Abstract
Recent studies have shown that activation of the signal transducer and activator of transcription-3 (Stat3) is required for decidualization, interacting with progesterone receptor (PR) in uterus. Based on previous reports, we hypothesized that crosstalk between STAT3 and PR signaling is required for successful implantation. To identify the interaction between STAT3 and PR isoforms, we performed immunoprecipitation following transient cotransfection and found that STAT3 physically interacted with PR-A, which is known to be important for uterine development and function, but not with PR-B. To further investigate the role of Stat3 in uterine function, Stat3 was conditionally ablated only in the PR-positive cells (PRcre/+ Stat3f/f; Stat3d/d). Our studies revealed that ovarian function and uterine development of Stat3d/d mice were normal. However, Stat3d/d female mice were infertile due to defective embryo implantation. Unlike Stat3f/f mice, Stat3d/d mice exhibited an unclosed uterine lumen. Furthermore, uteri of Stat3d/d mice were unable to undergo a well-characterized hormonally induced decidual reaction. The expression of stromal PR was decreased during decidualization and preimplantation period in Stat3d/d mice, and PR target genes were significantly down-regulated after progesterone induction. Our results suggest that STAT3 and PR crosstalk is required for successful implantation in the mouse uterus.—Lee, J. H., Kim, T. H., Oh, S. J., Yoo, J.-Y., Akira, S., Ku, B. J., Lydon, J. P., Jeong, J.-W. Signal transducer and activator of transcription-3 (Stat3) plays a critical role in implantation via progesterone receptor in uterus.
Keywords: animal model, early pregnancy, steroid hormone, transcription factor
The endometrium is a dynamic tissue that undergoes coordinated changes under the influence of ovarian steroid hormones progesterone (P4) and estrogen (E2) during implantation. In rodents, E2 stimulates the proliferation of uterine epithelial cells at 0.5 days postcoitus (dpc; vaginal plug) of pregnancy. On formation of corpus luteum at 2.5 dpc, P4 inhibits E2-mediated proliferation of uterine epithelial cells and leads to uterine stromal cell proliferation (1). At 3.5 dpc, an acute E2 spike in concert with elevated P4 levels further stimulates uterine stromal proliferation and induces production of leukemia inhibitory factor (LIF) from uterine glands, a critical step for uterine receptivity (2). Implantation begins with the attachment of the blastocyst to the luminal epithelium, followed by invasion by the embryonic trophoblasts at 4.5 dpc (3). On invasion, uterine stromal cells decidualize, a cellular transformation characterized by morphological changes and the development of a secretory phenotype, which creates a local environment permissive to implantation and supportive of the implanting embryo. The implantation process itself is characterized by changes in the expression of adhesion molecules, cytokines, and transcriptional factors, which are dependent on E2 and P4. Human endometrium is also tightly regulated by steroid hormones, including proliferation, secretion, and menstrual shedding. Endometrium becomes receptive for embryo implantation during the midsecretory phase, and decidualized stromal cells are seen in the late-secretory phase of the menstrual cycle (4, 5). Because of the complexity and dynamic nature of implantation, the molecular processes underlying these changes are poorly understood. Unexplained infertility accounts for ∼30% of all infertility in humans (6, 7), and a primary cause of infertility is thought to be defective implantation and dysfunctional decidualization (8). To improve fertility rates, identification of molecular mechanism for unexplained infertility is required.
Progesterone receptor (PR) is required for successful implantation in both humans and in rodents. PR signaling has been shown to regulate implantation-associated events such as the preparation of uterine epithelium for receptivity and the decidualization of endometrial stromal cells (9). The PR-knockout (PRKO) mouse model utilizing a null mutation of the PR gene demonstrates the essential role for PR in P4-mediated uterine responses (10, 11), and has led to the identification of several P4-regulated pathways within the uterus (12). PRs are composed of 2 isoforms, termed PR-A and PR-B (13, 14). The generation of transgenic mice lacking either PR-A and PR-B (by site-directed mutagenesis of the PR gene in vivo) has demonstrated that the PR-A isoform is the major mediator of P4 signaling in the mouse uterus, whereas the PR-B isoform is required for the normal proliferative and differentiative responses of the mammary gland to progesterone (15, 16). Previous studies have shown that, during decidualization, PR directly interacts with signal transducer and activator of transcription-3 (STAT3) through protein-protein interactions (17). However, the functional relationship between PR and STAT3 in reproductive biology has remained elusive.
STAT3 is a transcription factor that is localized in the cytoplasm until activated by phosphorylation. STAT3 can be activated by a variety of cytokines, including LIF (18), interleukin (IL)-6 (19), IL-11 (20), and epidermal growth factor (19); on activation, it forms homodimers or heterodimers and translocates to the nucleus, where it binds to promoter regions of target genes and induces target gene expression (21). Stat3 is required for embryonic development, as Stat3-deficient embryos die in utero between 6.5 and 7.5 dpc (22). Transient and local suppression of STAT3 activity using a STAT3-phosphorotioate-modified oligodeoxynucleotide transferred in utero resulted in decreased implantation and suppression of decidualization (23). These studies suggest that Stat3 has a critical role during decidualization and implantation. However, there are currently no useful animal models to investigate the specific molecular mechanism.
In this study, Stat3 was conditionally ablated in the uterus by crossbreeding floxed Stat3 (Stat3f/f) mice with PRcre mice to effectively investigate the molecular role of Stat3 during early pregnancy. Herein, we demonstrate that Stat3 plays a crucial role in the uterus during embryo attachment, implantation, and decidualization, that this role involves direct interaction with PR, and that it regulates PR-related signaling in the uterus.
MATERIALS AND METHODS
Animals and tissue collection
Animals were maintained in a designated animal care facility in accordance with Michigan State University's institutional guidelines for their care. All protocols related to animals were overseen and approved by the animal care and use committee. Stat3 conditional-knockout mice were generated by crossing PRcre/+ (24) with Stat3f/f (25) mice (PRcre/+ Stat3f/f; Stat3d/d). Pregnant uterine samples were obtained by mating Stat3f/f or Stat3d/d female mice with C57BL/6 male mice with the morning of a vaginal plug being designated as 0.5 dpc. Mice were euthanized at 3.5, 4.5, and 5.5 dpc, and the number of implantation sites was identified at 5.5 dpc. The mice were anesthetized with avertin (2,2,-tibromoethyl alcohol; Sigma-Aldrich, St. Louis, MO, USA) and euthanized by cervical dislocation under anesthesia. Uterine tissues were snap-frozen at the time of dissection and either stored at −80°C for RNA/protein extraction or fixed with 4% (v/v) paraformaldehyde for histology. Fertility was assessed by mating 8-wk-old female Stat3f/f (n=4) and Stat3d/d (n=4) mice with wild-type male mice for 6 mo and determining the number and size of litters delivered.
Ovulation and embryo development
Virgin females (n=3/genotype) were superovulated by i.p. injection of 5 IU of pregnant mare's serum gonadotropin followed 48 h later by 5 IU of human chorionic gonadotropin and mated with wild-type male mice. The following morning (0.5 dpc), ovulated eggs were flushed from the oviducts at 0.5 dpc. To investigate preimplantation embryonic development, Stat3f/f or Stat3d/d female mice were allowed to naturally mate with wild-type male mice until the appearance of a vaginal plug. Then, at 3.5 dpc, embryos were collected by uterine flushing with M2 medium (Sigma-Aldrich), rinsed into microdrops of M2 medium, and counted.
Induction of decidualization
The hormonally induced decidual response has been previously described (26). Briefly, Stat3f/f and Stat3d/d female mice at 6 wk of age were ovariectomized (n=3/genotype for d 1, n=4/genotype for d 5). At 2 wk after ovariectomy, Stat3f/f and Stat3d/d mice were subjected to the following hormonal regimen: 100 ng E2/d for 3 d; 2 d rest; then 3 daily injections of 1 mg P4 + 6.7 ng E2. At 6 h after the third P4 and E2 injection, the left uterine horn was mechanically stimulated by scratching the full length of the antimesometrial side with a burred needle. The other horn was left unstimulated as a control. Daily injections of P4 (1 mg/mouse) + E2 (6.7 ng/mouse) were continued for 5 d to maximize the decidual response. Then, mice were euthanized on d 1 or 5. The uteri were then excised, weighed, and fixed in 4% paraformaldehyde for histological analysis.
Progesterone treatment
Stat3f/f and Stat3d/d mice at 6 wk of age were ovariectomized. At 2 wk after ovariectomy, the mice received injections of either vehicle (sesame oil) or P4 (1 mg/mouse). Mice were euthanized at 6 h after the injection (n=5/genotype/treatment). Uteri were then excised, trimmed, and processed for real-time PCR analysis.
Immunoprecipitation
HEK 293T cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL, Gaithersburg, MD, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco BRL), 100 U/ml penicillin (Gibco BRL), and 0.1 mg/ml streptomycin (Gibco BRL) at 37°C under 5% CO2. Human STAT3, PR-A, and PR-B expression vectors were generated by PCR and cloned into the pcDNA3.1-V5/His and pcDNA3.1 (Invitrogen Corp., Carlsbad, CA, USA) plasmid vectors. Transient transfection of V5-tagging human STAT3, PR-A, and PR-B expression vectors was performed using Lipofectamine 2000 (Invitrogen). At 24 h post-transfection, lysates were sonicated in lysis buffer (20 mM Tris-Cl, pH 8.0; 0.5 mM EDTA; 0.5% Nonidet P-40; and 100mM NaCl) with phosphatase inhibitor cocktails (Sigma-Aldrich) to extract total proteins including nuclear proteins. Lysate (1 mg) was immunoprecipitated with 2 μg of antibodies to V5 (A190-220A; Bethyl Laboratories, Montgomery, TX, USA) and PR (sc-538; Santa Cruz Biotechnology, Santa Cruz, CA, USA) with 30 μl of resuspended protein A-agarose (Pierce Biotechnology, Rockford, IL, USA) and incubated overnight at 4°C. Immunocomplexs were captured by protein A-agarose after centrifugation at 3000 rpm for 3 min at 4°C. The supernatant was discarded, and the pellet was resuspended and washed 3 times in 1 ml of lysis buffer. The washed pellet was then resuspended in 20 μl of Laemmli buffer (Bio-Rad Laboratories, Hercules, CA, USA) and boiled for 5 min at 95°C. Immunocomplexes were subjected to polyacrylamide gel electrophoresis, transferred to PVDF membranes, and exposed to anti-PR and anti-V5 antibodies.
For in vivo immunoprecipitation, Stat3f/f and Stat3d/d mice were mated with C57BL/6 male mice and euthanized at 3.5 dpc of pregnancy. Whole uteri were pooled from 3 Stat3f/f and 3 Stat3d/d mice, and cell lysates were extracted in lysis buffer after embryo flushing and precleared by addition of normal mouse immunoglobulin G with protein A-agarose. After bead removal, the supernatant (1 mg/ml) was immunoprecipitated with an antibody to STAT3 (no. 4904; Cell Signaling, Danvers, MA, USA) and PR (Santa Cruz Biotechnology). Immunocomplexes were then subjected to polyacrylamide gel electrophoresis, transferred onto PVDF membranes, and subsequently exposed to PR and STAT3 antibodies.
Quantitative real-time PCR
RNA was extracted from the uterine tissues using the RNeasy total RNA isolation kit (Qiagen, Valencia, CA, USA). mRNA expression levels of Stat3, decidual marker genes (Wnt4 and Bmp2), and PR target genes (Lrp2, Fst, Areg, Il13ra2, and Cyp26a1) were measured by real-time PCR TaqMan analysis using an Applied Biosystems StepOnePlus system (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions and using prevalidated probes, primers, 18S RNA, and Universal Master Mix reagent purchased from Applied Biosystems. Template cDNA was produced from 1 μg of total RNA using random hexamers and MMLV Reverse Transcriptase (Invitrogen). All real-time PCRs were done by using 3 independent RNA sets. The mRNA quantities were normalized against 18S RNA using ABI rRNA control reagents (Applied Biosystems).
Immunohistochemistry
Uterine sections from paraffin-embedded tissues were cut at 5 μm and mounted on silane-coated slides, deparaffinized, and rehydrated in a graded alcohol series before blocking with 10% normal serum in PBS (pH 7.5) and incubating with primary antibody diluted in 10% normal goat serum in PBS (pH 7.5) overnight at 4°C at the following dilutions: 1:100 for anti-phospho-STAT3 (no. 9131; Cell Signaling), 1:500 for anti-STAT3 (4904; Cell Signaling), and 1:1000 for anti-total PR antibody (A0098; Dako, Carpinteria, CA, USA). On the following day, sections were washed in PBS and incubated with the appropriate species-specific horseradish peroxidase-conjugated secondary antibody (2 μg/ml; Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature. Immunoreactivity was detected using the Vectastain Elite DAB kit (Vector Laboratories).
Statistical analysis
Data are presented as means ± sd. Statistical analyses were performed using 1-way ANOVA followed by Tukey's post hoc multiple range test or Student's t tests using the Instat package from GraphPad (San Diego, CA, USA). Values of P < 0.05 were considered statistically significant.
RESULTS
Interaction of STAT3 with PR
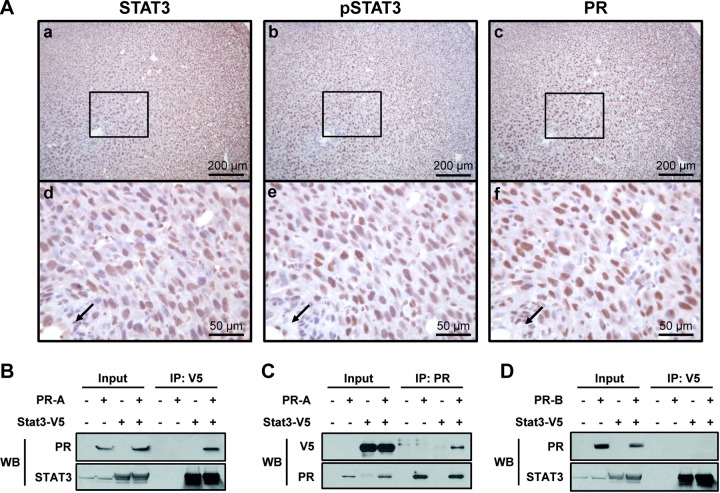
We examined the expression profile of Stat3 mRNA in the uteri of wild-type adult mice during early pregnancy via real-time RT-PCR. As shown in Supplemental Fig. S1A, the expression of Stat3 was gradually increased until 7.5 dpc, when it reached statistical significance (P<0.01). Next, protein expression profiles of STAT3 and phospho-STAT3 were analyzed during early pregnancy (Supplemental Fig. S1B). Immunohistochemical analysis of uterine cross-sections revealed that STAT3 was consistently expressed in the both the luminal and glandular epithelium and in some stromal cells of pregnant and nonpregnant uteri (Supplemental Fig. S1Ba–f). Strong expression of phospho-STAT3 was present in luminal and glandular epithelium at 3.5 dpc (Supplemental Fig. S1Bi) and markedly increased within the decidual stromal cells at the implantation site (Supplemental Fig. S1Bj, k). However, the level of phospho-STAT3 was weak in nonpregnant uteri (Supplemental Fig. S1Bl). Interestingly, this expression pattern was highly correlated with PR expression in decidual cells near the embryos (Fig. 1A, arrows). Levels of PR and phospho-STAT3 were increased at 5.5 dpc (Fig. 1Ae, f). These results show that the expression patterns of STAT3 and PR are highly correlated in decidual cells. To determine whether STAT3 physically interacts with PR-A or PR-B isoform, we cotransfected V5-tagged human STAT3 with PR constructs expressing either human PR-A or PR-B. Lysates were then immunoprecipitated with V5 or PR antibodies, blotted, and reprobed with PR and V5 antibodies. The results show that PR-A immunoprecipitated with V5 antibody (Fig. 1B), and V5-tagged STAT3 was present within the anti-PR immunoprecipitate (Fig. 1C). However, no PR-B was detected within the immunoprecipitate of the V5-tagged STAT3 (Fig. 1D), suggesting that STAT3 physically interacts with PR-A, not PR-B.
Figure 1.
Expression of STAT3 and PR in decidual cells and the immunoprecipitation of STAT3 with PR-A or PR-B. A) Localization pattern of STAT3 (a, d), phospho-STAT3 (b, e), and PR (c, f) proteins was investigated by immunohistochemistry in uteri of C57BL/6 mice at 5.5 dpc. Nuclei were counterstained with hematoxylin. Arrow indicates embryo. B) Immunoprecipitate with V5 was immunoblotted with PR and STAT3 antibodies. C) Immunoprecipitate with PR was immunoblotted with V5 and PR antibodies. D) Immunoprecipitate with V5 was immunoblotted with PR and STAT3 antibodies.
Ablation of Stat3 in the murine uterus
To explore the relevance of the interaction between PR and STAT3, we used a Cre-LoxP approach to generate a conditional-knockout mouse model in which Stat3 gene expression is ablated specifically in the PR-expressing cells. Ablation of Stat3 was confirmed by real-time PCR, Western blot, and immunohistochemical analysis of the uteri of artificially induced decidualized uterus on d 1. As seen in Fig. 2A, we found Stat3 mRNA expression was significantly lower in Stat3d/d mice compared to Stat3f/f mice, and STAT3 protein was reduced in Stat3d/d mice by Western blot (Fig. 2B). In line with our previous results, the uteri of Stat3f/f control mice expressed STAT3 protein within the luminal epithelium, glandular epithelium, and stroma, whereas this staining was absent in the Stat3d/d mice (Fig. 2C). These results confirm our successful ablation of Stat3 within the uterus of Stat3d/d mice.
Figure 2.
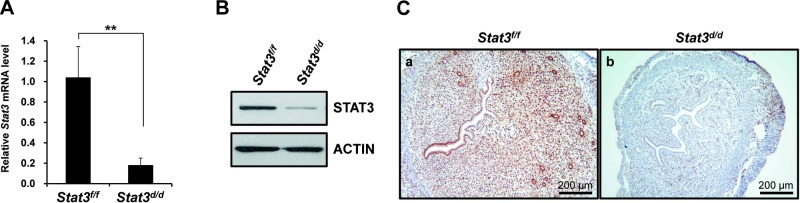
Ablation of Stat3 mRNA and STAT3 protein in the uterus of Stat3d/d mice. A) Expression of Stat3 was measured by real-time PCR in artificially induced decidualized uterus. Results represent means ± sd of 3 independent RNA sets. **P < 0.01. B) Expression of STAT3 protein was compared between uteri of Stat3f/f and Stat3d/d mice by Western blot after artificially induced decidualization. C) Expression of STAT3 protein in uteri of Stat3f/f (a) and Stat3d/d (b) mice after artificially induced decidualization, compared by immunohistochemistry.
Effect of Stat3 ablation on fertility and implantation
Female Stat3f/f and Stat3d/d mice were mated with C57BL/6 male mice to determine their fertility (n=4). After recording the number of pups over a 6-mo time period, we found that Stat3d/d mice were infertile (Table 1) whereas control mice exhibited normal fecundity (average 7.78±1.60 pups/litter). Because of reported Cre recombinase expression within the pituitary, ovary, uterus, and mammary gland of PRCre mice (24), we reasoned that Stat3d/d mice may display an ovulatory defect. To test for a possible ovarian defect, female Stat3f/f and Stat3d/d mice were examined for their ability to ovulate in response to a well-established superovulatory regimen of gonadotropin treatment. Stat3f/f and Stat3d/d mice yielded 22.2 ± 1.88 and 27 ± 3.79 oocytes, respectively. These values were in line with previously published data and not significantly different. In addition, histological analysis of the intact ovaries from Stat3f/f and Stat3d/d mice at 10 wk of age revealed no morphological defect (Supplemental Fig. S2). To test for embryonic defects, blastocysts generated by natural mating were flushed from the uterus at 3.5 dpc. We isolated 6.7 ± 0.33 and 8.3 ± 0.67 embryos from uteri of Stat3f/f and Stat3d/d mice, respectively. These results show that ovarian function and embryonic development are not affected in Stat3d/d females, suggesting that the fertility defect seen in Stat3d/d mice is primarily of uterine origin.
Table 1.
Infertility of Stat3d/d mice
| Genotype | Mice (n) | Litters (n) | Pups (n) | Average pups/litter (n) | Average litters/mouse (n) |
|---|---|---|---|---|---|
| Stat3f/f | 4 | 23 | 179 | 7.78 ± 1.60 | 5.75 ± 0.13 |
| Stat3d/d | 4 | 0 | 0 | 0 | 0 |
Female mice were mated with C57BL/6 male mice for 6 mo, and the number of pups and litters were counted.
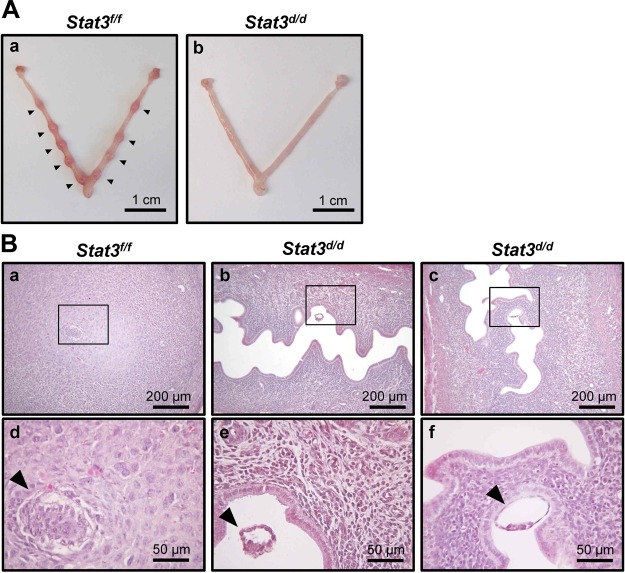
To further dissect the cause of infertility, we examined whether ablation of Stat3 alters implantation rates. Mice were euthanized at 5.5 dpc, and the number of implantation sites was identified. The uterine horns (n=5) of Stat3d/d mice had no implantation sites (Fig. 3Ab), whereas control Stat3f/f mice averaged 9 ± 0.26 implantation sites (n=6; Fig. 3Aa). Subsequent histological analysis revealed that all embryos were positioned as expected alongside the mesometrial luminal epithelium, and the stromal cells exhibited the decidual response surrounding the embryo (Fig. 3Ba, d). However, 85.71% of the embryos were observed free-floating within the uterine lumen of Stat3d/d mice (Fig. 3Bb, e), suggesting a lack of attachment. In addition, 14.29% of embryos were located near the luminal epithelium, but decidual cells were not observed in the uteri of Stat3d/d mice (Fig. 3Bc, f). These results demonstrate that the infertility seen in Stat3d/d mice is due to a uterine defect and caused by defective embryo attachment and decidualization.
Figure 3.
Implantation failure in Stat3d/d mice. Mice were euthanized at 5.5 dpc, and the number of implantation sites was counted. A) Implantation sites were not detected in the uteri of any Stat3d/d mice (n=5), compared with Stat3f/f mice (n=6). Arrowheads indicate implantation sites. B) Histology of implantation sites in Stat3f/f (a, d) and Stat3d/d (b, c, e, f) mice at 5.5 dpc was investigated by hematoxylin and eosin staining. Stat3d/d mice exhibited 6 floating embryos (b, e) and 1 attached embryo (c, f) at implantation site. Arrowheads indicate embryos.
Effect of Stat3 ablation on embryo-uterine attachment
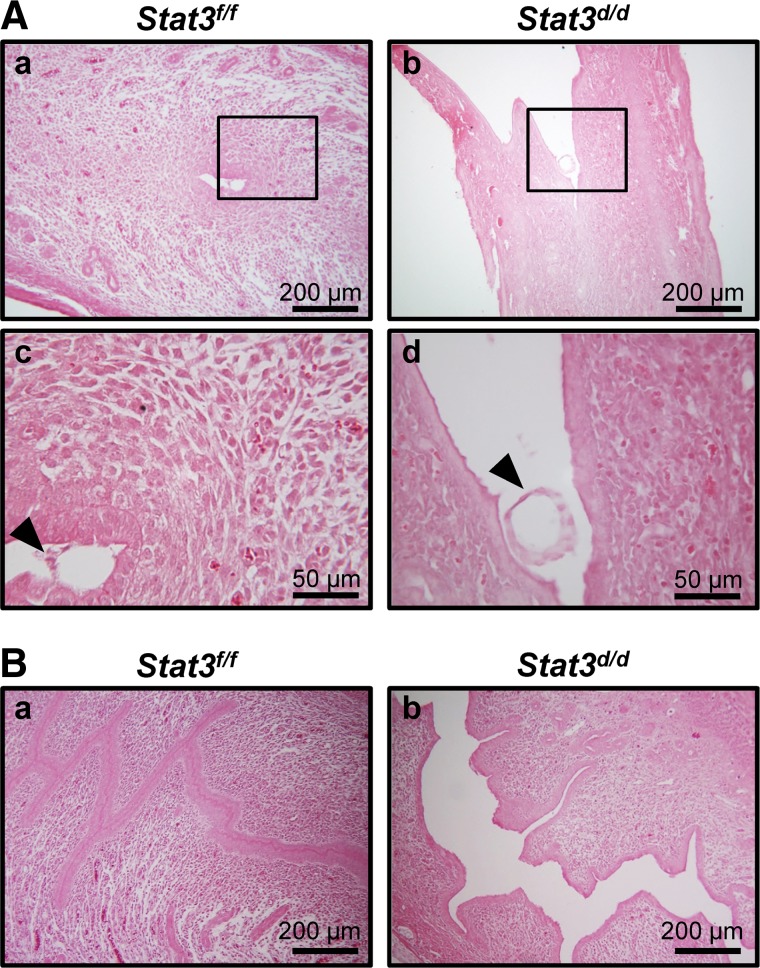
Successful blastocyst implantation requires attachment of the embryo to the luminal epithelium and a uterine environment permissive to this attachment. To address the defect of implantation, we investigated embryo attachment by analyzing uterine histology in Stat3f/f and Stat3d/d mice at 4.5 dpc. As shown in Fig. 4A, embryos failed to attach into luminal epithelium and floated in the uterine lumen of Stat3d/d mice (Fig. 4Ab, d), otherwise Stat3f/f mice exhibited well-attached embryo and differentiation of uterine stromal cells to decidual cells began (Fig. 4Aa, c). Interestingly, we also observed that Stat3d/d mice failed to assemble a closed uterine lumen, which is essential for successful embryo attachment, unlike control mice at 4.5 dpc (Fig. 4B). These results, in total, suggest that Stat3d/d mice have defects in uterine receptivity that may be caused, in part, by the failure of closed uterine lumen.
Figure 4.
Defective embryo attachment and abnormal structure of luminal epithelium in Stat3d/d mice. A) Histology of implantation site in Stat3f/f and Stat3d/d mice at 4.5 dpc was investigated by H&E staining. Stat3d/d mice exhibited floating embryos (b, d), whereas embryos were successfully attached into luminal epithelium in Stat3f/f mice (a, c). Arrowheads indicate embryos. B) Histological analysis by H&E staining. Uteri in Stat3d/d mice revealed a failure to close the lumen at 4.5 dpc (b), while Stat3f/f uteri had well-closed lumens (a).
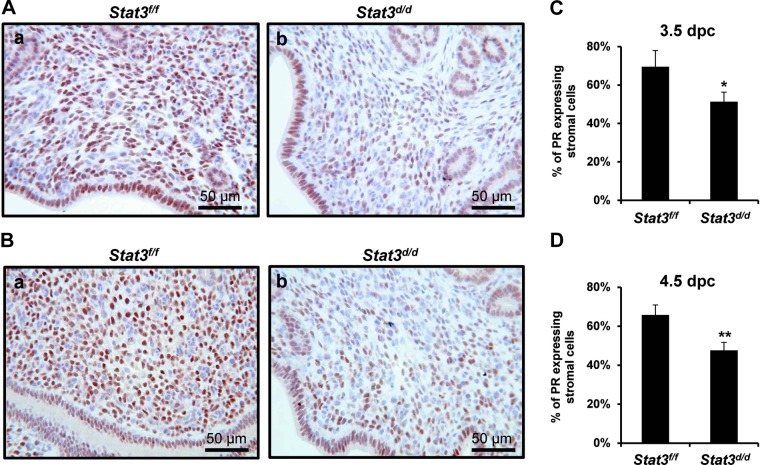
To identify the effect of Stat3 ablation on PR signaling during pre-, and peri-implantation, we examined PR protein levels at 3.5 and 4.5 dpc via immunohistochemistry. As shown in Fig. 5, PR levels were decreased in the stromal cells of Stat3d/d mice at both 3.5 dpc (Fig. 5Ab) and 4.5 dpc (Fig. 5Bb), and the percentages of PR-positive stromal cells were significantly reduced in Stat3d/d mice at 3.5 dpc (Fig. 5C) and 4.5 dpc (Fig. 5D). These data also support our hypothesis that Stat3 has a critical role during implantation via interaction with PR.
Figure 5.
PR protein expression in the uteri at 3.5 and 4.5 dpc. A) Decreased stromal PR protein expression was detected in the uterus of Stat3d/d mice at 3.5 dpc (b), compared to Stat3f/f mice (a). B) PR protein expression was reduced in the uterine stroma of Stat3d/d mice at 4.5 dpc (b), compared to Stat3f/f mice (a). C, D) Percentage of PR expressing stromal cells analyzed in the uteri at 3.5 (C) and 4.5 dpc (D). Results represent means ± sd of 3 animals/treatment. *P < 0.05; **P < 0.01.
Effect of Stat3 ablation on decidualization
Decidualization is required for successful embryo implantation and maintenance of pregnancy. Levels of Stat3 mRNA and activated STAT3 protein were increased on d 5 of induced decidualization in the wild-type uterus (Supplemental Fig. S3). To determine whether the infertility seen in our Stat3d/dmice is due to a decidualization defect, uterine function was assessed by examining the ability of Stat3d/d mice to undergo a well-characterized induced decidual response. Ovariectomized female Stat3f/f and Stat3d/d mice (n=3 for d 1, n=4 for d 5) were primed with E2 plus P4, and the uterus was mechanically stimulated to mimic the presence of an implanting embryo and induce decidualization (see Materials and Methods). Stat3f/f mice responded normally with the expected increase in decidual uterine horn weight; however, the Stat3d/d mice were unresponsive (Fig. 6A). This difference can be observed visually (Fig. 6B) and further confirmed by histological analysis of stromal differentiation (Fig. 6C). In addition, the expression of known decidualization markers, such as wingless-related mouse mammary tumor virus integration site 4 (Wnt4) and bone morphogenetic protein 2 (Bmp2), were significantly reduced in the decidual uterine horn of the Stat3d/d mice compared to Stat3f/f mice on d 1 and 5 of artificial induction of decidualization (Fig. 7A, B). As Wnt4 and Bmp2 are regulated by PR signaling, we also investigated PR expression levels after artificial induction of decidualization in Stat3f/f and Stat3d/d mice. PR expression was weak during artificially induced decidualization in Stat3d/d mice, whereas its expression was highly increased in Stat3f/f mice, as expected (Fig. 7C). These results clearly show that Stat3d/d mice exhibit a decidualization defect accompanied by alterations in downstream PR signaling during decidualization.
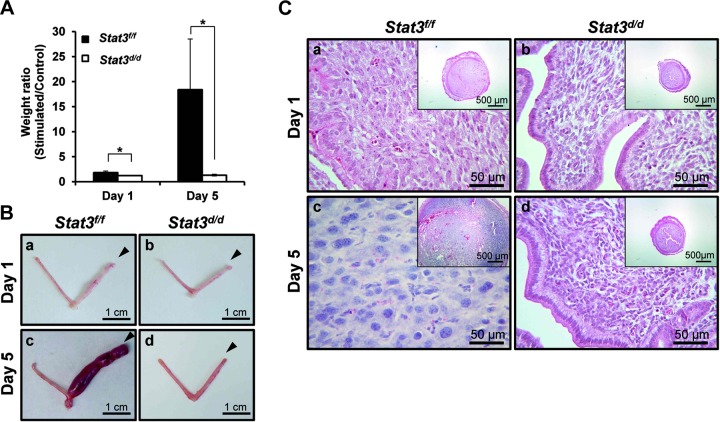
Figure 6.
Decidualization defect in Stat3d/d mice. A) Uterine weight ratios were measured after artificially induced decidualization at d 1 and 5 and not induced in Stat3d/d mice. Results represent means ± sd of 3 and 4 animals/group. *P<0.05. B) Uteri were dissected from Stat3f/f and Stat3d/d mice after artificially induced decidualization. Stimulated horns did not develop decidua in Stat3d/d mice (b, d), whereas Stat3f/f mice had a robust decidual response (a, c). Arrowheads indicate stimulated horns. C) Histology of uteri was investigated by hematoxylin and eosin staining. Stained sections show well-developed decidua in Stat3f/f mice (a, c), but not in Stat3d/d mice (b, d).
Figure 7.
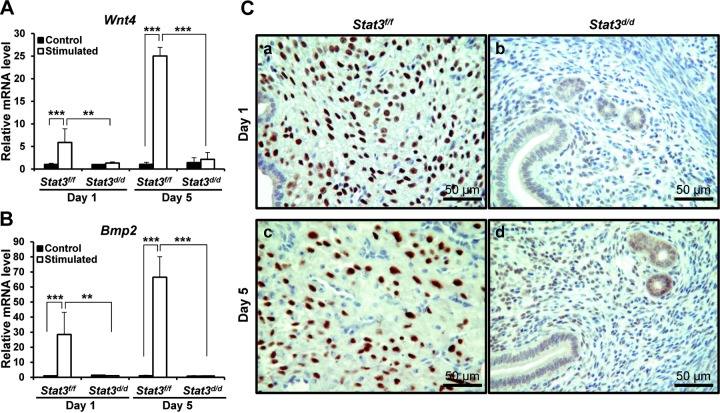
Expression of decidual marker genes and PR protein expression in induced decidual uterus. A, B) Wnt4 (A) and (B) Bmp2 mRNA expression were measured in uteri of artificially induced decidualization and compared in Stat3f/f and Stat3d/d mice on d 1 and 5 of stimulation. Results represent means ± sd of 3 and 4 animals/treatment. *P < 0.05; **P < 0.01. C) Decreasing PR protein expression was detected by immunohistochemistry in Stat3d/d mice (b, d), while Stat3f/f mice had increased PR expression in decidual cells (a, c).
Effect of Stat3 ablation on progesterone signaling
Since we observed the interaction of STAT3 and PR-A in vitro, we used uterine lysates from Stat3f/f and Stat3d/d mice at 3.5 dpc of pregnancy to examine whether STAT3 interacts with PR in vivo. When uterine lysates were immunoprecipitated with STAT3 and PR antibodies, a STAT3:PR complex was detected in Stat3f/f mice, but not in Stat3d/d mice (Supplemental Fig. S4). This result suggests that PR expression is altered in the Stat3-knockout uterus during the implantation period through protein-protein interaction.
To determine whether direct and rapid responsive targets of P4 activity are altered by the ablation of Stat3, the expressions of known PR target genes were analyzed after P4 treatment. Ovariectomized Stat3f/f and Stat3d/d female mice were treated with vehicle (sesame oil) or P4 (1 mg/mouse) for 6 h (n=5 mice/treatment/genotype), and whole uteri were subjected to real-time PCR. As seen in Fig. 8, the expression levels of cytochrome P450, family 26, subfamily A, polypeptide 1 (Cyp26a1), follistatin (Fst), amphiregulin (Areg), lipoprotein receptor-related protein 2 (Lrp2), and IL-13 receptor, α2 (Il13ra2) were significantly reduced in Stat3d/d mice as compared to levels in Stat3f/f mice in response to the P4 treatment. These data indicate that PR signaling is dysregulated by the ablation of Stat3.
Figure 8.
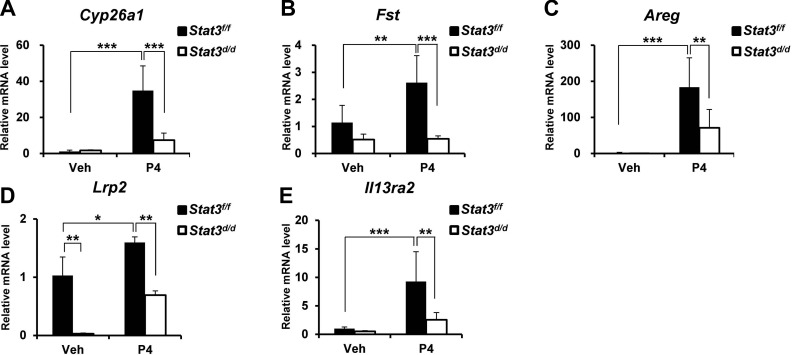
PR-regulated gene expression in uteri of Stat3d/d mice. Expressions of Cyp26a1 (A), Fst (B), Areg (C), Lrp2 (D), and Il13ra2 (E) were investigated by real-time PCR in uteri treated with P4 for 6 h. Results represent means ± sd of 5 animals/genotype. *P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
In this study, we found that STAT3 is highly expressed and activated in decidual cells expressing PR. To confirm our hypothesis that Stat3 crosstalk with PR signaling is required for successful pregnancy, we utilized a Cre-LoxP mouse model to specifically ablate Stat3 only in PR-expressing cells and found that the resultant Stat3d/d female mice were infertile (Table 1). Further studies confirmed that this defect was not due to altered ovarian function or embryonic development but rather implantation failure at 5.5 dpc (Fig. 3), including embryo-uterine attachment failure (Fig. 4) and a defective decidual response (Fig. 6). In addition, the down-regulation of known PR target genes was identified in uteri of Stat3d/d mice (Fig. 8).
During implantation, the embryonic trophectoderm layer first attaches to and then invades the maternal luminal epithelium. In this process, uterine stromal cells transform into large multinucleated cells with a secretory phenotype in a process known as decidualization (3). Decidualization is critical for establishment of a fetal-maternal interface during implantation and dependent on P4. PRKO mice demonstrate a decidualization defect, suggesting that PR has an important role in decidualization (10). Studies of isoform-specific PR ablation in mice have shown that the absence of PR-A is the major mediator of P4 signaling in murine reproductive tract (9) whereas the PR-B isoform is required for P4 signaling in the mammary gland (15). In human stroma, PR-A expresses throughout the cycle and implies a role for postovulatory events (27), whereas only PR-A isoform is present in endometriotic implants (28). This study confirmed a previous report (15) demonstrating that STAT3 and PR interact in the mesometrium at 3.5 dpc (Supplemental Fig. S4) and further identified that STAT3 specifically interacted with PR-A isoform, not PR-B (Fig. 1B, C). In this study, conditional ablation of uterine Stat3 caused a decidualization defect (Fig. 6) and prevented the induction of both PR protein expression and the induction of several known PR target genes in decidual cells (Fig. 7). Furthermore, Stat3d/d mice displayed a lack of decidual response at the implantation site, even when embryos were located adjacently to the luminal epithelium (Fig. 3Bc, f), which confirms the endometrial origin of the infertility phenotype. The expression of stromal PR was decreased during decidualization and the preimplantation period in Stat3d/d mice (Figs. 5 and 7), and PR target genes were significantly down-regulated after progesterone induction (Fig. 8). Our results suggest that STAT3 is critical for PR function in the uterus during embryo attachment, implantation, and decidualization. Female mice with ablation of PR (PRKO) are infertile due to defects in multiple reproductive tissues (10, 11). Female mice with epithelial specific ablation of PR are infertile due to defects in embryo attachment, stromal cell decidualization, and the inability to cease E2-induced epithelial cell proliferation (29). Therefore, future studies are needed to determine whether PR regulates STAT3 function in the uterus using PRKO and epithelial cell-specific PR-knockout mice.
P4-PR-regulated genes during implantation have been identified by oligonucleotide arrays following acute and chronic P4 treatment in wild-type and PRKO mice, and the genes encoding Cyp26a1, Il13ra2, Areg, and Lrp2 were identified (12). In this study, we analyzed these genes after 6 h of P4 administration to investigate the direct effect of Stat3 ablation on PR target genes (Fig. 8). Our real-time PCR results showed that the expression of Lrp2 was dramatically decreased in Stat3d/d mice with respect to the control. Lrp2 is an endocytic receptor expressed in steroid-responsive tissues, such as the epididymis, prostate, ovaries, and uterus (30). Cyp26a1 is an early response gene encoding an enzyme involved in retinoic acid metabolism that has an important role in the maintenance of pregnancy and embryonic development (31). Previous studies have shown that Cyp26a1 expression is induced within uterine luminal epithelium in response to acute P4-treatment, particularly between 3.5 and 4.5 dpc, and this response is absent in the PRKO uterus (32). Interestingly, we observed a similar pattern with PRKO in our Stat3d/d mice, with reduced expression of Cyp26a1 after acute (6 h) P4 treatment in this study. Areg, a member of the epidermal growth factor family, is known to be P4 regulated in the mouse uterus, where its expression is limited to the luminal epithelium of implantation sites (33). Suppression of these PR target genes in Stat3d/d mice following P4 treatment indicates that the P4-PR axis is altered by Stat3 ablation and may contribute to the observed implantation defect. However, we also found that several known PR target genes (Aldh1a1, Hoxa10, Ihh, Cited2, and Nfil3) did not change in Stat3d/d mice following P4 treatment (data not shown), suggesting that Stat3-independent PR target genes also exist.
Immunohistochemistry analysis for phospho-STAT3 demonstrated that STAT3 was highly activated in luminal and glandular epithelia at 3.5 dpc (Supplemental Fig. S1Bc, i) and its expression then moved to the decidualized stromal cells at 5.5 dpc, at which time phospho-STAT3 became coexpressed with PR (Fig. 1A). At 3.5 dpc, LIF, a known marker of uterine receptivity, is produced by uterine epithelial cells in response to an acute spike of E2 (2). Lif is known to be an E2-regulated gene in the glandular epithelium and a necessary effector of E2 action at the time of implantation (34, 35). To determine whether E2 signaling is altered by the ablation of Stat3 at preimplantation, the expression of LIF and E2 receptor α (ERα) was examined. However, the expression of LIF and ERα was not altered in Stat3d/d mice (data not shown). As Lif is an upstream molecule of Stat3 pathway, Lif expression level might not be changeable by the ablation of Stat3. The levels of E2 response genes, including mucin 1 (Muc1), chloride channel calcium activated 3 (Clca3), lactoferrin (Ltf), and Lif, were not altered in Stat3d/d mice at 3.5 dpc compared with Stat3f/f mice. These results suggest that E2 signaling is not altered in Stat3d/d mice at the preimplantation period.
Uterine function of Stat3 has been studied previously by expression analysis during pregnancy (36, 37) and the use of specific inhibitors to suppress the Stat3 expression in uterus (23). STAT3 has also been shown to be activated in both the luminal epithelium and stromal cells during preimplantation and in the decidua (36). In a previous study, STAT3 activity was transiently and locally suppressed by STAT3 phosphorotioate-modified oligodeoxynucleotides, resulting in decreased implantation and suppression of decidualization (23). On the basis of previous studies, we further identified that the defective embryo attachment and the failure of closed uterine lumen caused infertility in mice with a uterine conditional ablation of Stat3. In humans, STAT3 is highly activated in midsecretory stage epithelium, a time when the endometrium is receptive to incoming embryos, and intense expression of phospho-STAT3 was shown in decidual stromal cells in the late-secretory stage (5). However, STAT3 expression in glandular epithelium is significantly decreased in women with infertility as compared to fertile controls (38). These cumulative data suggest that Stat3 may represent a useful diagnostic or therapeutic target for infertility. In this study, we also confirmed STAT3 expression and activation are correlated with PR signaling in epithelial and stromal compartments of the adult uterus during the pre- and peri-implantation periods. Therefore, our mouse model may also represent a useful mechanism for the future study of unexplained infertility and the P4-PR signaling axis.
In summary, the conditional ablation of Stat3 results in infertility, due to implantation failure caused by defective uterine receptivity and decidual response. Based on our observation that the absence of Stat3 leads to dysregulation of PR-mediated pathways and decreased PR protein expression in utero, we suggest that Stat3 has a critical and PR-dependent role during embryo implantation. As the mechanisms regulating steroid hormone signaling are implicated in a variety of human uterine disorders, including infertility, endometriosis, and endometrial cancer, understanding the Stat3 regulation in uterine function may provide valuable insight into these diseases.
Supplementary Material
Acknowledgments
The authors thank Francesco J. DeMayo and CheMyong Jay Ko for invaluable discussions and Susan D. Ferguson, Sharra A. Poncil, and Thuy L. Tran for manuscript preparation.
This work was supported by U.S. National Institutes of Health grant R01HD057873; the World Class University (WCU) program (R31-10056) through the National Research Foundation of Korea, funded by the Ministry of Education, Science, and Technology (J.W.J.); and the Lalor Foundation's postdoctoral fellowship program (J.H.L).
The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- Areg
- amphiregulin
- Bmp2
- bone morphogenetic protein 2
- Cyp26a1
- cytochrome P450, family 26, subfamily A, polypeptide 1
- dpc
- days postcoitus
- E2
- estrogen
- ERα
- estrogen receptor α
- Fst
- follistatin
- IL
- interleukin
- Il13ra2
- interleukin-13 receptor, α2
- LIF
- leukemia inhibitory factor
- Lrp2
- lipoprotein receptor-related protein 2
- P4
- progesterone
- PR
- progesterone receptor
- PRKO
- progesterone receptor knockout
- STAT3
- signal transducer and activator of transcription-3
- Wnt4
- wingless-related mouse mammary tumor virus integration site 4
REFERENCES
- 1. Franco H. L., Jeong J. W., Tsai S. Y., Lydon J. P., DeMayo F. J. (2008) In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin. Cell. Dev. Biol. 19, 178–186 [DOI] [PubMed] [Google Scholar]
- 2. Stewart C. L., Kaspar P., Brunet L. J., Bhatt H., Gadi I., Kontgen F., Abbondanzo S. J. (1992) Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359, 76–79 [DOI] [PubMed] [Google Scholar]
- 3. Lee K. Y., DeMayo F. J. (2004) Animal models of implantation. Reproduction 128, 679–695 [DOI] [PubMed] [Google Scholar]
- 4. Von Wolff M., Thaler C. J., Strowitzki T., Broome J., Stolz W., Tabibzadeh S. (2000) Regulated expression of cytokines in human endometrium throughout the menstrual cycle: dysregulation in habitual abortion. Mol. Human Reprod. 6, 627–634 [DOI] [PubMed] [Google Scholar]
- 5. Dimitriadis E., Stoikos C., Tan Y. L., Salamonsen L. A. (2006) Interleukin 11 signaling components signal transducer and activator of transcription 3 (STAT3) and suppressor of cytokine signaling 3 (SOCS3) regulate human endometrial stromal cell differentiation. Endocrinology 147, 3809–3817 [DOI] [PubMed] [Google Scholar]
- 6. Evers J. L. (2002) Female subfertility. Lancet 360, 151–159 [DOI] [PubMed] [Google Scholar]
- 7. Smith S., Pfeifer S. M., Collins J. A. (2003) Diagnosis and management of female infertility. JAMA 290, 1767–1770 [DOI] [PubMed] [Google Scholar]
- 8. Sharkey A. M., Smith S. K. (2003) The endometrium as a cause of implantation failure. Best Pract. Res. Clin. Obstet. Gynaecol. 17, 289–307 [DOI] [PubMed] [Google Scholar]
- 9. Conneely O. M., Mulac-Jericevic B., Lydon J. P. (2003) Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids 68, 771–778 [DOI] [PubMed] [Google Scholar]
- 10. Lydon J. P., DeMayo F. J., Funk C. R., Mani S. K., Hughes A. R., Montgomery C. A., Jr., Shyamala G., Conneely O. M., O'Malley B. W. (1995) Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 9, 2266–2278 [DOI] [PubMed] [Google Scholar]
- 11. Lydon J. P., DeMayo F. J., Conneely O. M., O'Malley B. W. (1996) Reproductive phenotpes of the progesterone receptor null mutant mouse. J. Steroid Biochem. Mol. Biol. 56, 67–77 [DOI] [PubMed] [Google Scholar]
- 12. Jeong J. W., Lee K. Y., Kwak I., White L. D., Hilsenbeck S. G., Lydon J. P., DeMayo F. J. (2005) Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology 146, 3490–3505 [DOI] [PubMed] [Google Scholar]
- 13. Kastner P., Krust A., Turcotte B., Stropp U., Tora L., Gronemeyer H., Chambon P. (1990) Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 9, 1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kraus W. L., Montano M. M., Katzenellenbogen B. S. (1993) Cloning of the rat progesterone receptor gene 5′-region and identification of two functionally distinct promoters. Mol. Endocrinol. 7, 1603–1616 [DOI] [PubMed] [Google Scholar]
- 15. Mulac-Jericevic B., Lydon J. P., DeMayo F. J., Conneely O. M. (2003) Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc. Natl. Acad. Sci. U. S. A. 100, 9744–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mulac-Jericevic B., Mullinax R. A., DeMayo F. J., Lydon J. P., Conneely O. M. (2000) Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 289, 1751–1754 [DOI] [PubMed] [Google Scholar]
- 17. Liu T., Ogle T. F. (2002) Signal transducer and activator of transcription 3 is expressed in the decidualized mesometrium of pregnancy and associates with the progesterone receptor through protein-protein interactions. Biol. Reprod. 67, 114–118 [DOI] [PubMed] [Google Scholar]
- 18. Cheng J. G., Chen J. R., Hernandez L., Alvord W. G., Stewart C. L. (2001) Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proc. Natl. Acad. Sci. U. S. A. 98, 8680–8685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhong Z., Wen Z., Darnell J. E., Jr. (1994) Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264, 95–98 [DOI] [PubMed] [Google Scholar]
- 20. Marwood M., Visser K., Salamonsen L. A., Dimitriadis E. (2009) Interleukin-11 and leukemia inhibitory factor regulate the adhesion of endometrial epithelial cells: implications in fertility regulation. Endocrinology 150, 2915–2923 [DOI] [PubMed] [Google Scholar]
- 21. Darnell J. E., Jr. (1997) STATs and gene regulation. Science 277, 1630–1635 [DOI] [PubMed] [Google Scholar]
- 22. Takeda K., Noguchi K., Shi W., Tanaka T., Matsumoto M., Yoshida N., Kishimoto T., Akira S. (1997) Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc. Natl. Acad. Sci. U. S. A. 94, 3801–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakamura H., Kimura T., Koyama S., Ogita K., Tsutsui T., Shimoya K., Taniguchi T., Koyama M., Kaneda Y., Murata Y. (2006) Mouse model of human infertility: transient and local inhibition of endometrial STAT-3 activation results in implantation failure. FEBS Lett. 580, 2717–2722 [DOI] [PubMed] [Google Scholar]
- 24. Soyal S. M., Mukherjee A., Lee K. Y., Li J., Li H., DeMayo F. J., Lydon J. P. (2005) Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41, 58–66 [DOI] [PubMed] [Google Scholar]
- 25. Takeda K., Kaisho T., Yoshida N., Takeda J., Kishimoto T., Akira S. (1998) Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J. Immunol. 161, 4652–4660 [PubMed] [Google Scholar]
- 26. Finn C. A., Martin L. (1972) Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol. Reprod. 7, 82–86 [DOI] [PubMed] [Google Scholar]
- 27. Giudice L. C., Kao L. C. (2004) Endometriosis. Lancet 364, 1789–1799 [DOI] [PubMed] [Google Scholar]
- 28. Attia G. R., Zeitoun K., Edwards D., Johns A., Carr B. R., Bulun S. E. (2000) Progesterone receptor isoform A but not B is expressed in endometriosis. J. Clin. Endocrinol. Metab. 85, 2897–2902 [DOI] [PubMed] [Google Scholar]
- 29. Franco H. L., Rubel C. A., Large M. J., Wetendorf M., Fernandez-Valdivia R., Jeong J. W., Spencer T. E., Behringer R. R., Lydon J. P., Demayo F. J. (2012) Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J. 26, 1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zeng X., Goetz J. A., Suber L. M., Scott W. J., Jr., Schreiner C. M., Robbins D. J. (2001) A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature 411, 716–720 [DOI] [PubMed] [Google Scholar]
- 31. Han B. C., Xia H. F., Sun J., Yang Y., Peng J. P. (2010) Retinoic acid-metabolizing enzyme cytochrome P450 26a1 (cyp26a1) is essential for implantation: functional study of its role in early pregnancy. J. Cell. Physiol. 223, 471–479 [DOI] [PubMed] [Google Scholar]
- 32. Vermot J., Fraulob V., Dolle P., Niederreither K. (2000) Expression of enzymes synthesizing (aldehyde dehydrogenase 1 and reinaldehyde dehydrogenase 2) and metabolizaing (Cyp26) retinoic acid in the mouse female reproductive system. Endocrinology 141, 3638–3645 [DOI] [PubMed] [Google Scholar]
- 33. Das S. K., Chakraborty I., Paria B. C., Wang X. N., Plowman G., Dey S. K. (1995) Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol. Endocrinol. 9, 691–705 [DOI] [PubMed] [Google Scholar]
- 34. Ernst M., Inglese M., Waring P., Campbell I. K., Bao S., Clay F. J., Alexander W. S., Wicks I. P., Tarlinton D. M., Novak U., Heath J. K., Dunn A. R. (2001) Defective gp130-mediated signal transducer and activator of transcription (STAT) signaling results in degenerative joint disease, gastrointestinal ulceration, and failure of uterine implantation. J. Exp. Med. 194, 189–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen J. R., Cheng J. G., Shatzer T., Sewell L., Hernandez L., Stewart C. L. (2000) Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology 141, 4365–4372 [DOI] [PubMed] [Google Scholar]
- 36. Teng C. B., Diao H. L., Ma H., Cong J., Yu H., Ma X. H., Xu L. B., Yang Z. M. (2004) Signal transducer and activator of transcription 3 (Stat3) expression and activation in rat uterus during early pregnancy. Reproduction 128, 197–205 [DOI] [PubMed] [Google Scholar]
- 37. Teng C. B., Diao H. L., Ma X. H., Xu L. B., Yang Z. M. (2004) Differential expression and activation of Stat3 during mouse embryo implantation and decidualization. Mol. Reprod. Dev. 69, 1–10 [DOI] [PubMed] [Google Scholar]
- 38. Dimitriadis E., Sharkey A. M., Tan Y. L., Salamonsen L. A., Sherwin J. R. (2007) Immunolocalisation of phosphorylated STAT3, interleukin 11 and leukaemia inhibitory factor in endometrium of women with unexplained infertility during the implantation window. Reprod. Biol. Endocrinol. 5, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.