Abstract
Indolic and kynuric pathways of skin melatonin metabolism were monitored by liquid chromatography mass spectrometry in human keratinocytes, melanocytes, dermal fibroblasts, and melanoma cells. Production of 6-hydroxymelatonin [6(OH)M], N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and 5-methoxytryptamine (5-MT) was detected in a cell type-dependent fashion. The major metabolites, 6(OH)M and AFMK, were produced in all cells. Thus, in immortalized epidermal (HaCaT) keratinocytes, 6(OH)M was the major product with Vmax = 63.7 ng/106 cells and Km = 10.2 μM, with lower production of AFMK and 5-MT. Melanocytes, keratinocytes, and fibroblasts transformed melatonin primarily into 6(OH)M and AFMK. In melanoma cells, 6(OH)M and AFMK were produced endogenously, a process accelerated by exogenous melatonin in the case of AFMK. In addition, N-acetylserotonin was endogenously produced by normal and malignant melanocytes. Metabolites showed selective antiproliferative effects on human primary epidermal keratinocytes in vitro. In ex vivo human skin, both melatonin and AFMK-stimulated expression of involucrin and keratins-10 and keratins-14 in the epidermis, indicating their stimulatory role in building and maintaining the epidermal barrier. In summary, the metabolism of melatonin and its endogenous production is cell type-dependent and expressed in all three main cell populations of human skin. Furthermore, melatonin and its metabolite AFMK stimulate differentiation in human epidermis, indicating their key role in building the skin barrier.—Kim, T.-K., Kleszczyński, K., Janjetovic, Z., Sweatman, T., Lin, Z., Li, W., Reiter, R. J., Fischer, T. W., Slominski, A. T. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells.
Keywords: keratinocytes, melanocytes, 6-hydroxymelatonin, AFMK, 5-methoxytryptamine
N-acetyl-5-methoxytryptamine (melatonin) is ubiquitously distributed in nature and is detectable in vertebrates, invertebrates and plants, as well as in simple eukaryotes and prokaryotes. Because of its small size and amphiphilic properties, it reaches all cellular compartments (1–6). In vertebrates, melatonin exerts multiple pleiotropic actions through interactions with high-affinity membrane bound receptors, and acts as a circadian rhythm regulator, neurotransmitter, hormone, cytokine, and biological response modifier (3, 7–11).
Melatonin also acts as broad-spectrum direct antioxidant or shows indirect actions through precise regulation of antioxidative and cytoprotective pathways and acts as a metabolic regulator; some of these properties are shared by N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and possibly other melatonin metabolites (2, 3, 10, 12–17).
The indolic (main) pathway of melatonin degradation involves its hydroxylation to 6-hydroxymelatonin [6(OH)M] mediated by CYP1A1, CYP1A2, and CYP1B1 (predominantly in the liver), which, after sulfation or glucoronidation, is excreted in the urine (8, 18, 19). In the same pathway, melatonin is metabolized to 5-methoxytryptamine (5-MT) by melatonin deacetylase, with subsequent sequential transformation to 5-methoxyindoleacetaldehyde, 5-methoxyindole acetic acid, or 5-methoxytryptophol (20, 21). The kynuric pathway produces AFMK either enzymatically or through nonenzymatic transformation that involves the action of free radicals or ultraviolet B (UVB) radiation (6, 14, 22–25). Finally, melatonin can also be demethylated to N-acetylserotonin (NAS; also a precursor of melatonin) by either CYP2C19 or CYP1A (18, 19). In this context, organ-specific metabolism of melatonin should determine its bioactivity or final phenotypic affects, influenced by production of biologically active metabolites (2, 3, 6, 12, 24, 25).
Skin, the largest organ in the body, plays a crucial life-long role in the regulation of whole-body homeostasis and is continuously exposed to environmental beneficial and harmful factors; therefore, it needs to protect itself by autonomous self-regulation (26). Being at the interface between the external and internal environments, the skin has established local neuroendocrine activities to protect local and, by interference, global homeostasis from environmental stressors (26–28). These functions also involve expression of the local melatoninergic system (reviewed in refs. 6, 16, 17, 29, 30) that includes sequential transformation of tryptophan to serotonin and melatonin (31–34), the expression of a cutaneous melatonin receptor system (7, 35), and a melatoninergic antioxidative system (25, 36, 37). In vertebrate skin, melatonin exerts a variety of phenotypic effects affecting pigmentary (38, 39), adnexal (30), and defense (16, 17, 29) systems. In humans, there is a paucity of information on the role of melatonin in the regulation of epidermal functions in intact skin. Furthermore, skin cell type-specific melatonin metabolism, which would dictate the biological effects of this molecule, has not been established, except for immortalized HaCaT keratinocytes exposed to UVB radiation (25).
To fill these knowledge gaps and to better understand the role of melatonin and its metabolites in human skin, we investigated skin cell type-specific melatonin metabolism. Furthermore, we tested the effect of melatonin and its main biologically active metabolite, AFMK, on induction of epidermal differentiation, using a recently characterized human skin organ culture system (40).
MATERIALS AND METHODS
Human subject and animal protocols
All studies involving specimens from humans were obtained through protocols approved by the institutional review boards at the participating institutions. For metabolic studies, we used lines of epidermal HaCaT keratinocytes and SKMEL-188 melanoma cells (35, 41) and primary cultures of normal human epidermal keratinocytes, melanocytes, and dermal fibroblasts (42, 43). Skin samples for the recently established human organ culture model were full-thickness explants taken from different Caucasian individuals (age range 39–61 yr) undergoing abdominoplastic surgery after informed consent (40). All subjects were healthy individuals with no evidence of systemic disease.
Studies involving animals followed protocols approved by the Institutional Animal Care and Use Committee at University of Texas Health Science Center. For biochemical reactions, a 2-yr-old female white pig from Nicole Farm (Memphis, TN, USA) was euthanized, and kidneys were collected for the isolation of mitochondria.
Reagents
Reagents for melatonin metabolism and cell cultures: DMEM (Mediatech, Manassas, VA, USA) and Ham's F-10 (Mediatech) were used for growth of HaCaT, fibroblast, and SKMEL-188 cells. KBM-2 plus KGM-2 and MBM-4 plus MGM-4 (Lonza, Walkersville, MD, USA) were used for human normal keratinocytes and melanocytes, respectively. FBS was purchased from Atlanta Biologicals (Lawrenceville, GA, USA) and trypsin/EDTA solution from Lonza. Melatonin, 6(OH)M, 5-MT, and NAS were purchased from Sigma-Aldrich (St. Louis, MO, USA), and AFMK was purchased from Cayman Chemical (Ann Arbor, MI, USA). Paraformaldehyde, Tris aminomethane (Sigma-Aldrich), NaCl (Fisher Scientific, Pittsburgh, PA, USA), KCl (Fisher Scientific), CaCl2 (Sigma-Aldrich), MgSO4 (Sigma-Aldrich), KH2PO4 (Sigma-Aldrich), and glucose (Gibco, Grand Island, NY, USA) were used for the reaction of melatonin with human cells. For pig mitochondrial reaction, HEPES (Sigma-Aldrich), sucrose (Sigma-Aldrich), KCl (Fisher Scientific), MgSO4 (Sigma-Aldrich, St. Louis, MO, USA), EDTA (Sigma-Aldrich) and bovine serum albumin (BSA; Sigma-Aldrich) were used. [3H]-thymidine was from Moravek Biochemicals (Brea, CA, USA).
Reagents for organ culture: Williams' E medium containing NaHCO3 (2200 μg/ml), glucose (2000 μg/ml), CaCl2·2H2O (265 μg/ml), essential amino acids (Biochrom AG, Berlin, Germany); l-glutamine (100×), penicillin-streptomycin solution (10,000 U of penicillin and 10 mg of streptomycin in 1 ml 0.9% NaCl) (Invitrogen, Carlsbad, CA, USA); insulin from bovine pancreas, hydrocortisone, acetone, ethanol, 4′,6-diamidino-2-phenylindole (DAPI) (Sigma); normal mouse serum (NMS) and normal goat serum (NGS) (Dako, Carpinteria, CA, USA); cryomatrix formula (Thermo Scientific, Runcore, UK); Fluoromount-G mounting medium (SouthernBiotech, Birmingham, AL, USA); TSA Fluorescein System (Perkin Elmer, Boston, MA, USA). Primary antibodies: goat polyclonal anti-K14 IgG, mouse monoclonal anti-K10 IgG, and rabbit polyclonal anti-IVL IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse monoclonal anti-human Ki-67 antiserum, clone MIB-1 (Dako). Secondary antibodies: biotin-labeled mouse anti-goat IgG, biotin-labeled goat anti-rabbit IgG, biotin-labeled goat anti-mouse IgG (Beckman Coulter, Marseille, France), rhodamine-conjugated goat anti-mouse secondary IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA).
Melatonin metabolism
Mitochondria
Mitochondria were isolated from pig kidneys, and the reactions were run according to previously described protocols (19, 44). Briefly, mitochondrial fractions were suspended in 0.25 M sucrose in 50 mM HEPES buffer (pH 7.4) containing 20 mM KCl, 5 mM MgSO4, 0.2 mM EDTA and 2 mg/ml BSA, and preincubated for 5 min with 50 μM melatonin at 37°C. The reaction was started by addition of 0.5 mM NADPH/5 mM dl-isocitrate and carried out at 37°C for up to 90 min. Reactions were stopped by adding ice-cold methylene chloride with 2 extraction steps and drying of extracts under N2 gas (44).
Intact cells
Human lines of epidermal HaCaT keratinocytes and SKMEL-188 melanoma were cultured in DMEM and Ham's F10 medium, respectively, plus 5% FBS plus antimycotic antibiotic mixture, as described previously (35). Induction of melanin pigmentation in SKMEL-188 cells was achieved by supplementing medium with high levels of l-tyrosine (75% DMEM/25% Ham's F10), as described previously (41). Primary cultures of normal human epidermal keratinocytes, melanocytes, and dermal melanocytes were established, as described previously, and second passages were used for the experiments (42, 43, 45).
Cells were detached from semiconfluent flasks by treatment with trypsin/EDTA solution and washed twice with phosphate buffered saline (PBS) followed by washing with Tris-buffered medium (pH 7.4, 33 mM Tris aminomethane, 110 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1 mM MgSO4, 1 mM KH2PO4 and 2 mg/ml glucose), as described previously (44, 46). The cells (3×105) were incubated in 1 ml of Tris-buffered medium containing 50 μM melatonin, 1 mM isocitrate, and 0.1 mM NADPH at 37°C for 16 h and then extracted with methylene chloride (44, 46). The dose response of melatonin concentrations up to 500 μM showed good liquid chromatography-mass spectrometry (LC-MS) signals for all main products at 50 μM, which was biologically relevant in skin organ culture.
High-pressure liquid chromatography (HPLC) and LC-MS
HPLC and LC-MS analyses were performed as described previously (32, 44, 46). Briefly, the extracts were dissolved in methanol and subjected to HPLC analysis using an HPLC unit fitted with an Atlantis C18 column (100×4.6 mm, 5 μm particle size; all equipment from Waters, Milford, MA, USA). Separation was achieved using an isocratic mobile phase consisting of 15% acetonitrile and 0.1% acetic acid in water at a flow rate of 0.75 ml/min. Column eluate was monitored by flow fluorescence detection (excitation wavelength of 285 nm and an emission wavelength of 365 nm) The peaks with retention times of the corresponding standards were collected and submitted to LC-MS analysis. The purified samples were applied to an API-3000 or 4000 LC-MS/MS mass spectrometer (Applied Biosystems, Toronto, ON, Canada) equipped with an electrospray ionization (ESI) source and a Zorbax Eclipse Plus C18 column (2.1×50 mm, 1.8 μm; Agilent Technology, Santa Clara, CA, USA) using 25% acetonitrile and 0.1% acetic acid in water at a flow rate of 0.05 ml/min, unless otherwise indicated. To detect 5-MT in skin cells, a Waters Xevo G2-S qTOF mass spectrometer equipped with an ESI ionization source, a Waters Acquity I-class ultra-high-pressure LC (UPLC) system, and a Zorbax Eclipse Plus C18 column (2.1×50 mm, 1.8 μm; Agilent Technology) were used with an isocratic mobile phase consisting of 25% acetonitrile and 0.1% formic acid in water, at a flow rate of 0.05 ml/min.
Skin organ cultures
The skin was prepared for organ culture as described previously and validated (36, 40). Briefly, skin was defatted, cut by scalpel into sample pieces of 0.5 × 1.0 cm, rinsed abundantly with 1×PBS (pH 7.2) and placed epidermis up/dermis down into 6-well plates, 1 sample/well in air-liquid interface. Skin culture was carried out in a humidified atmosphere (5% CO2, 37°C) in 2 ml of serum-free Williams' E medium.
Skin samples were cultured the first 24 h in normal culture medium to allow recovery from possible preparation-induced tissue stress. Then, culture medium was replaced with fresh medium containing melatonin or AFMK and incubated for 1 or 24 h. Control was melatonin- or AFMK-free medium, respectively. Both compounds were dissolved in absolute ethanol (final ethanol concentration <0.2%) and further diluted with PBS to yield 10−2 M stock solution, while skin samples were preincubated with a wide range of concentrations from low to high (10−10, 10−8, 10−6, 10−4, and 10−3 M) in a time-dependent manner (1 or 24 h) in a volume of 2 ml culture medium. After the compound incubation period, skin was further incubated with normal (compound-free) medium for 24 or 48 h, respectively. At specific time points (0, 24, and 48 h) after the compound incubation time (1 or 24 h), the skin samples were removed from culture, embedded in freezing Cryomatrix formula (Thermo Scientific, Runcore, UK), snap-frozen in liquid nitrogen, and subjected to cryosectioning (d=6 μm) using a Leica CM 3050S research cryostat (Leica Mikrosysteme, Wetzlar, Germany) for further immunofluorescence staining.
Immunofluorescence labeling
For visualization of epidermal keratinocytes, the tyramide signal amplification (TSA) staining procedure was applied using cytokeratin-14 (K14), a marker of undifferentiated keratinocytes of the stratum basale, and the differentiation markers cytokeratin-10 (K10; stratum spinosum) and involucrin (IVL; stratum granulosum) (47). Briefly, cryosection slides were dried for 10 min at room temperature, fixed with ice-cold acetone for 10 min at −20°C, and washed 3 times for 5 min each with 1× Tris-NaCl-Tween (TNT; pH 7.5). Endogenous peroxidases were quenched using 3% H2O2 for 15 min at room temperature, washed with 1× TNT again, and blocked in incubation sequence: avidin, 1× TNT, biotin, and 1× TNT. The preincubation was carried out for 30 min at room temperature using 2% NMS (K14) or 2% NGS (K10, IVL). After that, sections were incubated overnight at 4°C with the goat polyclonal anti-K14 IgG (1:400) or mouse monoclonal anti-K10 IgG (1:800) or rabbit polyclonal anti-IVL IgG (1:800). Then, slides were washed 3 times, each for 5 min, with 1× TNT, and treated for 45 min at room temperature with 2% NMS containing the secondary biotin-labeled mouse anti-goat IgG (1:400) for K14. In case of K10 or IVL, labeling was carried out with the secondary biotin-labeled goat anti-mouse IgG (1:400) and the secondary biotin-labeled goat anti-rabbit IgG (1:400) for K10 and IVL, respectively. Sections were washed again with 1× TNT buffer, and staining was developed using the TSA Fluorescein System. For this purpose, slides were incubated with horseradish-peroxidase-conjugated streptavidin (1:100) for 30 min at room temperature, washed 3 times with 1× TNT, and labeled using fluorescein tyramide reagent (1:50) for 5 min at room temperature. Sections were counterstained for cell nucleus detection with DAPI solution (1 μl/ml) for 1 min, washed 3 times for 5 min, each with 1× PBS, and mounted in Fluoromount-G mounting medium. Finally, all fluorescent images were obtained using the Keyence inverted fluorescence microscope (BZ-8000; Keyence, Neu-Isenburg, Germany).
Ki-67 immunocytochemistry in epidermal keratinocytes
To evaluate proliferating cells during skin culture, immunofluorescence Ki-67 staining was applied as described earlier (40). Briefly, skin sections were fixed with 1% paraformaldehyde for 10 min at room temperature, washed 3 times with 1× PBS, and postfixed in an ethanol:acetic acid mixture (2:1) at −20°C for 5 min. Subsequently, tissue sections were preincubated with 10% NGS, followed by application of monoclonal mouse anti-human Ki-67 antiserum, clone MIB-1 (1:20). To detect Ki-67 immunoreactivity, rhodamine-conjugated goat anti-mouse secondary IgG (1:200) was used. Sections were counterstained for cell nucleus detection with DAPI solution (1 μl/ml) for 1 min, washed 3 times for 5 min each with 1× PBS, and mounted in Fluoromount-G mounting medium. All fluorescent images were then obtained using the Keyence BZ-8000 inverted fluorescence microscope.
DNA synthesis in cell culture
Normal human primary epidermal keratinocytes (passage 2) were grown in 24-well plates using KBM-2 supplemented with KGM-2 until 30% confluent (42). At time 0 h, the medium was changed to medium containing 10−5 to 10−11 M of melatonin metabolites plus 0.1% BSA for 48 or 72 h incubation. [3H]-thymidine was added to the medium in a final concentration of 1 μCi/ml for the final 4 h of incubation. After 4 h incubation, medium was removed, and cells were precipitated with 10% trichloroacetic acid in PBS for 30 min and washed twice with 1 ml PBS. The washed cells were incubated with 1 N NaOH/1% sodium dodecyl sulfate (250 μl/well) for 30 min at 37°C. The lysed cell extracts were collected in scintillation vials and 5 ml of scintillation cocktail was added to each vial. 3H-radioactivity incorporated into DNA was measured using a Packard Matrix 9600 direct β-counter (Packard, Meridan, CT, USA).
Statistical analysis
Data are expressed as pooled means ± se of 2–3 independent experiments. Quantification of K14, K10, IVL, and Ki-67 positivity from 6 images per experimental condition was carried out using Image J 1.38d software (U.S. National Institutes of Health, Bethesda, MD, USA). Comparative data were analyzed in multivariate analysis using 1-way ANOVA and adequate post hoc testing with GraphPad Prism 5.02 software (La Jolla, CA, USA). Values were normalized and expressed as a percentage of the control value, i.e., sample without tested compounds at 0 h of culture time. Values of P < 0.05 were considered statistically significant.
RESULTS
Metabolism of melatonin in skin cells
In the initial control experiments, kidney mitochondria were incubated with melatonin, and 3 major metabolites, including 6(OH)M, AFMK and 5-MT, were detected using HPLC analysis with fluorescent detection, with 6(OH)M being the most abundant product (Supplemental Fig. S1). Metabolite identities were confirmed by LC-MS in selective ion monitoring (SIM) mode; this revealed the presence of species with m/z = 249 [M+1]+, 265 [M+1]+ and 191 [M+1]+, corresponding to the retention times of 6(OH)M, AFMK, and 5-MT, respectively (Supplemental Fig. S1). Thus, major routes of melatonin metabolism in kidney mitochondria along indolic (predominant) and kynuric pathways were established with identification of their main metabolites.
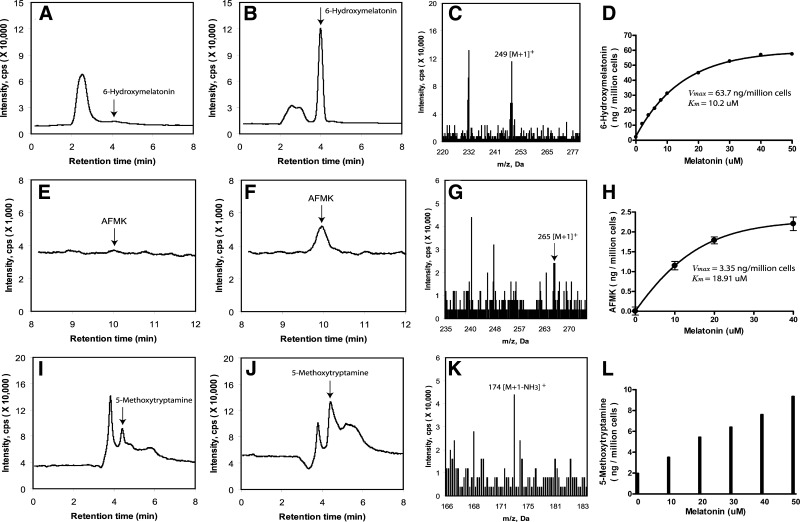
To investigate in depth the metabolism of melatonin in cells of epidermal origin, immortalized human epidermal keratinocytes (HaCaT) were used. Incubation of intact HaCaT keratinocytes with 50 μM melatonin led to the production of three main metabolites 6(OH)M, AFMK and 5-MT with 6(OH)M being the most abundant (Fig. 1). Two major metabolites [6(OH)M and AFMK] were produced in a dose-dependent manner with Vmax = 63.7 ng/106 cells and Km = 10.2 μM for 6(OH)M, and Vmax = 3.35 ng/106 cells and Km = 18.91 μM for AFMK (Fig. 1D, H). The production of 5-MT at the tested concentration range of 0–50 μM showed a linear response, preventing precise determination of the Vmax and Km values (Fig. 1L). However, it indicates a relatively high Km value for 5-MT. In addition, 5-MT was clearly detected in samples without the addition of melatonin substrate, indicating its relatively high endogenous production in comparison to 6(OH)M and AFMK.
Figure 1.
Melatonin metabolism in human epidermal (HaCaT) keratinocytes. Production of metabolites was monitored by LC-MS in SIM mode, m/z = 249 [M+1]+ for 6(OH)M (A, B, D), m/z = 265 [M+1]+ for AFMK (E, F, H) and m/z = 191 [M+1]+ for 5-MT (I, J, L). A, E, I) Cells incubated without substrate addition. B, F, J) Cells incubated with 50 μM melatonin. C, G, K) Mass spectra of the metabolites. D, H, L) Dose-dependent production of the metabolites.
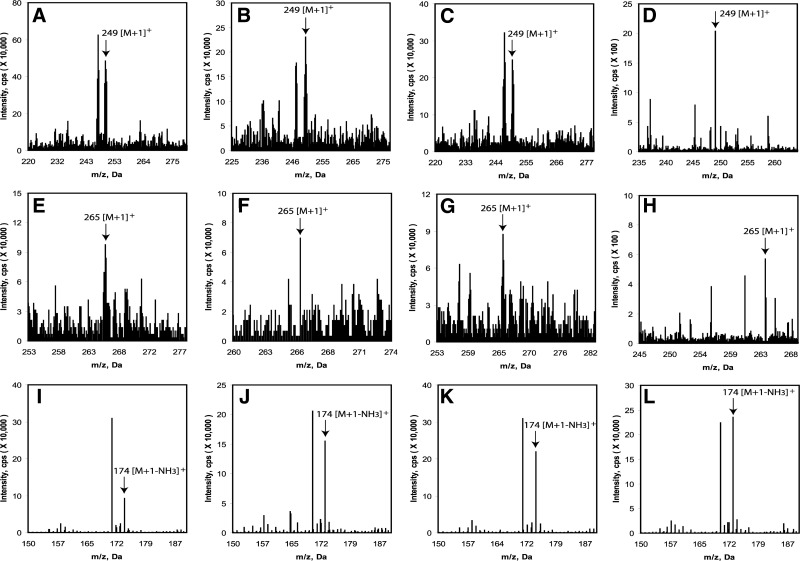
Production of 6(OH)M and AFMK after incubation with 50 μM melatonin was also detected in normal epidermal keratinocytes and melanocytes, dermal fibroblasts, and in pigmented and amelanotic melanoma cells, as determined by detection of ions with m/z = 249 [M+1]+ and m/z = 265 [M+1]+ corresponding to the 6(OH)M and AFMK (Fig. 2A–H). Again, the major product of metabolism in all cell types was 6(OH)M (Fig. 2A–D), with lower concentrations of AFMK (Fig. 2E–H). The levels of 5-MT were very low and required the use of a more sensitive LC-MS system, described in Materials and Methods, with precise detection of the same mass (m/z=174.0979 [M+1-NH3]+) both for the standard and experimental samples, with identical retention times (Fig. 2I–L).
Figure 2.
Detection of 6(OH)M, AFMK, and 5-MT in normal epidermal keratinocytes (A, E, I), melanocytes (B, F, J), dermal fibroblasts (C, G, K), and malignant melanoma cells (D, H, L). A–D) Ions with m/z = 249 [M+1]+ corresponding to 6(OH)M. E–H) Ions with m/z=265 [M+1]+ corresponding to AFMK. I–L) Mass identical to 5-MT (m/z=174.0979 [M+1-NH3]+ and eluting at the same retention time as the standard, where the more sensitive (better signal/noise ratio) LC-MS spectrometer (Xevo G2-S qTOF) was used.
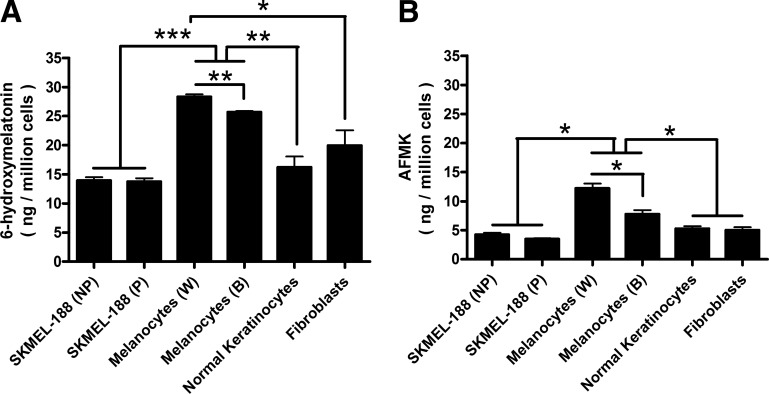
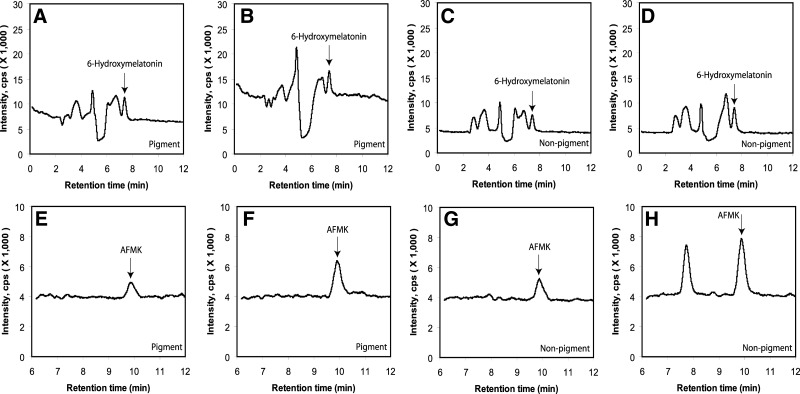
Relative production of 6(OH)M and AFMK was highest in normal epidermal melanocytes, being significantly higher in melanocytes from black compared to white patients (Fig. 3). There was no difference in their production in normal keratinocytes, dermal fibroblasts, and pigmented and nonpigmented melanoma cells (Fig. 3). Interestingly, endogenous production of 6(OH)M and AFMK was clearly detected in both pigmented and nonpigmented melanoma cells, with AFMK, but not 6(OH)M levels, significantly increasing after addition of exogenous melatonin (Fig. 4). Furthermore, both melanoma cells and normal epidermal melanocytes endogenously produced NAS, as identified by the detection of ion with m/z=219 [M+1]+ with a retention time identical to the corresponding standard (not shown). This production was unaffected by the addition of exogenous melatonin.
Figure 3.
Cell type-dependent production of 6(OH)M and AFMK in skin cells. Cells were incubated with 50 μM melatonin for 16 h at 37°C. ESI-equipped LC-MS was used to quantify the metabolites production using SIM mode (m/z=249 [M+1]+ for 6(OH)M (A) and m/z=265 [M+1]+ for AFMK (B). NP and P indicate nonpigmented and pigmented melanoma cells, respectively. W and B indicate normal melanocytes from white and black subjects, respectively. Data represent means ± se (n=3). *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 4.
Endogenous production of 6(OH)M and AFMK in human melanoma cells. Detection of 6(OH)M (A–D) and AFMK (E–H) by LC-MS in SIM mode, m/z = 249 [M+1]+ (A–D) or m/z = 265 [M+1]+ (E–H) in amelanotic (A, B, E, F) and melanotic (C, D, G, H) melanoma cells incubated without (A, C, E, G) or after (B, D, F, H) addition of 50 μM melatonin.
Melatonin and AFMK enhance epidermal barrier formation in human skin ex vivo
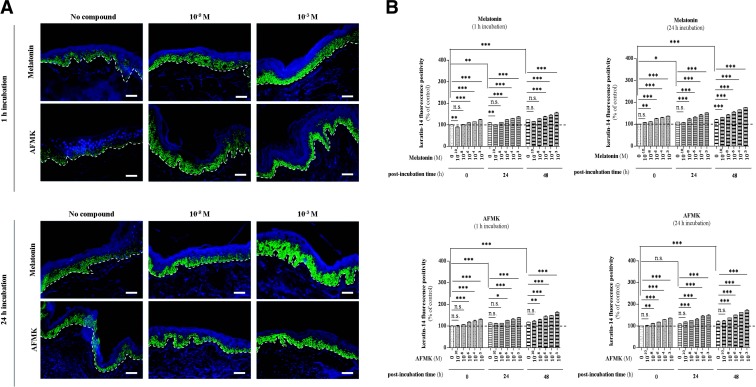
First, we found that melatonin and AFMK increase expression of K14 at the basal layer of the epidermis (Fig. 5A). Preincubation with melatonin caused a significant increase at 0 h postincubation time by 10% (P<0.001, 1 h incubation) and by 11% (P<0.01, 24 h incubation) at the concentrations of 10−6 M and 10−8 M, respectively (Fig. 5B). In the case of 1 h incubation with melatonin during prolonged culture of skin, we observed a prominent increase of K14 at the concentration of 10−6 M by 12% (P<0.001), both after 24 and 48 h postincubation time (Fig. 5B). Preincubation for 24 h caused a shift in the concentration of melatonin, which significantly enhanced K14 positivity. Compared to the nontreated skin at corresponding times, it increased by 13% (P<0.001, 24 h postincubation time) and by 9% (P<0.001, 48 h postincubation time) at the concentration of 10−8 M and 10−10 M, respectively (Fig. 5B). On the other hand, it should be underlined that the concentration of 10−10 M was found to inhibit K14 expression, particularly at 1 h incubation (Fig. 5B). Here, melatonin caused significant decrease of K14 positivity of epidermal keratinocytes by 10% (P<0.01) and 8.5% (P<0.01) after 1 and 24 h postincubation time, respectively. Similar to melatonin, AFMK also enhanced the number of proliferating keratinocytes. After 1 h of incubation, there were significant up-regulations by 18% (P<0.001, 10−6 M), 10% (P<0.05, 10−6 M), and 10% (P<0.01, 10−8 M) at 0, 24, and 48 h postincubation time, respectively (Fig. 5B). Extended incubation time with AFMK for 24 h caused significant up-regulation at lower concentration, i.e., 10−8 M, no matter the duration of postincubation time. It enhanced K14 positivity by 12% (P<0.01, 0 h postincubation time), 15% (P<0.001, 24 h postincubation time) and 14% (P<0.001, 48 h postincubation time) (Fig. 5B).
Figure 5.
Effect of melatonin and AFMK on keratin-14 expression. A)Localization of K14 immunoreactivity assessed by immunofluorescence in the human epidermis. Skin samples were incubated over the indicated concentration range with melatonin or AFMK for 1 or 24 h. Following these two incubation times, skin samples were collected immediately (0 h) and 24 or 48 h later, snap-frozen, subjected to cryosections (d=6 μm), and processed for immunolabeling. Detection of the protein was carried out according to the TSA technique as described in Materials and Methods using goat polyclonal anti-K14 IgG (1:400) and secondary biotin-labeled mouse anti-goat IgG (1:400), labeled with FITC (1:50; green). Nuclei were labeled with DAPI solution (blue). Presented images show the K14 expression 48 h after melatonin/AFMK incubation time at specific low (10−8 M) and high (10−3 M) concentrations. Dashed line shows the basement membrane. Scale bars = 50 μm. B) Evaluated data are presented as pooled means ± se of independent experiments showing the effect of melatonin or AFMK at particular concentrations. Values are expressed as a percentage of the control value, i.e., nonpreincubated skin with tested compound at 0 h postincubation. n.s., not significant. *P < 0.05, **P < 0.01, ***P < 0.001.
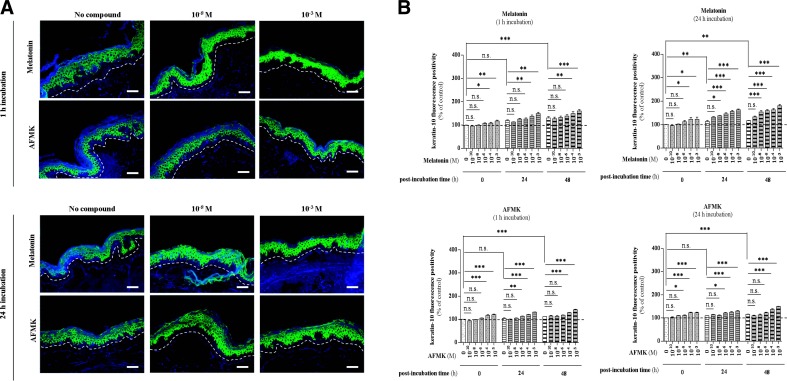
Notably, melatonin and AFMK enhanced differentiation of epidermal keratinocytes of human skin maintained ex vivo in culture as represented by K10 (stratum spinosum) and IVL (stratum granulosum) protein expression, observed over 48 h in a time- and concentration-dependent manner (Figs. 6A and 7A). Regarding K10, both melatonin and AFMK significantly induced K10 positivity within the stratum spinosum of the epidermis (Fig. 6). Directly after the incubation period (0 h postincubation time), skin preincubated with melatonin for 1 h showed a significant increase in concentration of 10−4 M by 9% (P<0.05), while the increase of K10 in skin preincubated with melatonin for 24 h was 23% (P<0.05) higher at the same melatonin concentration of 10−4 M, 24 h incubation (Fig. 6B). Comparatively, AFMK led to an initial significant increase directly after the incubation period (0 h postincubation time) of +17% (P<0.001, 1 h incubation) and +11% (P<0.05, 24 h incubation) at the concentration of 10−4 M and 10−6 M, respectively (Fig. 6B). Furthermore, later time points showed increasing effects of both compounds. Melatonin led to a 15% (P<0.01, 1 h incubation) and 20% (P<0.05, 24 h incubation) up-regulation at 10−4 M and 10−8 M concentrations, respectively at 24 h after incubation, while at 48 h postincubation, the first significant differences compared to the nontreated skin were evident at the same concentrations, increasing by 14% (P<0.01) and 32% (P<0.001) following 1 and 24 h incubations, respectively (Fig. 6B). On the other hand, AFMK caused prominent differences at concentrations of 10−6 and 10−4 at 24 and 48 h postincubation, respectively, regardless of the duration of incubation (Fig. 6B). After 1 h of incubation with AFMK, it led to a 9% (P<0.01, 10−6 M) and 16% (P<0.001, 10−4 M) up-regulation of K10 at 24 and 48 h postincubation, respectively (Fig. 6B). In addition, after 24 h incubation with AFMK, there was an increase by 11% (P<0.05, 10−6 M) and 19% (P<0.001, 10−4 M) at 24 and 48 h postincubation, respectively (Fig. 6B).
Figure 6.
Melatonin and AFMK increase the K10 expression of differentiating epidermal keratinocytes in the stratum spinosum. A) Localization of keratin-10 immunoreactivity was assessed by immunofluorescence in the human epidermis. Skin samples were incubated over the indicated concentration range with melatonin or AFMK for 1 or 24 h. Following these two incubation times, skin samples were collected immediately (0 h) and 24 or 48 h later, snap-frozen, subjected to cryosections (d=6 μm), and processed for immunolabeling. Detection of the protein was carried out according to the TSA technique, as described in Materials and Methods using mouse monoclonal anti-K10 IgG (1:800), secondary biotin-labeled goat anti-mouse IgG (1:400), labeled with FITC (1:50; green). Nuclei were labeled with DAPI solution (blue). Presented images show the K10 expression 48 h after melatonin/AFMK incubation time at specific low (10−8 M) and high (10−3 M) concentrations. Dashed line shows the basement membrane. Scale bars = 50 μm. B) Evaluated data are presented as pooled means ± se of independent experiments showing the effect of melatonin or AFMK at particular concentrations. Values are expressed as a percentage of the control value, i.e., nonpreincubated skin with tested compound at 0 h postincubation. n.s., not significant. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 7.
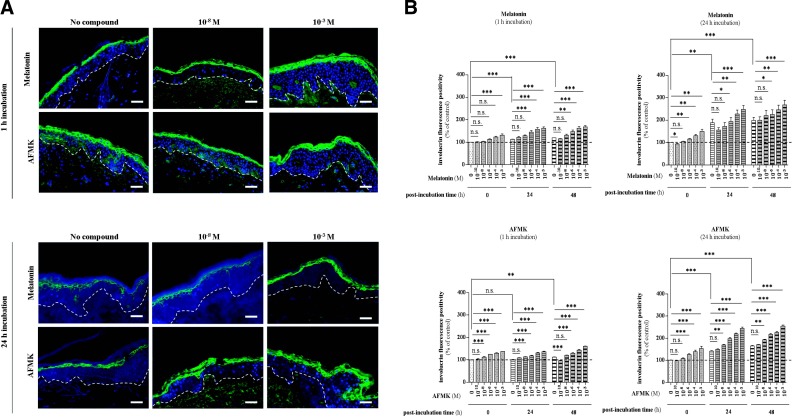
IVL expression of differentiating keratinocytes within the stratum granulosum is enhanced by melatonin and AFMK in human skin. A) Localization of involucrin immunoreactivity assessed by immunofluorescence detection in the human epidermis. Skin samples were incubated over the indicated concentration range with melatonin or AFMK for 1 or 24 h. Following these two incubation times, skin samples were collected immediately (0 h) and 24 or 48 h later, snap-frozen, subjected to cryosections (d=6 μm), and processed for immunolabeling. Detection of the protein was carried out according to the TSA technique, as described in Materials and Methods using primary rabbit polyclonal anti-IVL IgG (1:800), secondary biotin-labeled goat anti-rabbit IgG (1:400), labeled with FITC (1:50; green). Nuclei were labeled with DAPI solution (blue). Presented images show the IVL expression 48 h after melatonin/AFMK incubation time at specific low (10−8 M) and high (10−3 M) concentrations. Dashed line shows the basement membrane. Scale bars = 50 μm. B) Evaluated data are presented as pooled means ± se of independent experiments showing the impact of melatonin or AFMK at particular concentrations. Values are expressed as a percentage of the control value i.e., nonpreincubated skin at 0 h postincubation. n.s., not significant. *P < 0.05, **P < 0.01, ***P < 0.001.
In addition to a significant enhancement of K10, skin preincubated with melatonin for 1 h showed a significant increase of IVL positivity directly after the incubation period (0 h postincubation time) at the concentration of 10−3 M (+32%; P<0.001), while after 24 h of melatonin incubation, a concentration as low as 10−6 M led to a significant induction of IVL compared to the nonmelatonin-incubated control (+16%; P<0.01) (Fig. 7). Melatonin incubation for 24 h at the concentration of 10−3 M led to a markedly stronger increase than after 1 h of incubation (+50 vs. +32%). Comparative analysis using AFMK showed the first significant induction of IVL at a concentration of 10−8 (1 h incubation) and 10−6 M (24 h incubation) by 12% (P<0.001) and 26% (P<0.001), respectively (Fig. 7B). At later time points after the incubation period, melatonin and AFMK exhibited still more prominent effects. At 24 h postincubation, the presence of melatonin caused significant up-regulation at the concentration of 10−6 M by 28% (P<0.001) and by 3% (P<0.05) after 1 and 24 h preincubation, respectively, compared to the sample without melatonin at these time points. Similarly, at 48 h postincubation, the same concentration (10−6 M) showed the first significant effects in both preincubation time conditions (1 and 24 h; Fig. 7B). On the other hand, AFMK significantly up-regulated IVL positivity already at the concentration of 10−6 or 10−8 M after 1 or 24 h incubation, respectively. Compared to the skin without AFMK, differentiating activity increased by 13% (P<0.001, 1 h AFMK incubation, 10−8) and 20% (P<0.001, 24 h AFMK incubation, 10−8) at 24 h postincubation. At 48 h postincubation, significant increases of IVL expression by 16% (P<0.001, 1 h AFMK incubation, 10−6) and by 17% (P<0.01, 24 h AFMK incubation, 10−8) were observed (Fig. 7B).
Interestingly, the spontaneous up-regulation of differentiation capacity of human keratinocytes within the skin culture of up to 48 h postincubation reached 31% (P<0.01, K10) and 99% (P<0.001, IVL), compared to the control value at 0 h postincubation. This indicates that ex vivo skin preserves its endogenous capacity to build epidermal barrier.
Effects of melatonin on keratinocytes proliferation are context dependent
We have found that melatonin had a differential effect on keratinocyte proliferation depending on the context: in vitro culture in monolayer (Supplemental Fig. S2) vs. spatially restricted proliferation in the epidermis of intact human skin (Supplemental Fig. S3). In cell culture, exposure of normal epidermal keratinocytes to melatonin and its metabolites led to a slight but significant inhibition of DNA synthesis (Supplemental Fig. S2). Melatonin showed inhibition at low (10−10 M) and relatively high (10−5 M) concentrations without any effect at intermediate levels, while AFMK and 5-MT showed dose-dependent effects with inhibition evident between 10−10 M and 10−5 M. 6(OH)M exerted its inhibitory effects at relatively higher doses, ≥10−8 M (Supplemental Fig. S2).
To define the effect of melatonin on epidermal keratinocytes proliferation in intact human skin incubated ex vivo, we analyzed Ki-67 labeling, an indicator of keratinocyte proliferation in situ. Preincubation for 1 h with melatonin at the concentration of 10−3 M showed significant increase in number of Ki-67-positive epidermal keratinocytes in the basal and suprabasal layers (Supplemental Fig. S3A). It should be noted that the statistically significant difference was observed only on 48 h of culture, where melatonin up-regulated the number of proliferating cells by 114% (P<0.001) compared to the corresponding sample without preincubation (Supplemental Fig. S3B). In addition, the longest culture time after 1 h preincubation with melatonin also revealed significant difference compared to 0 and 24 h by 119% (P<0.001) and 133% (P<0.001), respectively.
DISCUSSION
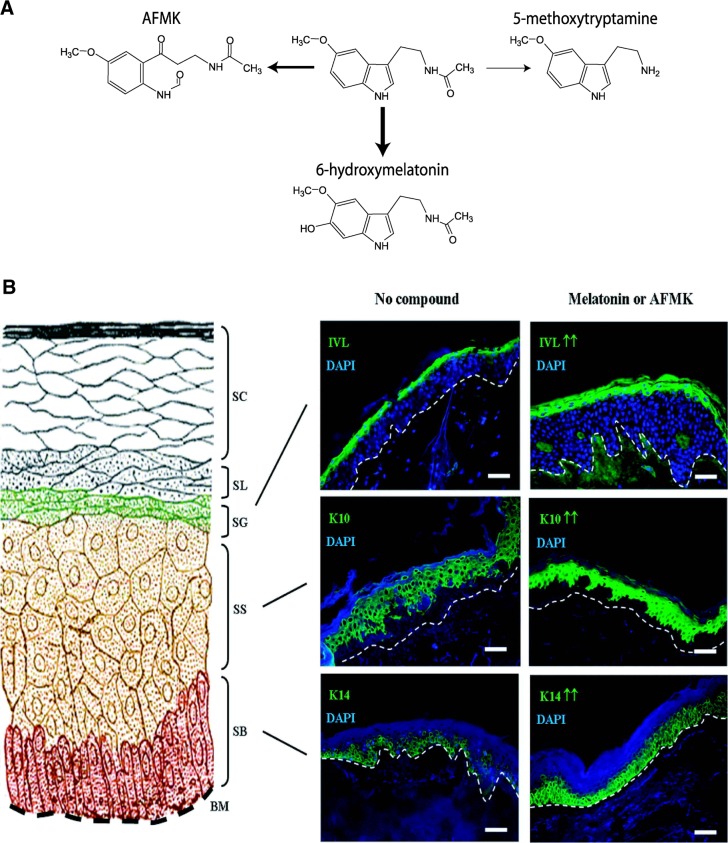
In this multidimensional experimental study, we define for the first time the pathways of melatonin degradation/metabolism in all main skin cell populations, with identification of 6(OH)M and AFMK as the main metabolites and representatives of the indolic and kynurenic melatonin degradation pathways, respectively (Fig. 8A). We also characterize the main phenotypic role of melatonin and its biologically active metabolite, AFMK, as stimulators of cell differentiation and formation of the epidermal barrier (Fig. 8B).
Figure 8.
Role of melatonin in the skin. A) Main pathways of melatonin metabolism in skin cells. B) Melatonin and its biologically active metabolite, AFMK, stimulate epidermal differentiation. On the left side, a scheme of the epidermis is displayed, and on the right side, staining of K14 (stratum basale) indicative for undifferentiated keratinocytes in the epidermal basal layer, while K10 (stratum spinosum) and IVL (stratum granulosum) are differentiating layers. BM, basement membrane; SB, stratum basale; SS, stratum spinosum; SG, stratum granulosum; SL, stratum lucidum; SC, stratum corneum. Dashed white lines show the basement membrane. Scale bars = 50 μm.
Experiments performed with normal epidermal keratinocytes and melanocytes, dermal fibroblasts, immortalized epidermal keratinocytes, and melanoma cells clearly demonstrate that melatonin in the skin is degraded and metabolized through both indolic and kynuric pathways (Fig. 8A). The indolic pathway produces 6(OH)M as the main product and 5-MT as a minor one, and these products represent the predominant route of melatonin metabolism in the skin (Figs. 1–3); similar effects are observed in the kidney (Supplemental Fig. S1). Thus, the predominant pathway of melatonin metabolism in all main skin populations (keratinocytes, melanocytes, and fibroblasts) and malignant melanoma cells is similar to that described at the systemic level, in which 6(OH)M is the main degradation product (reviewed in refs. 8 and 18).
Importantly, AFMK, a product of kynuric pathway, was detected as the second most abundant metabolite in all skin cell populations tested. This detection confirms and extends our previous studies on the UVB-induced melatonin degradation in vitro or in cultured immortalized line of HaCaT keratinocytes (25). AFMK is considered an original and evolutionary very ancient melatonin metabolite, produced either nonenzymatically or enzymatically in unicellular organisms and plants, as well as in invertebrate and vertebrate animals (12, 24, 48). Furthermore, in contrast to 6(OH)M, which is considered the main product of degradation, destined for secretion (18, 24), AFMK is a biologically active molecule with a higher potency than melatonin in relation to induction of responses against oxidative stress. Therefore, in concert with melatonin, it can help build a very potent antioxidative cascade against harmful free radical formation, such as under UV exposure, which is defined as the melatoninergic antioxidative system of the skin (12, 24, 25). The easily detectable production of AFMK reported in the present study, a process that does not require any additional treatment (UVB or exposure to noxious stimuli), is in contrast to the report showing that AFMK is an insignificant pathway of melatonin disposition in mice (49). It simply indicates an importance of the local kynuric pathway in organs exposed to environmental stress such as skin (25, 26) in contrast to the systemic metabolism, where AFMK may be below or at the level of detectability (49).
We have also noted that differences in the rate of melatonin metabolism depend on the cell type, as indicated by relatively higher production of 6(OH)M and AFMK in melanocytes in comparison to keratinocytes, fibroblasts, and melanoma cells (Fig. 3). There was also a slight, but significant, difference between melanocytes from black vs. white patients, which was, however, independent of the level of pigmentation, as production of 6(OH)M and AFMK was the same in amelanotic and melanotic malignant melanocytes (Fig. 3). Detection of endogenous production of 6(OH)M and AFMK, as well as of NAS is consistent with previous reports showing an endogenous tryptophan metabolic conversion toward melatonin operating predominantly in malignant and normal human melanocytes (17, 32, 33, 50, 51).
The importance of cell type-specific melatonin metabolism, e.g., termination or modification of its activity through, respectively, its metabolic inactivation or generation of biologically active molecules with changed properties, such as AFMK, is emphasized by previous studies showing that melatonin can regulate proliferation of epidermal keratinocytes, melanoma, and squamous cell carcinoma cells in a context-dependent fashion (35, 52–55). Accordingly, in primary epidermal keratinocytes, cultures of melatonin moderately inhibited proliferation only at the physiological (nanomolar and lower) and pharmacological (10 μM) concentrations, with no effect at intermediate concentrations, which is in accordance with previously published results on HaCaT keratinocytes (35) and human melanoma cells (52). Interestingly AFMK and 5-MT showed dose-dependent inhibition starting at 0.1 nM, while 6(OH)M was effective only at pharmacological concentrations (≥10 nM). These results indicate that a balance between local melatonin concentration and its metabolic consumption, with generation of intermediate products of indolic and kynuric pathways, will affect phenotypic activity. It has to be noted that melatonin plays an important role in skin physiology and pathology (29), with all major compartments, i.e., epidermis, dermis, and adnexa serving as targets for melatonin regulation (6, 16, 17, 30). The latter are mediated via interactions with melatonin receptors (7, 29). Specifically, melatonin can regulate hair cycling (30, 39, 56), cutaneous pigmentation (38), induce keratinocyte protective and antoxidative responses (16, 36, 37, 57), and it may play a role in development of vitiligo (58, 59). To better define the role of melatonin and the relative importance of its biologically active metabolite AFMK in the skin, we have investigated, for the first time, their role in epidermal differentiation using a human ex vivo full-thickness skin model. The biological observations of melatonin and AFMK activity in this skin model and their relevance are described and discussed below.
Specific concentrations of melatonin and its metabolite AFMK produced a time-dependent significant enhancement of K14 expression, a marker of undifferentiated keratinocytes in the basal epidermal layer, and K10 and IVL expression in the differentiating layers stratum spinosum and granulosum, respectively, over 48 h. K14 (together with K5) represent the primary keratin pair of keratinocytes in stratified squamous epithelia, including the epidermis (60). They are strongly associated with undifferentiated basal cell layer keratinocytes, which are, at the same time, proliferating and harboring the stem cell pools. Ultrastructurally, K5/K14 keratin filaments build bundles of tonofilaments that are attached to desmosomes and hemidesmosomes. Functionally, they are essential keratins for the physical stability and integrity of the epidermis, since dominant-negative mutations of the K14 gene have been shown to lead to the hereditary blistering disease epidermolysis bullosa simplex (EBS; ref. 61). Mutated K14 results in increased fragility of the basal keratinocytes, so that even mild physical trauma can cause intraepidermal cytolysis of basal cells and the formation of fluid-filled blisters. These patient-related findings were preceded by corresponding observations in mutant K14 transgenic mice showing skin abnormalities similar to EBS (62). Relating this clearly evident role of K14 in function and homeostasis of the epidermis to our observations in human full thickness skin, we can conclude that the melatonin and/or AFMK-mediated increase of K14 expression in basal layer keratinocytes represents a strong factor to maintain or increase epidermal stability to prevent mechanical friction-induced epidermal disconnection from basal lamina, or intraepidermal disruption of the basal layer keratinocytes from each other through hemidesmosome, and/or desmosome strengthening, respectively. In addition, the observed increase in the number of Ki-67-positive keratinocytes in the basal layers of the epidermis stimulated by melatonin/AFMK is consistent with induction of proliferation in this epidermal layer that is a part of epidermal differentiation program.
The phenomenon of K14 and Ki-67 regulation observed for the first time, to the authors knowledge, in human full skin is consistent with the observation reported earlier in mouse skin (63). Paradoxically, this is in contrast to cell culture data, demonstrating inhibition of keratinocyte proliferation shown in Supplemental Fig. S2 and reported previously (51). This difference may relate to different conditions and different roles, e.g., induction of cell division in basal layers of the epidermis is a prerequisite for turning on a precise differentiation program leading to the formation of the epidermal barrier (64). In cell culture, the epidermal structure is not formed, and action of melatonin and its metabolites is directed at restriction of proliferative activity of keratinocytes. In the intact skin, keratinocytes in the stratum spinosum and granulosum do not proliferate but differentiate, and melatonin and AFMK enhance this process, as illustrated by increased expression of K10 and IVL (Fig. 8B). Interestingly, our previous investigations (51) have revealed that the modulatory effect of melatonin on proliferation or differentiation of keratinocytes is strongly connected to the high expression of melatonin receptors in mammalian skin. Particularly, we detected predominant expression of MT1 receptor in normal skin, as well as in neonatal and adult epidermal keratinocytes, and it should be noted that exclusively the MT1 receptor, but not the MT2 receptor, was highly expressed in the epidermis within the stratum spinosum (+) and stratum granulosum (++) (17). Our present observations describe in these differentiating layers a strong and significant stimulatory effect of melatonin and AFMK on K10 and IVL expression. These results give strong support to the hypothesis that the inductive effect of melatonin and AFMK on differentiating keratinocytes and the expression of MT1 are mechanistically related, therefore, suggesting a receptor-mediated regulation of skin differentiation.
Regarding the effective concentrations required to stimulate K10 and IVL, the first significant increases were noticed at 10−4 M (K10) and 10−8 to 10−4 M (IVL) after 1 h of incubation with the agents. Moreover, 24 h of incubation caused more prominent effects at 10−8 to 10−6 M (K10) and 10−8 to 10−6 M (IVL). Such concentrations are consistent with the melatonin concentrations applied in serum-starved HaCaT keratinocytes, in which a concentration of 10−8 M led to a significant reduction of apoptotic subG1 signal (P<0.05) and reduced the number of early (FITC+/PI−) and late (FITC+/PI+) apoptotic cells by ∼40% (*P<0.05) (35). Here, the effect of pharmacological doses, including 10−3 M, leading to a significant increase of K14, K10, and IVL positivity requires explanation. Such high concentrations are required for nonreceptor-mediated metabolic mechanisms aimed at protection against harmful stressors, inducing oxidative stress and DNA damage (36, 65, 66). However, since melatonin and AFMK have to diffuse from the medium through dermis and basal membrane, their actual concentrations at the basal layers of the epidermis will likely be low, with the lowest values in the upper layers. Therefore, we propose that melatonin, in low concentrations, may exert its prodifferentiating capacity through the MT1 receptor within the upper layers of epidermis, while higher concentrations such as 10−3 M may use both receptor-mediated and receptor-independent mechanisms. The effectiveness of high concentrations of the compounds makes them perfect candidates for topical use in the treatment of epidermal pathology.
In summary, we define the indolic and kynuric pathways of melatonin metabolism in human skin cells, which is cell-type dependent, and we demonstrate melatonin and AFMK mediated stimulation of epidermal barrier formation with prodifferentiation effects in the stratum spinosum (+) and stratum granulosum (++).
Supplementary Material
Acknowledgments
This investigation was supported by U.S. National Institutes of Health (NIH)/National Institute of Arthritis and Muskuloskeletal and Skin Diseases grant 1R01AR056666-01A2 (A.S.), University of Lübeck (Lübeck, Germany) research grant E22-2012 (K.K.), experimental dermatology funds from the University of Lübeck medical faculty (T.W.F.), and NIH grants 1S10RR026377-01 and 1S10OD010678-01 (W.L.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- 5-MT
- 5-methoxytryptamine
- 6(OH)M
- 6-hydroxymelatonin
- AFMK
- N1-acetyl-N2-formyl-5-methoxykynuramine
- BSA
- bovine serum albumin
- DAPI
- 4′,6-diamidino-2-phenylindole
- EBS
- epidermolysis bullosa simplex
- ESI
- electrospray ionization
- HPLC
- high-pressure liquid chromatography
- IVL
- involucrin
- K-10
- cytokeratin-10
- K-14
- cytokeratin-14
- LC-MS
- liquid chromatography mass spectrometry
- MS
- mass spectrometry
- NAS
- N-acetylserotonin
- NGS
- normal goat serum
- NMS
- normal mouse serum
- PBS
- phosphate buffered saline
- SIM
- selective ion monitoring
- TNT
- Tris-NaCl-Tween
- TSA
- tyramide signal amplification
- UVB
- ultraviolet B
REFERENCES
- 1. Tan D. X., Manchester L. C., Hardeland R., Lopez-Burillo S., Mayo J. C., Sainz R. M., Reiter R. J. (2003) Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res. 34, 75–78 [DOI] [PubMed] [Google Scholar]
- 2. Hardeland R., Cardinali D. P., Srinivasan V., Spence D. W., Brown G. M., Pandi-Perumal S. R. (2011) Melatonin—a pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 93, 350–384 [DOI] [PubMed] [Google Scholar]
- 3. Reiter R. J., Tan D. X., Fuentes-Broto L. (2010) Melatonin: a multitasking molecule. Prog. Brain Res. 181, 127–151 [DOI] [PubMed] [Google Scholar]
- 4. Stehle J. H., Saade A., Rawashdeh O., Ackermann K., Jilg A., Sebesteny T., Maronde E. (2011) A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function, and chronobiological diseases. J. Pineal Res. 51, 17–43 [DOI] [PubMed] [Google Scholar]
- 5. Iriti M., Varoni E. M., Vitalini S. (2010) Melatonin in traditional Mediterranean diets. J. Pineal Res. 49, 101–105 [DOI] [PubMed] [Google Scholar]
- 6. Slominski A., Tobin D. J., Zmijewski M. A., Wortsman J., Paus R. (2008) Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol. Metab. 19, 17–24 [DOI] [PubMed] [Google Scholar]
- 7. Slominski R. M., Reiter R. J., Schlabritz-Loutsevitch N., Ostrom R. S., Slominski A. T. (2012) Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol. Cell. Endocrinol. 351, 152–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reiter R. J. (1991) Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 12, 151–180 [DOI] [PubMed] [Google Scholar]
- 9. Dubocovich M. L., Delagrange P., Krause D. N., Sugden D., Cardinali D. P., Olcese J. (2010) International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 62, 343–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lanoix D., Lacasse A. A., Reiter R. J., Vaillancourt C. (2012) Melatonin: the smart killer: the human trophoblast as a model. Mol. Cell. Endocrinol. 348, 1–11 [DOI] [PubMed] [Google Scholar]
- 11. Jockers R., Maurice P., Boutin J. A., Delagrange P. (2008) Melatonin receptors, heterodimerization, signal transduction and binding sites: what's new? Br. J. Pharmacol. 154, 1182–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tan D. X., Manchester L. C., Burkhardt S., Sainz R. M., Mayo J. C., Kohen R., Shohami E., Huo Y. S., Hardeland R., Reiter R. J. (2001) N1-acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 15, 2294–2296 [DOI] [PubMed] [Google Scholar]
- 13. Luchetti F., Canonico B., Betti M., Arcangeletti M., Pilolli F., Piroddi M., Canesi L., Papa S., Galli F. (2010) Melatonin signaling and cell protection function. FASEB J. 24, 3603–3624 [DOI] [PubMed] [Google Scholar]
- 14. Semak I., Naumova M., Korik E., Terekhovich V., Wortsman J., Slominski A. (2005) A novel metabolic pathway of melatonin: oxidation by cytochrome c. Biochemistry 44, 9300–9307 [DOI] [PubMed] [Google Scholar]
- 15. Nosjean O., Ferro M., Coge F., Beauverger P., Henlin J. M., Lefoulon F., Fauchere J. L., Delagrange P., Canet E., Boutin J. A. (2000) Identification of the melatonin-binding site MT3 as the quinone reductase 2. J. Biol. Chem. 275, 31311–31317 [DOI] [PubMed] [Google Scholar]
- 16. Fischer T. W., Slominski A., Zmijewski M. A., Reiter R. J., Paus R. (2008) Melatonin as a major skin protectant: from free radical scavenging to DNA damage repair. Exp. Dermatol. 17, 713–730 [DOI] [PubMed] [Google Scholar]
- 17. Slominski A., Wortsman J., Tobin D. J. (2005) The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 19, 176–194 [DOI] [PubMed] [Google Scholar]
- 18. Facciola G., Hidestrand M., von Bahr C., Tybring G. (2001) Cytochrome P450 isoforms involved in melatonin metabolism in human liver microsomes. Eur. J. Clin. Pharmacol. 56, 881–888 [DOI] [PubMed] [Google Scholar]
- 19. Semak I., Korik E., Antonova M., Wortsman J., Slominski A. (2008) Metabolism of melatonin by cytochrome P450s in rat liver mitochondria and microsomes. J. Pineal. Res. 45, 515–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grace M. S., Cahill G. M., Besharse J. C. (1991) Melatonin deacetylation: retinal vertebrate class distribution and Xenopus laevis tissue distribution. Brain Res. 559, 56–63 [DOI] [PubMed] [Google Scholar]
- 21. Rogawski M. A., Roth R. H., Aghajanian G. K. (1979) Melatonin: deacetylation to 5-methoxytryptamine by liver but not brain aryl acylamidase. J. Neurochem. 32, 1219–1226 [DOI] [PubMed] [Google Scholar]
- 22. Hirata F., Hayaishi O., Tokuyama T., Seno S. (1974) In vitro and in vivo formation of two new metabolites of melatonin. J. Biol. Chem. 249, 1311–1313 [PubMed] [Google Scholar]
- 23. Hardeland R., Reiter R. J., Poeggeler B., Tan D. X. (1993) The significance of the metabolism of the neurohormone melatonin: antioxidative protection and formation of bioactive substances. Neurosci. Biobehav. Rev. 17, 347–357 [DOI] [PubMed] [Google Scholar]
- 24. Tan D. X., Manchester L. C., Terron M. P., Flores L. J., Reiter R. J. (2007) One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 42, 28–42 [DOI] [PubMed] [Google Scholar]
- 25. Fischer T. W., Sweatman T. W., Semak I., Sayre R. M., Wortsman J., Slominski A. (2006) Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 20, 1564–1566 [DOI] [PubMed] [Google Scholar]
- 26. Slominski A. T., Zmijewski M. A., Skobowiat C., Zbytek B., Slominski R. M., Steketee J. D. (2012) Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv. Anat. Embryol. Cell. Biol. 212, 1–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Slominski A., Wortsman J. (2000) Neuroendocrinology of the skin. Endocr. Rev. 21, 457–487 [DOI] [PubMed] [Google Scholar]
- 28. Slominski A., Wortsman J., Tuckey R. C., Paus R. (2007) Differential expression of HPA axis homolog in the skin. Mol. Cell. Endocrinol. 265–266, 143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Slominski A., Fischer T. W., Zmijewski M. A., Wortsman J., Semak I., Zbytek B., Slominski R. M., Tobin D. J. (2005) On the role of melatonin in skin physiology and pathology. Endocrine 27, 137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fischer T. W., Slominski A., Tobin D. J., Paus R. (2008) Melatonin and the hair follicle. J. Pineal Res. 44, 1–15 [DOI] [PubMed] [Google Scholar]
- 31. Slominski A., Baker J., Rosano T. G., Guisti L. W., Ermak G., Grande M., Gaudet S. J. (1996) Metabolism of serotonin to N-acetylserotonin, melatonin, and 5-methoxytryptamine in hamster skin culture. J. Biol. Chem. 271, 12281–12286 [DOI] [PubMed] [Google Scholar]
- 32. Slominski A., Pisarchik A., Semak I., Sweatman T., Wortsman J., Szczesniewski A., Slugocki G., McNulty J., Kauser S., Tobin D. J., Jing C., Johansson O. (2002) Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 16, 896–898 [DOI] [PubMed] [Google Scholar]
- 33. Slominski A., Pisarchik A., Johansson O., Jing C., Semak I., Slugocki G., Wortsman J. (2003) Tryptophan hydroxylase expression in human skin cells. Biochim. Biophys. Acta 1639, 80–86 [DOI] [PubMed] [Google Scholar]
- 34. Slominski A., Pisarchik A., Semak I., Sweatman T., Szczesniewski A., Wortsman J. (2002) Serotoninergic system in hamster skin. J. Invest. Dermatol. 119, 934–942 [DOI] [PubMed] [Google Scholar]
- 35. Slominski A., Pisarchik A., Zbytek B., Tobin D. J., Kauser S., Wortsman J. (2003) Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J. Cell. Physiol. 196, 144–153 [DOI] [PubMed] [Google Scholar]
- 36. Fischer T. W., Kleszczynski K., Hardkop L. H., Kruse N., Zillikens D. (2012) Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. J. Pineal Res 23171152. [DOI] [PubMed] [Google Scholar]
- 37. Kleszczynski K., Hardkop L. H., Fischer T. W. (2011) Differential effects of melatonin as a broad range UV-damage preventive dermato-endocrine regulator. Dermato-endocrinology 3, 27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Slominski A., Tobin D. J., Shibahara S., Wortsman J. (2004) Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 84, 1155–1228 [DOI] [PubMed] [Google Scholar]
- 39. Slominski A., Wortsman J., Plonka P. M., Schallreuter K. U., Paus R., Tobin D. J. (2005) Hair follicle pigmentation. J. Invest. Dermatol. 124, 13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kleszczynski K., Fischer T. W. (2012) Development of a short-term human full-thickness skin organ culture model in vitro under serum-free conditions. Arch. Dermatol. Res. 304, 579–587 [DOI] [PubMed] [Google Scholar]
- 41. Slominski A., Zbytek B., Slominski R. (2009) Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int. J. Cancer 124, 1470–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Slominski A. T., Kim T. K., Janjetovic Z., Tuckey R. C., Bieniek R., Yue J., Li W., Chen J., Nguyen M. N., Tang E. K., Miller D., Chen T. C., Holick M. (2011) 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am. J. Physiol. Cell Physiol. 300, C526–C541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Slominski A. T., Li W., Bhattacharya S. K., Smith R. A., Johnson P. L., Chen J., Nelson K. E., Tuckey R. C., Miller D., Jiao Y., Gu W., Postlethwaite A. E. (2011) Vitamin D analogs 17,20S(OH)2pD and 17,20R(OH)2pD are noncalcemic and exhibit antifibrotic activity. J. Invest. Dermatol. 131, 1167–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Slominski A. T., Kim T.-K., Shehabi H. Z., Semak I., Tang E. K. Y., Nguyen M. N., Benson H. A. E., Korik E., Janjetovic Z., Chen J., Yates C. R., Postlethwaite A., Li W., Tuckey R. C. (2012) In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 26, 3901–3915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Janjetovic Z., Tuckey R. C., Nguyen M. N., Thorpe E. M., Jr., Slominski A. T. (2010) 20,23-dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-κB activity in human keratinocytes. J. Cell. Physiol. 223, 36–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Slominski A. T., Kim T. K., Chen J., Nguyen M. N., Li W., Yates C. R., Sweatman T., Janjetovic Z., Tuckey R. C. (2012) Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int. J. Biochem. Cell Biol. 44, 2003–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schoop V. M., Mirancea N., Fusenig N. E. (1999) Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J. Invest. Dermatol. 112, 343–353 [DOI] [PubMed] [Google Scholar]
- 48. Hardeland R., Tan D. X., Reiter R. J. (2009) Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 47, 109–126 [DOI] [PubMed] [Google Scholar]
- 49. Niu S., Li F., Tan D. X., Zhang L., Idle J. R., Gonzalez F. J., Ma X. (2010) Analysis of N1-acetyl-N2-formyl-5-methoxykynuramine/N1-acetyl-5-methoxy-kynuramine formation from melatonin in mice. J. Pineal Res. 49, 106–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Slominski A., Semak I., Pisarchik A., Sweatman T., Szczesniewski A., Wortsman J. (2002) Conversion of l-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett. 511, 102–106 [DOI] [PubMed] [Google Scholar]
- 51. Slominski A., Pisarchik A., Semak I., Sweatman T., Wortsman J. (2003) Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur. J. Biochem. 270, 3335–3344 [DOI] [PubMed] [Google Scholar]
- 52. Fischer T. W., Zmijewski M. A., Zbytek B., Sweatman T. W., Slominski R. M., Wortsman J., Slominski A. (2006) Oncostatic effects of the indole melatonin and expression of its cytosolic and nuclear receptors in cultured human melanoma cell lines. Int. J. Oncol. 29, 665–672 [DOI] [PubMed] [Google Scholar]
- 53. Slominski A., Pruski D. (1993) Melatonin inhibits proliferation and melanogenesis in rodent melanoma cells. Exp. Cell Res. 206, 189–294 [DOI] [PubMed] [Google Scholar]
- 54. Yu H. S., Reiter R. J. (1993) Melatonin Biosynthesis, Physiological Effects, and Clinical Implications, CRC Press, Boca Raton [Google Scholar]
- 55. Hipler U. C., Fischer T. W., Elsner P. (2003) HaCaT cell proliferation influenced by melatonin. Skin Pharmacol. Appl. Skin Physiol. 16, 379–385 [DOI] [PubMed] [Google Scholar]
- 56. Kobayashi H., Kromminga A., Dunlop T. W., Tychsen B., Conrad F., Suzuki N., Memezawa A., Bettermann A., Aiba S., Carlberg C., Paus R. (2005) A role of melatonin in neuroectodermal-mesodermal interactions: the hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB J. 19, 1710–1712 [DOI] [PubMed] [Google Scholar]
- 57. Fischer T. W., Zbytek B., Sayre R. M., Apostolov E. O., Basnakian A. G., Sweatman T. W., Wortsman J., Elsner P., Slominski A. (2006) Melatonin increases survival of HaCaT keratinocytes by suppressing UV-induced apoptosis. J. Pineal Res. 40, 18–26 [DOI] [PubMed] [Google Scholar]
- 58. Schallreuter K. U., Bahadoran P., Picardo M., Slominski A., Elassiuty Y. E., Kemp E. H., Giachino C., Liu J. B., Luiten R. M., Lambe T., Le Poole I. C., Dammak I., Onay H., Zmijewski M. A., Dell'Anna M. L., Zeegers M. P., Cornall R. J., Paus R., Ortonne J. P., Westerhof W. (2008) Vitiligo pathogenesis: autoimmune disease, genetic defect, excessive reactive oxygen species, calcium imbalance, or what else? Exp Dermatol 17, 139–140; discussion 141–160 [DOI] [PubMed] [Google Scholar]
- 59. Slominski A., Paus R., Bomirski A. (1989) Hypothesis: possible role for the melatonin receptor in vitiligo: discussion paper. J. R. Soc. Med. 82, 539–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Moll R., Divo M., Langbein L. (2008) The human keratins: biology and pathology. Histochem. Cell Biol. 129, 705–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Omary M. B., Coulombe P. A., McLean W. H. (2004) Intermediate filament proteins and their associated diseases. N. Engl. J. Med. 351, 2087–2100 [DOI] [PubMed] [Google Scholar]
- 62. Vassar R., Coulombe P. A., Degenstein L., Albers K., Fuchs E. (1991) Mutant keratin expression in transgenic mice causes marked abnormalities resembling a human genetic skin disease. Cell 64, 365–380 [DOI] [PubMed] [Google Scholar]
- 63. Slominski A., Chassalevris N., Mazurkiewicz J., Maurer M., Paus R. (1994) Murine skin as a target for melatonin bioregulation. Exp. Dermatol. 3, 45–50 [DOI] [PubMed] [Google Scholar]
- 64. Elias P. M., Menon G., Wetzel B. K., Williams J. J. (2010) Barrier requirements as the evolutionary “driver” of epidermal pigmentation in humans. Am. J. Hum. Biol. 22, 526–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fischer T. W., Zmijewski M. A., Wortsman J., Slominski A. (2008) Melatonin maintains mitochondrial membrane potential and attenuates activation of initiator (casp-9) and effector caspases (casp-3/casp-7) and PARP in UVR-exposed HaCaT keratinocytes. J. Pineal Res. 44, 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kleszczynski K., Tukaj S., Kruse N., Zillikens D., Fischer T. W. (2013) Melatonin prevents ultraviolet radiation-induced alterations in plasma membrane potential and intracellular pH in human keratinocytes. J. Pineal Res. 54, 89–99 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.