Zhao Y. (2012) F-box protein FBXL19-mediated ubiquitination and degradation of the receptor for IL-33 limits pulmonary inflammation. Nat. Immunol. 13, 651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oberoi T. K., Dogan T., Hocking J. C., Scholz R. P., Mooz J., Anderson C. L., Karreman C., Meyer zu Heringdorf D., Schmidt G., Ruonala M., Namikawa K., Harms G. S., Carpy A., Macek B., Koster R. W., Rajalingam K. (2012) IAPs regulate the plasticity of cell migration by directly targeting Rac1 for degradation. EMBO J. 31, 14–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torrino S., Visvikis O., Doye A., Boyer L., Stefani C., Munro P., Bertoglio J., Gacon G., Mettouchi A., Lemichez E. (2011) The E3 ubiquitin-ligase HACE1 catalyzes the ubiquitylation of active Rac1. Dev. Cell 21, 959–965 [DOI] [PubMed] [Google Scholar]
- 19. Zhao Y., He D., Saatian B., Watkins T., Spannhake E. W., Pyne N. J., Natarajan V. (2006) Regulation of lysophosphatidic acid-induced epidermal growth factor receptor transactivation and interleukin-8 secretion in human bronchial epithelial cells by protein kinase Cdelta, Lyn kinase, and matrix metalloproteinases. J. Biol. Chem. 281, 19501–19511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Desai L. P., Aryal A. M., Ceacareanu B., Hassid A., Waters C. M. (2004) RhoA and Rac1 are both required for efficient wound closure of airway epithelial cells. Am. J. Physiol. 287, L1134–L1144 [DOI] [PubMed] [Google Scholar]
- 21. Wang Y., Wang A., Wang F., Wang M., Zhu M., Ma Y., Wu R. (2008) IQGAP1 activates Tcf signal independent of Rac1 and Cdc42 in injury and repair of bronchial epithelial cells. Exp. Mol. Pathol. 85, 122–128 [DOI] [PubMed] [Google Scholar]
- 22. Chen B. B., Glasser J. R., Coon T. A., Mallampalli R. K. (2011) FBXL2 is a ubiquitin E3 ligase subunit that triggers mitotic arrest. Cell Cycle 10, 3487–3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boulter E., Garcia-Mata R., Guilluy C., Dubash A., Rossi G., Brennwald P. J., Burridge K. (2010) Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat. Cell Biol. 12, 477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kwon T., Kwon D. Y., Chun J., Kim J. H., Kang S. S. (2000) Akt protein kinase inhibits Rac1-GTP binding through phosphorylation at serine 71 of Rac1. J. Biol. Chem. 275, 423–428 [DOI] [PubMed] [Google Scholar]
- 25. Zhao J., He D., Berdyshev E., Zhong M., Salgia R., Morris A. J., Smyth S. S., Natarajan V., Zhao Y. (2011) Autotaxin induces lung epithelial cell migration through lysoPLD activity-dependent and -independent pathways. Biochem. J. 439, 45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gustin C., Van Steenbrugge M., Raes M. (2008) LPA modulates monocyte migration directly and via LPA-stimulated endothelial cells. Am. J. Physiol. Cell Physiol. 295, C905–C914 [DOI] [PubMed] [Google Scholar]
- 27. Zolk O., Schenke C., Sarikas A. (2006) The ubiquitin-proteasome system: focus on the heart. Cardiovasc. Res. 70, 410–421 [DOI] [PubMed] [Google Scholar]
- 28. Vodermaier H. C. (2004) APC/C and SCF: controlling each other and the cell cycle. Curr. Biol. 14, R787–796 [DOI] [PubMed] [Google Scholar]
- 29. Tanaka K., Kawakami T., Tateishi K., Yashiroda H., Chiba T. (2001) Control of IkappaBalpha proteolysis by the ubiquitin-proteasome pathway. Biochimie (Paris) 83, 351–356 [DOI] [PubMed] [Google Scholar]
- 30. Hoeck J. D., Jandke A., Blake S. M., Nye E., Spencer-Dene B., Brandner S., Behrens A. (2010) Fbw7 controls neural stem cell differentiation and progenitor apoptosis via Notch and c-Jun. Nat. Neurosci. 13, 1365–1372 [DOI] [PubMed] [Google Scholar]
- 31. Chen B. B., Coon T. A., Glasser J. R., Mallampalli R. K. (2011) Calmodulin antagonizes a calcium-activated SCF ubiquitin E3 ligase subunit, FBXL2, to regulate surfactant homeostasis. Mol. Cell. Biol. 31, 1905–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zou C., Butler P. L., Coon T. A., Smith R. M., Hammen G., Zhao Y., Chen B. B., Mallampalli R. K. (2011) LPS impairs phospholipid synthesis by triggering beta-transducin repeat-containing protein (beta-TrCP)-mediated polyubiquitination and degradation of the surfactant enzyme acyl-CoA: lysophosphatidylcholine acyltransferase I (LPCAT1). J. Biol. Chem. 286, 2719–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ten Klooster J. P., Leeuwen I., Scheres N., Anthony E. C., Hordijk P. L. (2007) Rac1-induced cell migration requires membrane recruitment of the nuclear oncogene SET. EMBO J. 26, 336–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Filippi M. D., Szczur K., Harris C. E., Berclaz P. Y. (2007) Rho GTPase Rac1 is critical for neutrophil migration into the lung. Blood 109, 1257–1264 [DOI] [PubMed] [Google Scholar]
- 35. Hirshberg M., Stockley R. W., Dodson G., Webb M. R. (1997) The crystal structure of human rac1, a member of the rho-family complexed with a GTP analogue. Nat. Struct. Biol. 4, 147–152 [DOI] [PubMed] [Google Scholar]
- 36. Skowyra D., Craig K. L., Tyers M., Elledge S. J., Harper J. W. (1997) F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 91, 209–219 [DOI] [PubMed] [Google Scholar]
- 37. Lawlor M. A., Alessi D. R. (2001) PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 114, 2903–2910 [DOI] [PubMed] [Google Scholar]
- 38. Kim D., Kim S., Koh H., Yoon S. O., Chung A. S., Cho K. S., Chung J. (2001) Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 15, 1953–1962 [DOI] [PubMed] [Google Scholar]
- 39. Tang X., Jang S. W., Wang X., Liu Z., Bahr S. M., Sun S. Y., Brat D., Gutmann D. H., Ye K. (2007) Akt phosphorylation regulates the tumour-suppressor merlin through ubiquitination and degradation. Nat. Cell Biol. 9, 1199–1207 [DOI] [PubMed] [Google Scholar]
- 40. Lin H. K., Wang L., Hu Y. C., Altuwaijri S., Chang C. (2002) Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 21, 4037–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ogawara Y., Kishishita S., Obata T., Isazawa Y., Suzuki T., Tanaka K., Masuyama N., Gotoh Y. (2002) Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J. Biol. Chem. 277, 21843–21850 [DOI] [PubMed] [Google Scholar]
- 42. Dogan T., Harms G. S., Hekman M., Karreman C., Oberoi T. K., Alnemri E. S., Rapp U. R., Rajalingam K. (2008) X-linked and cellular IAPs modulate the stability of C-RAF kinase and cell motility. Nat. Cell Biol. 10, 1447–1455 [DOI] [PubMed] [Google Scholar]
- 43. Klotz K., Cepeda D., Tan Y., Sun D., Sangfelt O., Spruck C. (2009) SCF(Fbxw7/hCdc4) targets cyclin E2 for ubiquitin-dependent proteolysis. Exp. Cell Res. 315, 1832–1839 [DOI] [PubMed] [Google Scholar]
- 44. Welcker M., Orian A., Grim J. E., Eisenman R. N., Clurman B. E. (2004) A nucleolar isoform of the Fbw7 ubiquitin ligase regulates c-Myc and cell size. Curr. Biol. 14, 1852–1857 [DOI] [PubMed] [Google Scholar]
- 45. Welcker M., Orian A., Jin J., Grim J. E., Harper J. W., Eisenman R. N., Clurman B. E. (2004) The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl. Acad. Sci. U. S. A. 101, 9085–9090 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.