Abstract
HSV triggers intracellular calcium release to promote viral entry. We hypothesized that Akt signaling induces the calcium responses and contributes to HSV entry. Exposure of human cervical and primary genital tract epithelial, neuronal, or keratinocyte cells to HSV serotype 2 resulted in rapid phosphorylation of Akt. Silencing of Akt with small interfering RNA prevented the calcium responses, blocked viral entry, and inhibited plaque formation by 90% compared to control siRNA. Susceptibility to infection was partially restored if Akt was reintroduced into silenced cells with an Akt-expressing plasmid. HSV-2 variants deleted in glycoproteins B or D failed to induce Akt phosphorylation, and coimmunoprecipitation studies indicated that Akt interacts with glycoprotein B. Cell-surface expression of Akt was rapidly induced in response to HSV exposure. Miltefosine (50 μM), a licensed drug that blocks Akt phosphorylation, inhibited HSV-induced calcium release, viral entry, and plaque formation following infection with acyclovir-sensitive and resistant clinical isolates. Miltefosine blocked amplification of HSV from explanted ganglia to epithelial cells; viral yields were significantly less in miltefosine compared to control-treated cocultures (P<0.01). Together, these findings identify a novel role for Akt in viral entry, link Akt and calcium signaling, and suggest a new target for HSV treatment and suppression.—Cheshenko, N., Trepanier, J. B., Stefanidou, M., Buckley, N., Gonzalez, P., Jacobs, W., and Herold, B. C. HSV activates Akt to trigger calcium release and promote viral entry: novel candidate target for treatment and suppression.
Keywords: glycoprotein B, glycoprotein D, miltefosine, genital herpes
Herpes simplex viruses (HSVs) type 1 and type 2 are a major global health problem, the leading cause of genital ulcerative disease and neonatal and sporadic infectious encephalitis, and a major cofactor for HIV infection (1). Acyclovir treatment reduces the morbidity and mortality associated with encephalitis, and suppressive therapy with acyclovir or its prodrug, valacyclovir, reduces mucocutaneous recurrences and the risk of transmission (2). However, acyclovir resistance is a problem in some populations, and there is no effective vaccine (3). Thus, novel approaches to prevent, suppress, and treat HSV infection are needed (4). Development of new strategies requires an understanding of the cellular and molecular events required for infection.
HSV entry is complex, and both serotypes (HSV-1 and HSV-2) require the concerted activities of the envelope glycoproteins D and B (gD and gB) and heterooligomers of gH and gL (gH-gL) (5, 6). However, the precise role each plays and the cellular pathways that mediate entry are not fully understood. In prior studies, we demonstrated that both serotypes trigger a rapid increase in the intracellular calcium concentration ([Ca2+]) in human epithelial and neuronal cells and that blockade of this response prevents viral entry (7). Specifically, the increase in [Ca2+] and viral entry was blocked following chelation of intracellular (but not extracellular) Ca2+; treatment with the inositol triphosphate receptor (IP3R) antagonist, 2-aminoethoxydiphenyl borate; or silencing of IP3R with small interfering RNA (siRNA) (7, 8). Two spatially distinct Ca2+ responses were subsequently identified by confocal microscopy, one at the plasma membrane and the other a global cytosolic response (8).
Using siRNA strategies to silence cellular proteins in CaSki cells (cervical epithelial cell line) and HSV variants deleted in the essential envelope glycoproteins (gB-2, gD-1 and gH-1) (7, 8), we found that binding of viral particles to syndecan-2, a major family of heparan sulfate proteoglycans, and engagement of the gD coreceptor, nectin-1, triggered the release of a small amount of Ca2+ near the plasma membrane, which may initiate the entry process. However, completion of the entry process and intracellular delivery of viral capsids required the release of global intracellular Ca2+ stores, activation of IP3Rs, and the full complement of essential viral envelope glycoproteins (gB, gD, and gH-gL). The IP3Rs are located predominantly in the endoplasmic reticulum (ER) and are the major components of the cytosolic Ca2+ regulatory machinery. Confocal studies demonstrated that silencing of IP3Rs resulted in virus being trapped within the plasma membrane (8). Together, these findings suggest a central role for Ca2+ in promoting HSV entry. However, the initial signals at the cell surface that trigger the plasma membrane Ca2+ response and how it is linked to the release of IP3R-regulated ER Ca2+ stores have not been delineated.
Two signaling pathways are involved in the intracellular generation of inositol phosphates (9). The first is initiated by one of several phosphoinositide-specific phospholipase C (PLC) proteins, which trigger the hydrolysis of phosphatidylinositol-4,5-bisphosphate to release the second messengers, diacylglycerol and IP3. IP3 binds to its receptors, located primarily on the ER, to activate Ca2+ release, which is characterized by temporal oscillations and propagating waves (10). The second signaling pathway is initiated by phosphoinositide 3-kinase (PI3K), an enzyme that phosphorylates inositol lipids generating phosphatidylinositol 3,4-bisphosphate (PIP2) and phosphatidylinositol 3,4,5-trisphosphate (PIP3) (11). These molecules play critical roles in regulating the serine/threonine protein kinase, Akt (also called protein kinase B). Akt possesses a pleckstrin homology (PH) domain, which when bound by PIP3, becomes anchored at the plasma membrane, where it may be activated in response to phosphorylation by cellular kinases or following autophosphorylation at the Ser-473 site (12–15). Activated Akt, in turn, phosphorylates a number of key substrates involved in the promotion of cell survival, proliferation, and growth. Recent studies demonstrated that the IP3Rs are one of the substrates activated by Akt and that Akt potentiates IP3R-mediated Ca2+ release (16). Thus, we hypothesized that Akt might participate in the early cellular Ca2+ response to HSV.
MATERIALS AND METHODS
Cells and viruses
CaSki [human cervical epithelial cell line; CRL-1550; American Type Culture Collection (ATTC), Manassas, VA, USA] and Vero (ATCC CCL-81) cells were passaged in DMEM supplemented with 10% fetal bovine serum (FBS); SK-N-SH (human neuroblastoma cell line; ATCC HTB11) cells were passaged in DMEM-F12 supplemented with 10% FBS; HaCAT (human keratinocyte cell line; CLS 300493) cells were propagated in DMEM supplemented with 10% FBS (17). Primary genital tract epithelial cells were isolated from cervical tissue obtained from women undergoing hysterectomy for benign conditions, as described previously (18). The laboratory isolates HSV-2(G), HSV-2(G)gC-2-deletion virus (ΔgC; ref. 19), and HSV-1(KOS),HSV-1(KVP26GFP), which contains a green fluorescent protein (GFP)-VP26 fusion protein (20), and HSV-1(F-GS2822), which encodes the red fluorescent protein 1 fused to the N terminus of VP26 (ref. 21; gift from Greg Smith, Northwestern University, Evanston, IL, USA), were propagated on Vero cells, and viral stocks were stored at −80°C. The HSV-2 clinical isolates 4674, DT1 (acyclovir susceptible), and DT2 (acyclovir resistant) were propagated on CaSki cells (22, 23). Stocks of ΔgD-HSV-2, a gD-2-deletion virus constructed from HSV-2(4674), as described below, were propagated on complementing VD60 cells (24) and stocks of HSV-2(G)ΔgB2, a previously described gB-2-deletion virus, were propagated on VgB2 cells (25). One passage of each of the deletion viruses through Vero cells yields glycoprotein-negative virions. The complemented and noncomplemented viruses were purified on dextran gradients, and relative particle numbers were determined by performing Western blots and probing with antibody to the viral capsid protein, VP5 (8). HSV-2(G) was inactivated by exposure to UV light at a distance of 10 cm from the light source for 8 min (26).
Construction of a HSV-2 gD-deletion virus
Plasmid pcDNA3-eGFP (13031; Addgene, Cambridge, MA, USA) was used as a template to PCR amplify the pCMV-eGFP-Neor and OriE-Ampr regions flanked by Van91I restriction enzyme sites. The pCMV-eGFP-Neor region was PCR-amplified using primers Fwd-pCMV and Rev-NeoR-Term (See Supplemental Table S1 for a list of primers). The OriE-Ampr region was PCR amplified using primers Fwd-Origin and Rev-AmpR. In parallel, genomic regions flanking the left and right of the US6 gene (gD) in HSV-2 were PCR amplified using purified viral DNA (HSV-2 strain 4674) as a template and primers LL-V91I-US6 plus LR-V91I-US6 for the left homology arm and primers RL-V91I-US6 and RR-V91I-US6 for the right homology arm. All four PCR fragments were gel purified, digested with Van91I (Fermentas Molecular Biology Tools, Thermo Scientific, West Palm Beach, FL, USA), ligated with Quick-Ligase [New England Biolabs (NEB), Ipswich, MA, USA], and transformed into NEB 5-α competent cells. The resulting plasmid (eKO2-US6) was sequence verified and extracted from E. coli using an endotoxin-free miniprep kit (MO-BIO Laboratories, Carlsbad, CA, USA). HSV-2 DNA (1 μg) was cotransfected with 100 ng of eKO2-US6 into VD60 cells using Effectene (Qiagen, Valencia, CA, USA), according to manufacturer recommendations. At 4 d after transfection, plates were screened for green plaques (Supplemental Fig. S1A), and supernatants were collected and overlaid on fresh VD60 cells for 1 h, then washed and covered with 4% low-melting agarose prepared in Optimem (Invitrogen, Carlsbad, CA, USA). Single green fluorescent plaques were picked and purified 3 times using this method. Viral stocks were grown on VD60 cells, and noncomplemented virus was generated by harvesting infected cell lysates from Vero cells, as described for HSV-1gD-deletion viruses (24).
Genotypic confirmation of the gD deletion in ΔgD-HSV-2 was performed by PCR (Supplemental Fig. S1B). A primer set was used to confirm the presence of wild-type (WT) and ΔgD virus DNA in the samples (primers RL-V91I-US6 and RL-V91I-US6), while another set of primers (Neo-Out and US8-Out) was used to amplify a DNA region comprising eK02-US6 and the genomic target region. To confirm deletion of gD expression, Vero or VD60 cells were infected with parental WT or ΔgD virus (grown on VD60 cells and thus competent for entry) at a multiplicity of infection (MOI) of 10 plaque-forming units (PFU)/cell (based on VD60 titer). After 1-h incubation, cells were washed twice with PBS, incubated in Optimem for 48 h at 37°C, and harvested and evaluated for gD expression by Western blot by loading 50 μg protein/well for SDS-PAGE. Proteins were transferred onto a PVDF membrane, blocked with 5% nonfat milk in PBS, and incubated with a mAb to either gB (H1359; Virusys Corp., Taneytown, MD, USA) or gD (H170, Virusys) overnight at 4°C. Membranes were washed and incubated with a secondary horseradish perioxidase-conjugated anti-mouse IgG Ab (GE Healthcare Life Sciences, Pittsburgh, PA, USA). A chemiluminescent substrate (Western lightening ECL; Perkin Elmer, Waltham, MA, USA) was then added to the membrane to visualize bands (Supplemental Fig. S1C).
siRNA, plasmids, and transfections
Cells (CaSki, HaCAT, SK-N-SH, or primary) were transfected with siRNA sequences (final concentration 10 nM) in 12-well plates. The transfections were performed with the Effectene transfection reagent (Qiagen), according to the manufacturer's protocol, using human Akt1 siRNA (633), Akt2 siRNA (103305), Akt3 siRNA (110901), or a cocktail of siAkt1, siAkt2, and siAkt3 (3.3 nM each) and control siRNA (AM4636; Applied Biosystems, Foster City, CA, USA). To reintroduce Akt1, siRNA-treated cells were transfected with 0.3 μg of pCMV-AC-GFP plasmid expressing Akt1 fused to GFP (RG201850; OriGene, Rockville, MD, USA) or a control plasmid expressing only GFP (pS100010; OriGene). Transfections with plasmids were performed 72 h after the introduction of siRNA.
Viral labeling and purification
Vero cells were infected with KVP26GFP (GFP labeled; ∼0.001 PFU/cell) and after 48 h, the infected cells were lysed and, if indicated, incubated with the lypophilic tracer DiI (1 μM; Invitrogen Molecular Probes) for 10 min at room temperature before purification on sucrose gradients, as described previously (8). Titers of the purified viruses were determined by plaque assays.
Western blots of Akt phosphorylation
Cells were serum starved for 24 h and then exposed to live or UV-inactivated virus (or medium as a mock-infection control), and at different times after viral exposure, the cells were harvested and lysed in buffer containing 20 mM Tris (pH 7.5), 50 mM NaCl, 1% Nonidet P-40, and 0.05% DOC supplemented with fresh protease and phosphatase inhibitors (118735, Roche Diagnostics, Indianapolis, IN, USA; and P0044, P5726, Sigma-Aldrich, St. Louis, MO, USA, respectively). Proteins were separated by SDS-PAGE and were transferred to membranes for immunoblotting with the indicated antibodies. Membranes were stripped between antibodies by incubating the membranes in Restore Western blot stripping buffer (21059; Thermo Scientific) for 10 min at room temperature and washing 3 times in TBS-Tween. Blots were scanned, and the band intensities were analyzed using the GelDoc2000 system (Bio-Rad, Hercules, CA, USA).
Antibodies and chemical reagents
Primary antibodies and dilutions were as follows: mouse anti-VP16 mAb, 1:500 (sc7545; Santa Cruz Biotechnology, Santa Cruz, CA, USA); anti-VP5 mAb,1:200 (sc-56989; Santa Cruz Biotechnology); anti-gB mAb, 1:500 (sc-69799; Santa Cruz Biotechnology); anti-gD mAb, 1:1000 (sc-56988; Santa Cruz Biotechnology); anti-β-actin mAb, 1:5000 (A-5441; Sigma-Aldrich); anti-phospho-Akt (Ser-473) mAb, 1:500 (4051; Cell Signaling Technology, Danvers, MA, USA); anti-Akt1 mAb, 1:500 (sc-55523; Santa Cruz Biotechnology); anti-histone H1 mAb, 1:250 (sc-8030; Santa Cruz Biotechnology), rabbit anti-total Akt123, 1:1000 (sc-8312; Santa Cruz Biotechnology); and rabbit anti-goat IgG, 1:500 (HAF017; R&D Systems, Minneapolis, MN, USA). The secondary antibodies for Western blots were horseradish peroxidase-conjugated goat anti-mouse (170-5047; Bio-Rad), goat anti-rabbit (170-5046; Bio-Rad). The secondary antibodies for confocal microscopy were anti-mouse Alexa Fluor 488, Alexa Fluor 555, or Alexa Fluor 350 and anti-rabbit Alexa Fluor 488 or Alexa Fluor 350 (A11001, A211422, A11045, A11008, A21068; Invitrogen Molecular Probes). All secondary antibodies were diluted 1:1000.
Staurosporine (PHZ1271) and ionomycin (I24222) were purchased from Invitrogen Molecular Probes; wortmannin (1232) was obtained from Tocris (Bristol, UK); miltefosine (sc-203135) was obtained from Santa Cruz Biotechnology; perifosine (s1037) and MK-2206 (s1078) were obtained from Selleck Chemicals (Houston, TX, USA), and inhibitor VIII (124018) was obtained from Calbiochem (EMD Millipore, Billerica, MA, USA).
Viral binding, entry, and plaque assays
For binding studies, cells were exposed to serial dilutions of purified virus for 5 h at 4°C. Unbound virus was removed by washing, and the cell-bound virus was analyzed by preparing Western blots of cell lysates and probing with anti-gD mAb (1103; Virusys; ref. 25). For plaque assays, cells were exposed to serial dilutions of virus for 1 h and then washed 3 times with PBS (pH 7.4) and overlaid with 199 medium containing 1% pooled human IgG. Plaques were counted by immunoassay using an anti-human IgG antibody peroxidase conjugate (Calbiochem; ref. 27). In select experiments, cells were pretreated for 15 min with drugs that block Akt signaling or with DMEM containing 0.1% DMSO as a control. For comparison, acyclovir, which acts postentry, was added to the overlay medium. The ability of VP16, a viral tegument protein, to translocate to the nucleus following infection was assessed as a marker of viral entry. CaSki cells were infected with the indicated MOI of virus at 37°C for 45 min, washed 3 times with PBS, and overlaid with fresh medium, and nuclear extracts were prepared 45 min later and analyzed by Western blot for VP16 and histone H1 (7).
Confocal microscopy
Viral entry was also monitored by confocal microscopy. Cells were grown on glass coverslips in 12- or 24-well plates and, where indicated, were transfected with siRNA, incubated for 48 h, and then infected with KVP26GFP or DiI-labeled KVP26GFP (MOI 5 PFU/cell). To label plasma membranes, the cells were stained for 30 min with EZ-Link sulfosuccinimidobiotin reagent (0.1 mM; Pierce Chemical, Rockford, IL, USA), which reacts with primary amines on cell-surface proteins before infection, and fixed with 4% paraformaldehyde solution (Electron Microscopy, Hatfield, PA, USA) at the indicated times postinfection, and the biotinylated cells were allowed to react with Alexa Fluor 350-conjugated streptavidin (1:1000 dilution; S11225, S11249; Invitrogen). Nuclei were stained blue with DAPI (1 μg/ml; Invitrogen) after fixation. Images were acquired by Zeiss Live DuoScan laser confocal microscope (Carl Zeiss, Oberkochen, Germany) equipped with an oil-immersion objective (100×1.4). Images were captured in an optical slice of ∼0.5 μm with appropriate filters, and Alexa Fluor 488 and GFP were excited using the 488-nm line of a krypton/argon laser and viewed with a 505- to 530-nm bandpass micrometer. Alexa Fluor 360 was excited with a 405-nm diode laser and was collected with a 420- to 475-nm filter; Alexa Fluor 555 was excited using a 561-nm helium/neon laser and was collected with a 575- to 655-nm filter. All images were captured using the multitrack mode of the microscope to decrease crosstalk of fluorescent signals. In experiments with primary cells, the plasma membranes and nuclei were stained for 10 min with red fluorescent Alexa Fluor 594 wheat germ agglutinin and blue fluorescent Hoechst 33342 using the Image-IT Live plasma membrane and nuclear labeling kit (Invitrogen).
Calcium live-image microscopy
Cells were grown in glass-bottom 35-mm culture dishes (P35G-1.5-10-C; MatTek Corp., Ashland, MA, USA), and cellular membranes were stained with the lipophilic red fluorescent CellTrace BODIPY TR methyl ester and nuclei with Hoechst (blue) using Image-iT LIVE intracellular membrane and nuclear labeling kit (Invitrogen). The cells were then loaded with Calcium Green (2.5 μg/ml; Invitrogen) for 1 h at 37°C and then infected with purified virus for 4 h at 4°C. Cells were washed 3 times with PBS, overlaid with 25-mM HEPES buffer, and placed into a temperature-regulated 37°C environmental chamber in a Zeiss Live DuoScan confocal microscope fitted with a 100 × 1.4 oil objective. Images were acquired 3 min after the dishes were placed in the chamber; Z sections were captured in an optical slice of 0.5 μm, and 15–20 cells were scanned per experiment. Image analysis was conducted using the LSM confocal software package (Carl Zeiss), and quantification of intensity staining with ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA). Three-dimensional images were generated using the Volocity 5 confocal software (Improvision, Lexington, MA, USA).
Calcium kinetic measurements
CaSki cells (5×104) were seeded in 96-well black plates with clear bottoms (3340, CellBind surface; Corning, Corning, NY, USA) and incubated with 25 μM Fura-2 AM diluted in PBS (F1221; Invitrogen Molecular Probes) for 60 min at 37°C, rinsed with PBS thrice, placed on ice, and then exposed to cold purified HSV-2 (MOI ∼5 PFU/cell) or control buffer (PBS). In select experiments, cells were pretreated with 5 nM wortmannin or 50 μM miltefosine prior to infection. Additional controls included cells exposed to 1 μM of ionomycin. The cells were then transferred to SpectraMaxMFe temperature-regulated chamber at 37°C (Invitrogen Molecular Devices) without washing; photometric data for [Ca2+] were generated by exciting cells at 340 and 380 nm and measuring emission at 510 nm every minute for 1 h. An intracellular calibration was performed with each experiment by determining the fluorescence ratio (340:380) in the presence of Ca-free 10 mM K2 EGTA buffer (Rmin) and 10 mM CaEGTA buffer containing 10 μM ionomycin (Rmax; C-3008, calcium calibration buffer kit 1; Invitrogen Molecular Probes). The mean [Ca2+] was determined from 4 wells, according to the manufacturer's recommendations, using the following equation: [Ca2+] = Kd Q (R − Rmin)/(Rmax − R), where R represents the fluorescence intensity ratio Fλ1/Fλ2; λ1 (340 nm) and λ2 (380 nm) are the fluorescence detection wavelengths for ion-bound and ion-free indicators; Kd is the Ca2+ dissociation constant (0.14 μM; Fura and Indo ratiometric calcium indicators; Invitrogen Molecular Probes); and Q is the ratio of Fmin to Fmax at λ2.
Immunoprecipitation assay
Cells were serum starved for 24 h prior to being synchronously infected with purified HSV-2 (MOI 5 PFU/cell), and at 15 min postinfection, cells were lysed by sonication in RIPA buffer (Thermo Scientific) supplemented with complete protease inhibitors (Roche Diagnostics). The lysates were incubated with equal amounts (based on protein concentration provided by manufacture) of rabbit polyclonal anti-Akt or control rabbit IgG (sc-2763; Santa Cruz Biotechnology) overnight at 4°C, and then immune complexes were isolated following 2 h incubation with protein A agarose beads (Thermo Scientific). The precipitated complexes (pellet), supernatants, or aliquots of the nonimmunoprecipitated cell lysate were analyzed by Western blot for gB or gD. Reciprocal immunoprecipitation studies were performed by immunoprecipitating lysates from infected cells with anti-gB (sc-52425, Santa Cruz Biotechnology) or anti-gD (sc-21719; Santa Cruz Biotechnology) mAbs and probing for gB and Akt by Western blot analysis.
Biotinylation of cell surface proteins of HSV-1-infected cells
CaSki cells were exposed to HSV-2 (MOI 10 PFU/cell) or mock infected at 4°C for 4 h. At 2 and 15 min after transfer of the cells to 37°C, cell samples were washed 4 times with ice-cold PBS and biotinylated with sulfo-NHS-SS-Biotin (F20650; Invitrogen Molecular Probes) for 1 h at 4°C. After 3 washes with PBS supplemented with 1% BSA, cells were harvested, solubilized in PBS containing a proteinase inhibitor cocktail, precipitated with streptavidin magnetic beads (Dynabeads M-280 Streptavidin; Life Technologies, Gaithersburg, MD, USA) and analyzed by immunoblotting with a polyclonal rabbit anti-Akt Ab.
Toxicity assays
CaSki cells were transfected and grown in 96-well plates, and cell viability was determined 72 h after transfection using a cell proliferation assay (CellTiter96; Promega, San Luis Obispo, CA, USA). Controls included cells exposed to medium alone or exposed to 0.01% nonoxynol-9 (N-9). Optical density was determined using a Beckman Coulter DTX880 multidetection microplate reader. To determine whether transfections induced apoptosis, CaSki cells were transfected for 72 h and either mock treated or incubated with 1 μM staurosporine (EMD Chemicals, Gibbstown, NJ, USA) for 4 h, then fixed in 4% paraformaldehyde and permeabilized in 1% Triton-X. The cells were stained with the Click-iT TUNEL Alexa Fluor 594 (C10246; Invitrogen Molecular Probes). The percentage of cell death was determined by quantifying a total of 100 cells visualized in 4 random fields.
Flow cytometry
HaCAT cells (1×106 cells/sample) were trypsinized, washed, exposed to virus (MOI 10) or mock infected for 4 h at 4°C in suspension, immediately transferred to a 37°C water for 15 min, transferred back to ice, centrifuged at 700 rpm for 10 min at 4°C, stained with violet Live/Dead marker (L34955; Invitrogen) for 1 h at 4°C, and then subsequently stained with anti-Akt FITC-conjugated Ab (1:50, sc5298; Santa Cruz Biotechnology) for 1 h at 4°C. Cells were subsequently fixed with 4% paraformaldehyde at 4°C and analyzed by flow cytometry, after gating on the live populations using a Becton Dickinson LSRII analyzer (BD Biosciences, San Jose, CA, USA). Data were processed by FlowJo 9.3.1 software (Tree Star, Ashland, OR, USA). Ten thousand events were acquired per sample.
Ganglion-epithelial cell ex vivo cocultures
At 7 d postinfection, sacral ganglia were excised from animals that had been intravaginally infected with ∼105 PFU of HSV-2 (4674). The extracted tissue was cocultured with confluent Vero cells in 60-mm Petri dishes containing 5 ml of DMEM supplemented with 0.1% DMSO (control) or with 20 μM of miltefosine. Culture supernatants (200 μl) were collected on d 2–7 postcoculture, and the amount of infectious virus released into the medium was determined by plaque assay on Vero cells.
Statistical analyses
Statistical analyses were performed by using ANOVA and Student's t tests; values of P < 0.05 were considered significant. Bonferroni adjustments were applied for multiple comparisons between each treatment group and the control. All analyses were performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA).
RESULTS
HSV triggers rapid phosphorylation of Akt
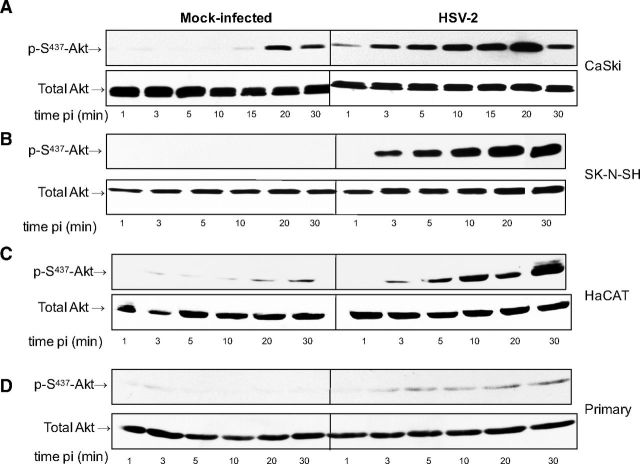
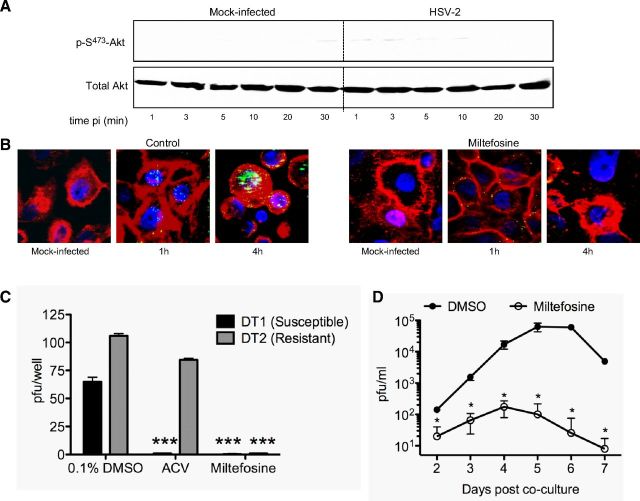
To assess whether exposure to HSV activates Akt signaling, serum-starved CaSki human cervical epithelial cells were infected with HSV-2 diluted in serum-free medium or serum-free medium alone (mock-infection), and cell lysates were prepared at different times postinfection. Phosphorylation of Akt was assessed by Western blot; blots were first probed with a monoclonal antibody specific for phosphorylated Akt and then stripped and probed with a rabbit polyclonal antibody to total Akt. An increase in p-S473-Akt relative to mock-infected cells was consistently observed as early as 1 min following exposure to HSV-2 and peaked ∼10–20 min after viral exposure (Fig. 1A). A more modest increase in pAkt was also observed in mock-infected CaSki cells at the later time points, which may reflect the physiology of this cell line. Indeed, CaSki cells harbor multiple copies of integrated HPV DNA and may have altered Akt expression. Therefore, we extended the observations to include SK-N-SH human neuroblastoma cells, HaCAT cells (a spontaneously immortalized human kerinatocyte cell line), and primary cells isolated from human ectocervical tissue. HSV-2 induced Akt phosphorylation in all of the cell types, although the relative amounts of Akt expressed and the kinetics of the response varied (Fig. 1B–D). Similar results were also obtained in End2/E6E7 cells, human endocervical cells immortalized with a vector containing HPV-16 E6/E7 (28), and in response to HSV-1 (not shown).
Figure 1.
HSV induces Akt phosphorylation. CaSki (A), SK-N-SH (B), HaCAT (C), or primary genital tract (D) cells were exposed to serum-free medium (mock infection) or infected with HSV-2(G) diluted in serum-free medium (MOI 10 PFU/cell), and cell lysates were prepared for Western blot analysis at the indicated times postinfection (pi). Blots were incubated with anti-pS473-Akt123 and then stripped and probed with anti-total Akt123. Representative blots from ≥3 independent experiments are shown.
Silencing of Akt reduces HSV infection
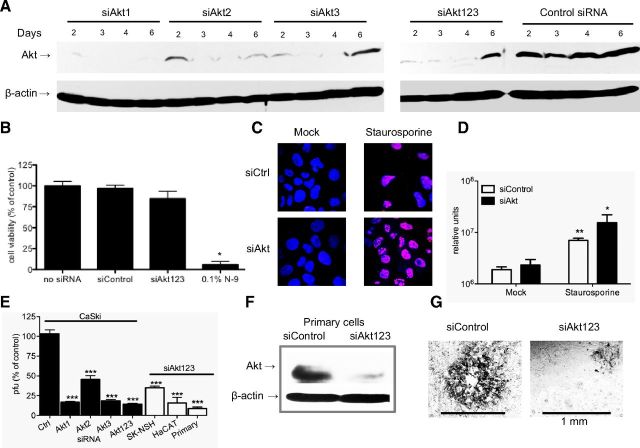
To explore the role that Akt phosphorylation plays in infection and, specifically, viral entry, CaSki cells were transfected with siRNA targeting each isoform of Akt, a cocktail of siRNA targeting the 3 isoforms of Akt (Akt123; ref. 29), or a nonspecific control siRNA. The effect of siRNA on Akt protein expression was evaluated by performing Western blots. A reduction in Akt protein expression was observed as early as 2 d post-transfection, which persisted for ≤6 d; siAkt1 was the most effective (Fig. 2A). Silencing was not cytotoxic (N-9 was used as a positive toxicity control; Fig. 2B) and had little effect on the number of cells undergoing spontaneous apoptosis, as assessed by DNA fragmentation (Fig. 2C, D). Silencing also did not induce the expression of IFN-α (not shown). However, plaque assays demonstrated that silencing significantly inhibited HSV-2 infection by as much as 90% relative to cells transfected with nonspecific control siRNA (P<0.001, ANOVA followed by post hoc t test with Bonferroni adjustment for multiple comparisons; Fig. 2E). Transfection of SK-N-SH, HaCAT, or primary genital tract epithelial cells with siRNA targeting Akt123 (confirmed by Western blots as shown for primary cells; Fig. 2F) also significantly inhibited HSV-2 infection (P<0.001; Fig. 2E). Silencing of Akt reduced both the number and size of viral plaques (Fig. 2G). Similar results were obtained with HSV-1 (not shown).
Figure 2.
siRNAs targeting Akt silence protein expression, are not cytotoxic, and inhibit HSV-2 plaque formation. A) Cells were transfected with individual siRNA targeting Akt1, Akt2, Akt3, or a mixture of the 3 different sequences of Akt or a control siRNA at a concentration of 10 nM (3.30 nM of each siRNA for the cocktail), and protein expression was evaluated by Western blot probing for total Akt and β-actin. B) Cellular proliferation was evaluated 72 h after transfection using the CellTiter96 kit. N-9 was included as a toxicity control. Optical density was measured, and the percentage of viability was determined relative to the no siRNA control level. C, D) CaSki cells were transfected with siAkt123 or siControl (siCtrl) for 72 h and either mock treated or incubated with 1 μM staurosporine for 4 h. Cells were stained for apoptosis; percentages of late apoptotic cells were determined by counting 5 random fields/treatment. Apoptotic cells are magenta; nuclei are blue. Images are representative (C); graphed values are means ± se (D). E) Cells were transfected with the indicated siRNAs and, at 72 h post-transfection, were infected with serial dilutions of HSV-2(G). Viral plaques were counted 2 d postinfection. Results are presented as PFU on siRNA-transfected cells as a percentage of PFU on cells transfected with control siRNA. Data are means ± se of a minimum of 3 independent experiments conducted in duplicate. Only wells in which the number of plaques ranged between 25 and 200 plaques were used to calculate the viral titer. F) Primary cells were transfected with siControl or siAkt123 cocktail; protein expression was evaluated by Western blot probing for total Akt and β-actin at 72 h post-transfection. G) Representative plaques on primary cells. *P < 0.05, **P < 0.01, ***P < 0.001 vs. respective control after Bonferroni adjustment.
Silencing of Akt prevents HSV entry postbinding
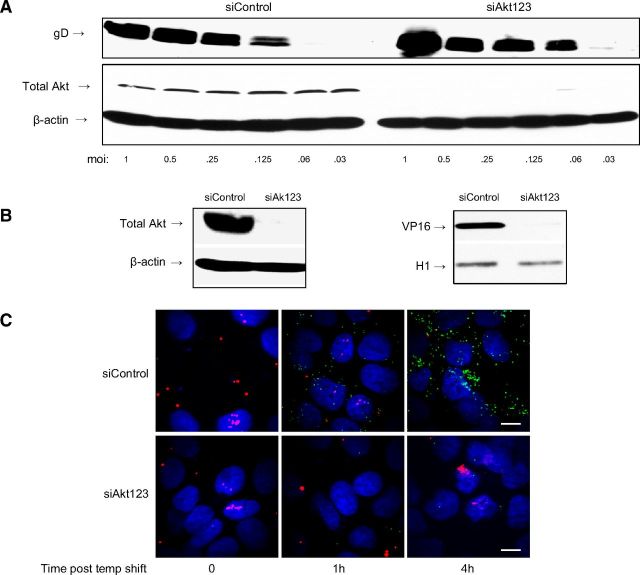
To identify whether Akt silencing impaired viral binding or entry, specific assays were performed with CaSki cells. Cells were transfected with control siRNA or siRNAs targeting Akt123 and then exposed to serial dilutions of HSV-2 at 4°C, a temperature permissive for binding, but not entry. Binding was monitored by preparing Western blots and probing for gD, as described previously (25). Silencing of Akt123 had no discernible effect on HSV-2 binding (Fig. 3A).
Figure 3.
Silencing of Akt inhibits viral entry independent of viral binding. A) Transfected CaSki cells were exposed to serial dilutions of HSV-2(G) at the MOI (PFU/cell) indicated for 5 h at 4°C. Cell-bound viral particles were detected by analyzing Western blots of cell lysates for gD; blots were also probed with antibodies to total Akt (to assess silencing) and β-actin (control for protein loading). Blots are representative of 3 independent experiments. B) Left panel: cells were transfected with siControl or siAkt123, and silencing was confirmed by Western blots 48 h post-transfection, probing for total Akt and β-actin. Right panel; siRNA-transfected cells were inoculated with HSV-2(G), and nuclear extracts were prepared and analyzed for the presence of the viral tegument protein VP16 and histone-H1 (as nuclear extract control) by Western blot. Blots shown are representative of results obtained in 3 independent experiments. C) Transfected CaSki cells (siControl or siAkt123) were exposed to purified dual-labeled HSV-1 K26GFP (capsids are green; viral envelopes are labeled red with Dil) at a MOI of 5 PFU/cell and 1 or 4 h postinfection, and cells were fixed and viewed by confocal microscopy; nuclei were stained with DAPI (blue). Representative images are shown. Scale bars = 10 μm.
Two independent approaches were applied to determine whether Akt contributed to viral entry. VP16 is a viral tegument protein, which is delivered to the nucleus following HSV entry, and thus its nuclear transport provides a surrogate of entry. Cells were transfected with siRNA (Akt123 or control) and, 72 h later, were infected with HSV-2. After 45 min incubation, nuclear extracts were prepared and evaluated for VP16 by Western blot; histone H1 was included as a control for the nuclear extracts. Silencing of Akt reduced the nuclear transport of VP16, which is consistent with a block to viral entry (Fig. 3B). To further assess the effect of Akt silencing on viral entry, confocal studies were conducted with purified K26GFP, an HSV-1 viral variant in which GFP has been fused in frame with the gene for the viral capsid protein VP26 (20). For these studies, the viral envelope was also labeled with the fluorescent long-chain carbocyanine dye DiI (30). This enables discrimination between viral envelopes (red) and capsids (green). Cells were transfected with siRNA (control or Akt123); 72 h later, the cells were synchronously infected (MOI 5 PFU/cell; virus was allowed to first bind for 4 h at 4°C, then temperature was shifted to 37°C to allow entry). Cells were harvested at 0, 1, and 4 h after temperature shift and viewed by confocal microscopy; nuclei were stained with DAPI (blue). While viral envelopes (red) were detected in both siControl and siAkt cells, capsids (green) were readily detected in the nuclei of siControl, but not siAkt cells. Specifically, colocalization of GFP (capsid) and DAPI (nuclear) staining was observed in 29 and 55% of siControl compared to 0 and 1% of siAkt cells (n=100 cells each) at 1 and 4 h after temperature shift, respectively (Fig. 3C). In other studies, the cells were harvested at 15, 30, or 60 min postinfection, fixed, and stained with EZ-Link to detect cellular plasma membranes rather than staining nuclei. Viral envelopes (red) were easily detected colocalized with the plasma membrane (blue; merge, purple) in both siControl and siAkt cells. However, capsids released from the viral envelope (green) were only observed in the siControl cells (Supplemental Fig. S2).
Akt is required for HSV-induced Ca2+ responses
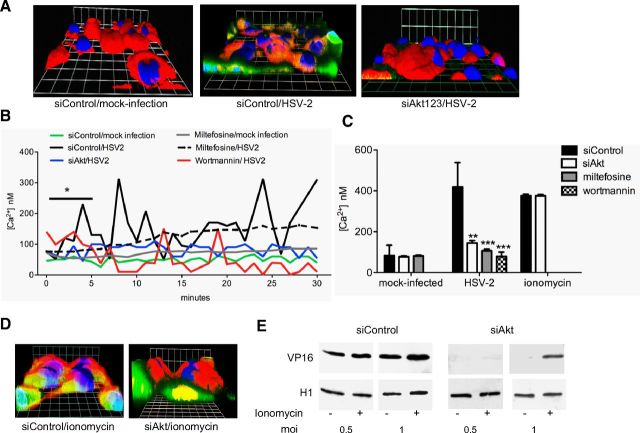
To determine whether Akt played a role in the HSV-induced Ca2+ responses associated with HSV entry, cells were transfected with siControl or siAkt123, and at 48 h post-transfection, the cells were loaded with Calcium Green, and then synchronously infected with HSV-2 (MOI 5 PFU/cell). The virus was allowed to bind for 4 h at 4°C, unbound virus was removed by washing, and the cells were transferred to temperature-regulated chamber (37°C) for imaging; ∼50–100 cells from random fields were imaged. Cellular membranes were stained with the lipophilic red fluorescent CellTrace BODIPY TR methyl ester and nuclei with Hoechst (blue). Within 3 min following temperature shift, intracellular Ca2+ responses (green) were detected in the majority of HSV-infected cells transfected with control siRNA. In contrast, Ca2+ release was observed in 5–10% of the HSV-infected siAkt-transfected cells (Fig. 4A).
Figure 4.
Akt is required for HSV-induced calcium release, and treatment with ionomycin partially overcomes the block to infection in Akt-silenced cells. A) CaSki cells were loaded with Calcium Green 72 h post-transfection with the indicated siRNA and synchronously mock-infected or infected with purified HSV-2(G) (5 PFU/cell). Live images were acquired 3 min after temperature shift to 37°C. Membranes were stained red (CellTrace), nuclei blue (Hoechst) and Ca2+ green. Representative xyz images from 4 independent experiments are shown. Grid bars = 9.2 μm. B) Transfected cells were loaded with Fura-2, infected with purified HSV-2 (MOI 2 PFU/cell) or mock-infected, and the kinetics of Ca2+ response was monitored. Mean intracellular [Ca2+] (nM) for 5 × 104 cells over the first 30 min was quantified. Control (nontransfected) cells were also pretreated with 50 μM miltefosine or 5 nM wortmannin for 15 min prior to infection with HSV-2. *P < 0.05 for HSV-2- vs. mock-infected cells over the first 5 min; t test. C) Cumulative [Ca2+] released over 1 h was calculated from 4 wells, each containing 5 × 104 cells for each of the following conditions: mock-infected siControl siAkt or miltefosine-pretreated nontransfected cells; HSV-2-infected siControl, siAkt, or nontransfected cells pretreated with miltefosine or wortmannin; or transfected, uninfected cells following treatment with 1 μM of ionomycin. **P < 0.01, ***P < 0.001 vs. siControl; ANOVA with post hoc t test with Bonferroni adjustment. D) CaSki cells were loaded with Calcium Green at 72 h after transfection with the indicated siRNA, then treated with 1 μM of ionomycin, and live images were acquired. Cellular membranes are stained red; nuclei are blue. E) Silenced cells were treated with ionomycin or buffer (0.4% DMSO) immediately prior to infection with 0.5 or 1 PFU/cell of HSV-2; nuclear extracts were prepared 45 min postinfection and analyzed by Western blot for the presence of VP16 (and histone H1 as nuclear extract control) as a surrogate for viral entry.
While confocal microscopy allows one to detect responses in individual cells and identify the subcellular localization of Ca2+ responses, it is not quantitative. Therefore, quantitative kinetic fluorometric studies were conducted in parallel. Transfected CaSki cells (siControl or siAkt123) were loaded with Fura-2, infected with purified HSV-2(G) (MOI 2 PFU/cell), and the kinetics of Ca2+ response were monitored; intracellular [Ca2+] for 5 × 104 cells/well at each time point was quantified. HSV-2 triggered a rapid and significant increase in intracellular [Ca2+] released from siControl, but not siAkt cells within the first 5 min compared to mock-infected cells (P<0.05, t test), and additional waves were observed over 30 min (Fig. 4B). The response to virus was significantly reduced if the cells were pretreated with miltefosine or wortmannin, inhibitors of the PI3K/Akt signaling pathway (31). The mean [Ca2+] released over 1 h was calculated from 4 wells, each containing 5 × 104 cells (Fig. 4C).
In parallel studies, the transfected cells were treated with 1 μM of ionomycin, a Ca2+ ionophore. Ionomycin triggered the release of a comparable amount of Ca2+ from the siRNA-transfected cell populations, as evidenced by fluorometry (Fig. 4C) and confocal microscopy (Fig. 4D), indicating intact intracellular stores. Moreover, ionomycin treatment partially restored the susceptibility to HSV entry in Akt-silenced cells, as evidenced by the detection of nuclear VP16 in ionomycin treated, but not buffer-treated cells, following exposure to 1 PFU/cell (Fig. 4E).
Introduction of a plasmid expressing Akt into silenced cells restores susceptibility to HSV infection
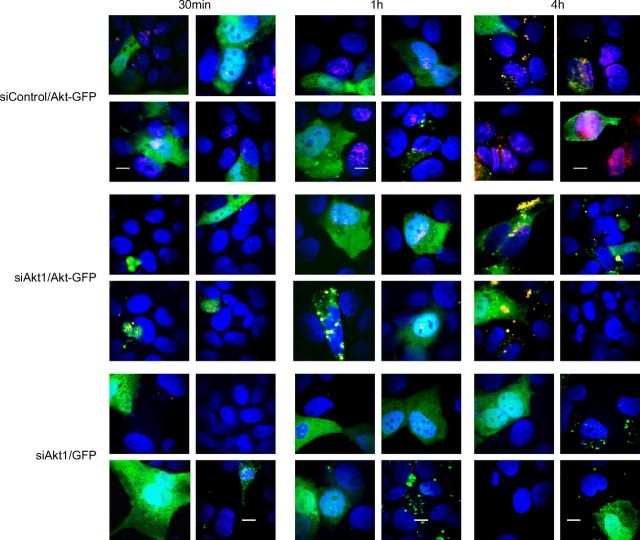
One of the limitations of the siRNA approach is the possibility that results obtained could reflect off-target silencing. To address this possibility, we reintroduced Akt1 into siRNA-transfected cells. Cells were transfected with siAkt1 and, 72 h later, were transfected with a plasmid expressing an Akt1-GFP fusion protein or GFP alone. After 72 h in culture, confocal imaging and Western blots were performed to assess the efficiency of Akt1 silencing and plasmid transfection. GFP was detected in 34% of siControl cells transfected with either the GFP or Akt1-GFP plasmid and in 24% and 43% of siAkt1 cells transfected with the GFP or Akt1-GFP plasmid, respectively (Fig. 5A; 50–70 cells counted for each condition). Native Akt1 (red) was detected in the majority (96%) of siControl cells, but in only 1.4% of siAkt1 cells transfected with a control GFP plasmid. Akt1 was detected in ∼43% of siAkt1 cells transfected with the Akt1-GFP plasmid and partially restored susceptibility to HSV-2 infection, as evidenced by live confocal imaging of Ca2+ at 5 min after viral exposure (Ca2+ detected with Calcium Crimson, red; Fig. 5B), Ca2+ kinetics (Fig. 5C), VP16 nuclear transport (Fig. 5D), and viral plaque assay (Fig. 5E).
Figure 5.
Reintroducing Akt into silenced cells restores susceptibility to infection. A) Cells were transfected with siAkt1 or siControl, and 72 h later were transfected with a plasmid expressing GFP or Akt1-GFP. Cells were fixed 72 h after plasmid transfection, immunostained with antibodies to Akt1 (red), nuclei stained with DAPI (blue), and analyzed by confocal microscopy. Images (xy) obtained from 4 independent fields are shown. Scale bars = 20 μm. B) Cells were transfected with siAkt1 and 72 h later transfected with the plasmid expressing GFP-Akt1 or GFP. Cells were loaded with Calcium Crimson 48 h after introduction of the plasmids and mock-infected or infected with purified HSV-2(G) (5 PFU/cell). Live images were acquired 3 min after temperature shift to 37°C. Nuclei were stained blue with Hoechst. Colocalization of Ca2+ (red) and GFP-transfected cells (green) is yellow. Representative images are shown. Scale bars = 10 μm. C) Kinetics of the Ca2+ response were monitored as in Fig. 3B, C. **P < 0.01, ***P < 0.001 vs. corresponding mock-infected (mi) cells; ANOVA with post hoc t test with Bonferroni adjustment. D) Transfected cells (siRNA followed by plasmid transfection) were inoculated with HSV-2(G) (MOI∼1 PFU/cell), and nuclear extracts were prepared 45 min after infection and analyzed for VP16 (and histone H1) by Western blot. Blots shown are representative of results obtained in 2 independent experiments. E) Silenced cells (siControl or siAkt) were transfected with the indicated plasmids (GFP or Akt-GFP) and then. 72 h later, exposed to HSV-2 (MOI selected to yield ∼100 PFU/well on control plates). Plaques were counted, and results are presented as PFU/well and are means from 2 independent experiments performed in duplicate. *P < 0.05 vs. siAkt cells transfected with control GFP plasmid; t test.
To determine whether susceptibility mapped to the cells expressing Akt1, additional confocal studies were performed with an HSV-1 variant that expresses red fluorescent protein fused to VP16 (21). Viral capsids (red) were detected in the majority of GFP+ and GFP-negative cells in siControl cells transfected with the Akt1-GFP plasmid (Fig. 6, top panels), but only in the GFP+ cells (merge of red capsids with Akt1-GFP, yellow) in siAkt1 cells transfected with the Akt1-GFP plasmid (Fig. 6, middle panels). Red capsids were rarely detected in siAkt1 cells transfected with the control plasmid (Fig. 6, bottom panels). Together, these findings indicate that the impediment to HSV entry in cells transfected with siAkt1 is attributable to silencing of Akt1 and not an off-target effect.
Figure 6.
Susceptibility to infection is restricted to cells expressing Akt. Silenced and plasmid-transfected (siControl or siAkt1 followed by transfection with Akt1-GFP or GFP-expressing plasmids) CaSki cells were exposed to purified HSV-F-GS2822 (capsids, red; MOI 1 PFU/cell) and then at the indicated times postinfection, cells were fixed with 4% of paraformaldehyde, nuclei were stained with DAPI (blue), and confocal images were acquired. Four independent fields are shown; and results are representative of 2 independent experiments. Scale bars = 10 μm.
Akt interacts with gB
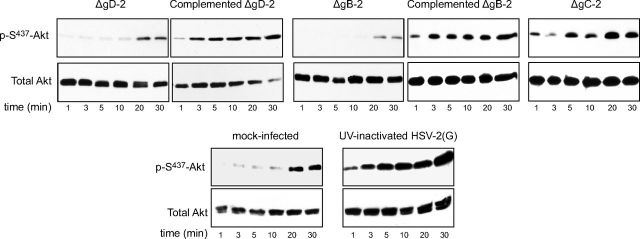
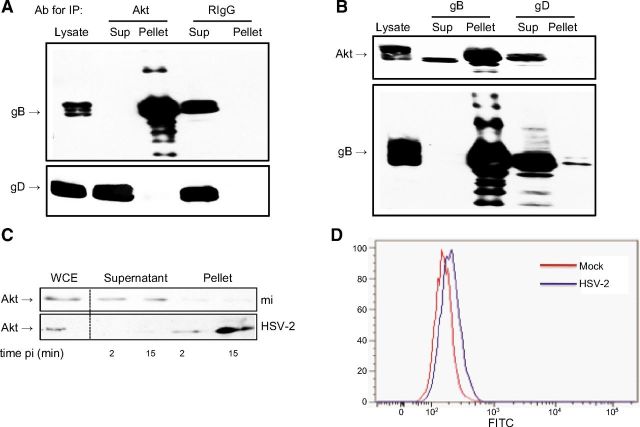
To explore whether the viral induced phosphorylation of Akt occurs in response to binding or entry, we tested HSV-2 viruses impaired in binding (ΔgB-2) or entry ΔgD-2. Controls included the two deletion viruses grown on respective complementing cells, a gC-2-deletion virus, and UV-inactivated HSV-2(G); the latter are all competent for binding and entry. There was little or no increase in Akt phosphorylation in response to ΔgD-2 or ΔgB-2 viruses. In contrast, the complemented, ΔgC-2, and UV-inactivated viruses induced rapid phosphorylation of Akt (Fig. 7). Together, these studies indicate that binding alone is not sufficient to induce Akt phosphorylation and suggest that gB and/or gD may directly interact with Akt. To explore this possibility, coimmunoprecipitation studies were performed. CaSki cells were synchronously infected with purified HSV-2(G) (MOI 5 PFU/cell), and cell lysates were prepared 15 min after the shift in temperature and were incubated with a rabbit polyclonal antibody to Akt or a control rabbit IgG. The immune complexes were precipitated with protein A agarose and analyzed by Western blot with mAbs to gB or gD (Fig. 8A). gB, but not gD, was detected in the pellet following precipitation with anti-Akt antibodies, but was retained in the supernatant when proteins were precipitated with a control antibody. Conversely, Akt was detected in the pellet following precipitation with anti-gB, but was retained in the supernatant when proteins were precipitated with anti-gD (Fig. 8B).
Figure 7.
Viruses deleted in HSV-2 gD and gB fail to trigger Akt phosphorylation. CaSki cells were serum starved for 24 h and then exposed to serum-free medium (mock-infection) or infected with ΔgD-2 grown on noncomplementing or complementing cells, ΔgB-2 grown on noncomplementing or complementing cells, ΔgC-2, or UV-inactivated HSV-2(G) (MOI 10 PFU/cell or equivalent number of particles for the ΔgD-2 and ΔgB-2 viruses), and cell lysates were prepared for Western blotting at the indicated times postinfection. Blots were incubated with anti-pS473-Akt123 and then stripped and probed with anti-total Akt123. Representative blots from ≤3 independent experiments are shown.
Figure 8.
Akt coimmunoprecipitates glycoprotein B. A) CaSki cells were synchronously infected with purified HSV-2 (MOI 5); cell lysates were harvested 15 min post-temperature shift and incubated with rabbit polyclonal anti-Akt or a control rabbit polyclonal antibody (RIgG). Immune complexes were precipitated, and an equivalent volume of cell lysates prior to precipitation, supernatant (sup), and pellet were subjected to Western blotting with anti-gB or anti-gD monoclonal antibodies. B) In reciprocal experiments, lysates from HSV-infected cells were precipitated with anti-gB or anti-gD mAbs and analyzed by Western blotting with antibodies to Akt or gB. C) CaSki cells were mock infected (mi) or exposed to HSV-2 (MOI 10 PFU/cell) at 4°C for 4 h and then transferred to 37°C for 2 or 15 min. Cell surface proteins were biotinylated and precipitated with streptavidin magnetic beads on ice and analyzed by immunoblotting; controls include whole cell extract (WCE). Results are representative of 3 independent experiments. D) Mean fluorescence intensity (MFI) staining of cell surface Akt 15 min after cells were shifted to 37°C following synchronous infection of HaCAT cells with HSV-2 (MOI 10 PFU/cell; MFI=208) or medium (MFI=171). Results are representative of 3 independent experiments.
The observation that gB and Akt coimmunoprecipitate suggests that Akt is accessible at the plasma membrane. To explore this, CaSki cells were exposed to HSV-2 (MOI 10 PFU/cell) or mock infected at 4°C for 4 h and then transferred to 37°C for 2 or 15 min, placed back on ice, and cell surface proteins were biotinylated and precipitated with streptavidin magnetic beads and analyzed by immunoblotting with an anti-Akt antibody. Akt was detected in the supernatant in mock-infected cells, but in the pellet in HSV-2 exposed cells, suggesting that HSV-2 Akt triggers relocalization of Akt to the cell surface (Fig. 8C). An increase in cell-surface Akt following HSV exposure was also observed by flow cytometry. HaCAT cells were mock-infected or exposed to virus (MOI 10 PFU/cell) in suspension for 4 h at 4°C and then transferred to 37°C for 2 or 15 min, placed back on ice, and stained with live/dead marker and for Akt. There was a 22.2 ± 1.57% increase in mean fluorescence intensity of Akt staining after gating on live cell population in HSV-exposed compared to mock-exposed cells 15 min after temperature shift (P<0.01, t test; Fig. 8D).
Akt-viral interactions are a potential target for HSV treatment
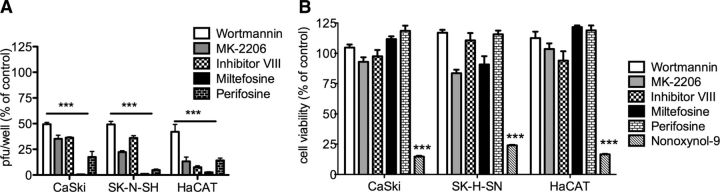
We tested a series of Akt pharmacological inhibitors being developed as adjunct therapy for cancer, including MK-2206, an allosteric Akt inhibitor (32); inhibitor VIII, which binds to the pleckstrin homology domain of Akt and prevents subsequent phosphorylation, as well as translocation of Akt to the plasma membrane (33); miltefosine, a drug licensed for treatment of leishmaniasis and other protozoal infections that blocks Akt phosphorylation (31), and perifosine, a structural analog of miltefosine in phase 3 clinical trials for treatment of several cancers (34). Cells were pretreated with each drug for 15 min and then exposed to HSV-2 for 1 h. The cells were washed, fresh medium was added, and plaques were counted at 48 h postinfection; toxicity assays were conducted in parallel with N-9 included as a positive toxicity control. All of the drugs significantly inhibited HSV infection in each of the cell types at concentrations that were not cytotoxic (Fig. 9). Miltefosine was the most potent and reduced viral plaques by >90% in all cell types.
Figure 9.
Pharmacological inhibitors of Akt signaling inhibit HSV-2 plaque formation. A) Cells (CaSki, SK-N-SH, or HaCAT) were pretreated for 15 min with wortmannin (10 nM), MK-2206 (50 μM), inhibitor VIII (50 μM), miltefosine (50 μM), or pirefosine (10 μM) and then challenged with dilutions of HSV-2 to achieve ∼100 plaques on control wells. Inoculum (and drug) was removed after 1 h by washing, and then the cells were overlaid with fresh medium; plaques were counted after 48 h incubation. Results are presented as percentage PFU relative to control wells (treated with 0.1% DMSO). B) Alternatively, cells were treated for 24 h with the same concentrations of the drugs alone (or 0.01% N-9 as a positive toxicity control), and cell viability was determined by CellTiter assay. ***P < 0.001; ANOVA with post hoc t test with Bonferroni adjustment.
Subsequent studies with miltefosine demonstrated that the drug blocked viral induced phosphorylation of Akt (Fig. 10A), prevented viral induced Ca2+ responses (Fig. 4C), and prevented viral entry into primary epithelial cells, as indicated by confocal imaging with GFP-labeled virus (Fig. 10B). Miltefosine was active against an acyclovir-susceptible (DT1) and acyclovir-resistant (DT2) clinical isolate in a plaque assay (Fig. 10C) and blocked viral amplification in a ganglion explant coculture model. Sacral ganglia were removed from HSV-2-infected mice on d 7 and were cocultured with Vero cells in the presence of 20 μM miltefosine or DMSO (control). Medium was removed on the indicated days postcoculture and assayed for the presence of infectious virus by titering on Vero cells. The viral yields were significantly less in miltefosine compared to control buffer (DMSO)-treated cocultures over the 7-d study period (Fig. 10D).
Figure 10.
Miltefosine prevents HSV-induced Akt signaling, entry, infection by acyclovir resistant and susceptible clinical isolates, and amplification of virus in a ganglion ex vivo coculture model. A) HaCAT cells were pretreated with miltefosine (50 μM) and then exposed to serum-free medium (mock-infection) or infected with HSV-2(G) diluted in serum-free medium (MOI 10 PFU/cell). Cell lysates were analyzed by Western blot at the indicated times postinfection (pi) with anti-pS473-Akt123 and then stripped and probed with anti-total Akt123. Results are representative of those found in 2 independent experiments with each of the cell types. B) Primary cells were pretreated with control buffer (0.1% DMSO) or miltefosine (50 μM) and then infected with HSV-1(K26GFP); at the indicated times postinfection, cells were fixed and viewed by confocal microscopy. Plasma membranes and nuclei were stained red and blue, respectively. C) CaSki cells were pretreated with control buffer or with miltefosine (50 μM) prior to infection with an acyclovir susceptible (DT1) or acyclovir-resistant (DT2) isolate. Alternatively, cells were pretreated with 0.1% DMSO (control buffer), and acyclovir (10 μg/ml) was added to the overlay medium for the 48-h incubation period. Results are means of 2 independent experiments conducted in duplicate and are presented as percentage inhibition of PFU relative to the control wells. ***P < 0.001; ANOVA with post hoc t test with Bonferroni adjustment. D) Sacral ganglia were extracted from mice 7 d following intravaginal infection and cocultured ex vivo with Vero cells in 5 ml of medium containing 0.1% DMSO or 20 μM miltefosine. Culture supernatants (200 μl) were collected on the indicated days postcoculture, and viral growth was monitored by plaque assay. Results are presented as PFU/ml (means±sd) obtained from 2 independent experiments, each with 3 sacral ganglia. *P < 0.05 vs. DMSO.
DISCUSSION
These studies demonstrate for the first time that Akt plays an important role in viral entry and link Akt activation to Ca2+ signaling at the plasma membrane. Exposure of primary or transformed genital tract epithelial, neuronal, or keratinocyte cells to either serotype of HSV triggered Akt phosphorylation and silencing of Akt expression or pharmacological blockade of its phosphorylation blocked the viral-induced Ca2+ response and prevented viral entry. Susceptibility to HSV entry was partially restored when Akt was reintroduced into siAkt-silenced cells by transfection with a plasmid expressing Akt or by independently triggering the release of intracellular Ca2+ by treating the cells with ionomycin.
Coimmunoprecipitation studies suggest that Akt interacts with gB at the plasma membrane and that this response occurs following viral binding and engagement of a gD coreceptor. The observation that the virus could interact with Akt at the plasma membrane was initially unanticipated as Akt typically localizes to the inner leaflet of plasma membranes. However, biotinylation of cell surface proteins and flow cytometry studies (Fig. 8) suggest that HSV-2 may trigger Akt translocation to the outer plasma membrane, perhaps within lipid rafts. Akt has been shown to localize to plasma membrane microdomains such as lipid rafts, which are found on both the inner and outer leaflet of plasma membrane (35). Consistent with our findings is an earlier observation that the ectodomain of gB (but not gD or its receptors, HVEM and nectin) associated with lipid rafts during viral entry; this observation led the authors to speculate that gB may interact with lipid raft components to activate cell signaling and promote viral entry (36). Crystallography studies demonstrate that gB possesses PH domains (37), which could interact with the PH domains of Akt directly or indirectly through PIP2 or PIP3, and promote Akt phosphorylation by cellular kinases, such as phosphoinositide-dependent kinase 1 (PDK1) and/or Akt autophosphorylation.
The link between Akt phosphorylation and release of local plasma membrane Ca2+ stores and the subsequent release of IP3R-mediated cytosolic stores is consistent with studies demonstrating that Akt potentiates IP3R-mediated Ca2+ release (16, 38). Notably, we previously found that transfection of cells with siRNA targeting integrin αv blocked the HSV-induced release of ER Ca2+ stores, but not the initial release of Ca2+ at the plasma membrane (8). These findings suggest that one of the downstream effects of Akt phosphorylation may be to promote integrin signaling, which, in turn, could activate PLC, resulting in the hydrolysis of PIP2 and generation of IP3, linking Akt to the release of IP3R-dependent ER Ca2+ stores. The Ca2+ responses may provide a positive feedback loop to further stimulate the activity of IP3Rs. This Ca2+-induced Ca2+ release mechanism enables Ca2+ signals to be rapidly amplified and spread throughout the whole cell, as observed in confocal images (spatial), and by fluorometry, which revealed several waves of Ca2+ release of varying amplitude and duration over the first hour following infection (Fig. 4).
Importantly, Akt signaling plays a role in HSV entry into multiple cell types, including primary cells isolated from the female genital tract and by both serotypes, suggesting that targeting this interaction could provide a novel strategy for HSV treatment or suppression. We screened several pharmacological inhibitors of Akt signaling and found that miltefosine was a potent inhibitor and blocked viral entry and infection by acyclovir-susceptible and acyclovir-resistant clinical isolates at concentrations that were not cytotoxic to cells. No cytotoxicity was observed when cells were cultured in miltefosine for 1 wk in the ganglion ex vivo coculture model. Miltefosine was originally developed as an antineoplastic agent and has been successfully used to treat leishmaniasis and other protozoal infections (39, 40). Miltefosine is also effective for treatment of leishmaniasis in HIV-infected patients. For example, oral miltefosine (100 mg/d) was administered to 39 HIV+ patients with leishmaniasis who had failed standard therapy; an initial response was achieved in 25 patients (64%), including 16 (43%) with parasitological cure (41). In all of the clinical studies, the drug was well tolerated.
Recent studies suggest that miltefosine may also be active against HIV through mechanisms independent of viral entry. Miltefosine inhibited HIV-1 replication in cocultures of human dendritic cells (DCs) and CD4+ T cells by triggering the release of type 1 interferons and other soluble factors from DCs (42). In a second study, miltefosine significantly reduced HIV production from macrophages by promoting cell death in HIV-infected cells without adversely affecting uninfected cells (42, 43). The study suggested that HIV activates Akt to prevent apoptosis in primary human macrophages. Similarly, it has been previously shown that HSV activates Akt early in the viral life cycle, presumably to block apoptosis in infected epithelial cells (44). In addition, recent work demonstrates that the viral tegument protein, VP11/12, triggers PI3K-Akt signaling in Jurkat T cells and primary fibroblasts at later time points following HSV-1 infection, although the downstream targets of the VP11/12-dependent Akt phosphorylation have yet to be determined (45). Taken together, these findings indicate that inhibitors of the Akt signaling pathway may impede HSV infection through multiple mechanisms, including the prevention of Ca2+ signaling responses required for HSV entry.
The findings in this study expand our understanding of the complex model of HSV entry, which likely reflects the ability of both seroptypes to enter multiple cell types through diverse mechanisms. The findings support the following paradigm for HSV entry: HSV gC (HSV-1) or gB (HSV-2) bind to heparan sulfate moieties on syndecan proteoglycans (8, 25, 27), and this may facilitate the subsequent engagement of a gD coreceptor, typically nectin-1 on epithelial cells, which induces conformational changes (46, 47). These changes may then trigger the translocation of Akt to microdomains on the outer leaflet of the plasma membrane, rendering Akt accessible to the viral envelope; Akt is then phosphorylated in a process that involves gB, triggering the release of an intracellular Ca2+ store near the plasma membrane to initiate the fusion process. The release of plasma membrane Ca2+ coupled with the activation of Akt and activation of downstream events promotes the subsequent release of IP3R-ER Ca2+ stores, culminating in the intracellular delivery of viral capsids. The role that heterooligomeric complexes of gH-gL, which have been previously shown to regulate the fusogenic activity of gB (48, 49), play in this Akt-Ca2+ pathway requires further study. In addition, there may be other cellular components that participate in this complex cascade. For example, a recent study found that HSV-1 (HSV-2 was not evaluated) triggers translocation of nonmuscle myosin heavy chain IIA to the plasma membrane, which was suggested to facilitate HSV-1 fusion in Vero cells through interactions with gB (35).
It is also noteworthy that other herpesviruses also interact with Akt signaling pathways during viral entry. For example, CMV has been shown to activate PI3K and Akt signaling through interactions between CMV gB and platelet-derived growth factor-α receptor; blockade of this pathway inhibited CMV entry (50). Thus, defining the role of Akt signaling in viral entry may have broad implications. The salient findings in this study are that Akt plays a central role in promoting HSV entry by triggering Ca2+ responses. Defining the gB domains that interact with Akt should promote the identification of drugs that specifically target this interaction and provide a novel strategy for treatment or suppression of HSV infection. This approach could be of particular importance for the subset of patients with recalcitrant disease or acyclovir-resistant viruses.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
Acknowledgments
The authors thank Briana Nixon and Theodore Segarra for assistance with animal experiments, Prashant Desai (Johns Hopkins University, Baltimore, MD, USA) for the KVP26GFP virus and Greg Smith (Northwestern University, Evanston, IL, USA) for HSV-1 (F-GS2822). The authors also thank Yungtai Lo for statistical advice. The authors thank the Analytical Imaging Facility at Albert Einstein College of Medicine for assistance with confocal laser and E. A. Eugenin (Albert Einstein College of Medicine) for scientific advice.
This work was supported in part by U.S. National Institutes of Health (NIH) grant AI-061679 and the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (NIH AI-51519).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ER
- endoplasmic reticulum
- FBS
- fetal bovine serum
- gB
- glycoprotein B
- gC
- glycoprotein C
- gD
- glycoprotein D
- GFP
- green fluorescent protein
- gH-gL
- glycoproteins H and L
- HSV
- herpes simplex virus
- IP3
- inositol triphosphate
- IP3R
- inositol triphosphate receptor
- MOI
- multiplicity of infection
- N-9
- nonoxynol-9
- PFU
- plaque-forming unit
- PH
- pleckstrin homology
- PI3K
- phosphoinositide 3-kinase
- PIP2
- phosphatidylinositol 3,4-bisphosphate
- PIP3
- phosphatidylinositol 3,4,5-trisphosphate
- PLC
- phospholipase C
- siRNA
- small interfering RNA
- WT
- wild type
REFERENCES
- 1.Tronstein E., Johnston C., Huang M. L., Selke S., Magaret A., Warren T., Corey L., Wald A. (2011) Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA , 1441–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corey L., Wald A., Patel R., Sacks S. L., Tyring S. K., Warren T., Douglas J. M., Paavonen J., Morrow R. A., Beutner K. R., Stratchounsky L. S., Mertz G., Keene O. N., Watson H. A., Tait D., Vargas-Cortes M. (2004) Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N. Engl. J. Med. , 11–20 [DOI] [PubMed] [Google Scholar]
- 3.Belshe R. B., Leone P. A., Bernstein D. I., Wald A., Levin M. J., Stapleton J. T., Gorfinkel I., Morrow R. L., Ewell M. G., Stokes-Riner A., Dubin G., Heineman T. C., Schulte J. M., Deal C. D. (2012) Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. , 34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson S. S., Fakioglu E., Herold B. C. (2009) Novel approaches in fighting herpes simplex virus infections. Expert Rev. Anti. Infect. Ther. , 559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spear P. G. (2004) Herpes simplex virus: receptors and ligands for cell entry. Cell. Microbiol. , 401–410 [DOI] [PubMed] [Google Scholar]
- 6.Heldwein E. E., Krummenacher C. (2008) Entry of herpesviruses into mammalian cells. Cell. Mol. Life Sci. , 1653–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheshenko N., Del Rosario B., Woda C., Marcellino D., Satlin L. M., Herold B. C. (2003) Herpes simplex virus triggers activation of calcium-signaling pathways. J. Cell Biol. , 283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheshenko N., Liu W., Satlin L. M., Herold B. C. (2007) Multiple receptor interactions trigger release of membrane and intracellular calcium stores critical for herpes simplex virus entry. Mol. Biol. Cell. , 3119–3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berridge M. J. (2009) Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta. , 933–940 [DOI] [PubMed] [Google Scholar]
- 10.Venkatachalam K., van Rossum D. B., Patterson R. L., Ma H. T., Gill D. L. (2002) The cellular and molecular basis of store-operated calcium entry. Nat. Cell Biol. , E263–E272 [DOI] [PubMed] [Google Scholar]
- 11.Patterson R. L., van Rossum D. B., Kaplin A. I., Barrow R. K., Snyder S. H. (2005) Inositol 1,4,5-trisphosphate receptor/GAPDH complex augments Ca2+ release via locally derived NADH. Proc. Natl. Acad. Sci. U. S. A. , 1357–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alessi D. R., James S. R., Downes C. P., Holmes A. B., Gaffney P. R., Reese C. B., Cohen P. (1997) Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. , 261–269 [DOI] [PubMed] [Google Scholar]
- 13.Harlan J. E., Hajduk P. J., Yoon H. S., Fesik S. W. (1994) Pleckstrin homology domains bind to phosphatidylinositol-4,5-bisphosphate. Nature , 168–170 [DOI] [PubMed] [Google Scholar]
- 14.McManus E. J., Collins B. J., Ashby P. R., Prescott A. R., Murray-Tait V., Armit L. J., Arthur J. S., Alessi D. R. (2004) The in vivo role of PtdIns(3,4,5)P3 binding to PDK1 PH domain defined by knockin mutation. EMBO J. , 2071–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toker A., Newton A. C. (2000) Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J. Biol. Chem. , 8271–8274 [DOI] [PubMed] [Google Scholar]
- 16.Hwang J. Y., Duncan R. S., Madry C., Singh M., Koulen P. (2009) Progesterone potentiates calcium release through IP3 receptors by an Akt-mediated mechanism in hippocampal neurons. Cell Calcium , 233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fichorova R. N., Rheinwald J. G., Anderson D. J. (1997) Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol. Reprod. , 847–855 [DOI] [PubMed] [Google Scholar]
- 18.Fahey J. V., Wira C. R. (2002) Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J. Infect. Dis. , 1606–1613 [DOI] [PubMed] [Google Scholar]
- 19.Gerber S. I., Belval B. J., Herold B. C. (1995) Differences in the role of glycoprotein C of HSV-1 and HSV-2 in viral binding may contribute to serotype differences in cell tropism. Virology , 29–39 [DOI] [PubMed] [Google Scholar]
- 20.Desai P., Person S. (1998) Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J. Virol. , 7563–7568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antinone S. E., Smith G. A. (2010) Retrograde axon transport of herpes simplex virus and pseudorabies virus: a live-cell comparative analysis. J. Virol. , 1504–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segarra T. J., Fakioglu E., Cheshenko N., Wilson S. S., Mesquita P. M., Doncel G. F., Herold B. C. (2011) Bridging the gap between preclinical and clinical microbicide trials: blind evaluation of candidate gels in murine models of efficacy and safety. PLoS One , e27675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oram R. J., Marcellino D., Strauss D., Gustafson E., Talarico C. L., Root A. K., Sharma P. L., Thompson K., Fingeroth J. D., Crumpacker C., Herold B. C. (2000) Characterization of an acyclovir-resistant herpes simplex virus type 2 strain isolated from a premature neonate. J. Infect. Dis. , 1458–1461 [DOI] [PubMed] [Google Scholar]
- 24.Ligas M. W., Johnson D. C. (1988) A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. , 1486–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheshenko N., Herold B. C. (2002) Glycoprotein B plays a predominant role in mediating herpes simplex virus type 2 attachment and is required for entry and cell-to-cell spread. J. Gen. Virol. , 2247–2255 [DOI] [PubMed] [Google Scholar]
- 26.Fakioglu E., Wilson S. S., Mesquita P. M., Hazrati E., Cheshenko N., Blaho J. A., Herold B. C. (2008) Herpes simplex virus downregulates secretory leukocyte protease inhibitor: a novel immune evasion mechanism. J. Virol. , 9337–9344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herold B. C., WuDunn D., Soltys N., Spear P. G. (1991) Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J. Virol. , 1090–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fichorova R. N., Anderson D. J. (1999) Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol. Reprod. , 508–514 [DOI] [PubMed] [Google Scholar]
- 29.Testa J. R., Bellacosa A. (2001) AKT plays a central role in tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. , 10983–10985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lakadamyali M., Rust M. J., Babcock H. P., Zhuang X. (2003) Visualizing infection of individual influenza viruses. Proc. Natl. Acad. Sci. U. S. A. , 9280–9285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiter G. A., Zerp S. F., Bartelink H., van Blitterswijk W. J., Verheij M. (2003) Anti-cancer alkyl-lysophospholipids inhibit the phosphatidylinositol 3-kinase-Akt/PKB survival pathway. Anticancer Drugs , 167–173 [DOI] [PubMed] [Google Scholar]
- 32.Yap T. A., Yan L., Patnaik A., Fearen I., Olmos D., Papadopoulos K., Baird R. D., Delgado L., Taylor A., Lupinacci L., Riisnaes R., Pope L. L., Heaton S. P., Thomas G., Garrett M. D., Sullivan D. M., de Bono J. S., Tolcher A. W. (2011) First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J. Clin. Oncol. , 4688–4695 [DOI] [PubMed] [Google Scholar]
- 33.Calleja V., Laguerre M., Parker P. J., Larijani B. (2009) Role of a novel PH-kinase domain interface in PKB/Akt regulation: structural mechanism for allosteric inhibition. PLoS Biol. , e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson P. G., Eng C., Kolesar J., Hideshima T., Anderson K. C. (2012) Perifosine, an oral, anti-cancer agent and inhibitor of the Akt pathway: mechanistic actions, pharmacodynamics, pharmacokinetics, and clinical activity. Expert Opin. Drug Metab. Toxicol. , 623–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arii J., Goto H., Suenaga T., Oyama M., Kozuka-Hata H., Imai T., Minowa A., Akashi H., Arase H., Kawaoka Y., Kawaguchi Y. (2010) Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1. Nature , 859–862 [DOI] [PubMed] [Google Scholar]
- 36.Bender F. C., Whitbeck J. C., Ponce de Leon M., Lou H., Eisenberg R. J., Cohen G. H. (2003) Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J. Virol. , 9542–9552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heldwein E. E., Lou H., Bender F. C., Cohen G. H., Eisenberg R. J., Harrison S. C. (2006) Crystal structure of glycoprotein B from herpes simplex virus 1. Science , 217–220 [DOI] [PubMed] [Google Scholar]
- 38.Koulen P., Madry C., Duncan R. S., Hwang J. Y., Nixon E., McClung N., Gregg E. V., Singh M. (2008) Progesterone potentiates IP(3)-mediated calcium signaling through Akt/PKB. Cell. Physiol. Biochem. , 161–172 [DOI] [PubMed] [Google Scholar]
- 39.Jha T. K., Sundar S., Thakur C. P., Bachmann P., Karbwang J., Fischer C., Voss A., Berman J. (1999) Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N. Engl. J. Med. , 1795–1800 [DOI] [PubMed] [Google Scholar]
- 40.Soto J., Arana B. A., Toledo J., Rizzo N., Vega J. C., Diaz A., Luz M., Gutierrez P., Arboleda M., Berman J. D., Junge K., Engel J., Sindermann H. (2004) Miltefosine for new world cutaneous leishmaniasis. Clin. Infect. Dis. , 1266–1272 [DOI] [PubMed] [Google Scholar]
- 41.Sindermann H., Engel K. R., Fischer C., Bommer W. (2004) Oral miltefosine for leishmaniasis in immunocompromised patients: compassionate use in 39 patients with HIV infection. Clin. Infect. Dis. , 1520–1523 [DOI] [PubMed] [Google Scholar]
- 42.Garg R., Tremblay M. J. (2012) Miltefosine represses HIV-1 replication in human dendritic cell/T-cell cocultures partially by inducing secretion of type-I interferon. Virology , 271–276 [DOI] [PubMed] [Google Scholar]
- 43.Chugh P., Bradel-Tretheway B., Monteiro-Filho C. M., Planelles V., Maggirwar S. B., Dewhurst S., Kim B. (2008) Akt inhibitors as an HIV-1-infected macrophage-specific anti-viral therapy. Retrovirology , 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benetti L., Roizman B. (2006) Protein kinase B/Akt is present in activated form throughout the entire replicative cycle of deltaU(S)3 mutant virus but only at early times after infection with wild-type herpes simplex virus 1. J. Virol. , 3341–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner M. J., Smiley J. R. (2011) Herpes simplex virus requires VP11/12 to activate Src family kinase-phosphoinositide 3-kinase-Akt signaling. J. Virol. , 2803–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carfi A., Gong H., Lou H., Willis S. H., Cohen G. H., Eisenberg R. J., Wiley D. C. (2002) Crystallization and preliminary diffraction studies of the ectodomain of the envelope glycoprotein D from herpes simplex virus 1 alone and in complex with the ectodomain of the human receptor HveA. Acta Crystallogr. D Biol. Crystallogr. , 836–838 [DOI] [PubMed] [Google Scholar]
- 47.Connolly S. A., Landsburg D. J., Carfi A., Whitbeck J. C., Zuo Y., Wiley D. C., Cohen G. H., Eisenberg R. J. (2005) Potential nectin-1 binding site on herpes simplex virus glycoprotein d. J. Virol. , 1282–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Atanasiu D., Whitbeck J. C., Cairns T. M., Reilly B., Cohen G. H., Eisenberg R. J. (2007) Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion. Proc. Natl. Acad. Sci. U. S. A. , 18718–18723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atanasiu D., Saw W. T., Cohen G. H., Eisenberg R. J. (2010) Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J. Virol. , 12292–12299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soroceanu L., Akhavan A., Cobbs C. S. (2008) Platelet-derived growth factor-α receptor activation is required for human cytomegalovirus infection. Nature , 391–395 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.