Abstract
Background
Non-Hodgkin’s lymphoma (NHL) has been widely reported to be associated with autoimmune and pro-inflammatory response, and genetic polymorphisms of candidate genes involved in autoimmune and pro-inflammatory response may influence the survival and prognosis of NHL patients. To evaluate the role of such genetic variations in prognosis of NHL, we conducted this study in a Chinese population.
Methods
We used the TaqMan assay to genotype six single nucleotide polymorphisms (SNPs) (TNF rs1799964T>C, LTA rs1800683G>A, IL-10 rs1800872T>G, LEP rs2167270G>A, LEPR rs1327118C>G, TNFAIP8 rs1045241C>T) for 215 NHL cases. Kaplan-Meier analysis was performed to compare progression free survival among two common genotypes. Cox proportional hazard models were used to identify independent risk factors.
Results
We observed that LTA rs1800683G>A was significantly associated with risk of progression or relapse in NHL patients (HR = 1.63, 95%CI = 1.06–2.51; P = 0.028), particularly in Diffuse large B cell lymphoma (DLBCL) cases (HR = 1.50, 95%CI = 1.10–2.04, P = 0.01). Both univariate and multivariate Cox regression analysis showed that in DLBCL patients, Ann Arbor stage III/IV, elevated LDH level before treatment and LTA rs1800683 AA genotype carrier were independent risk factors for progression or relapse. While in NK/T cell lymphoma, Ann Arbor stage III/IV and elevated β2-MG level before treatment indicated poorer prognosis.
Conclusions
The polymorphism of LTA rs1800683G>A contributes to NHL prognosis in a Chinese population. Further large-scale and well-designed studies are needed to confirm these results.
Introduction
Non-Hodgkin’s lymphomas (NHLs) represent a heterogeneous group of malignancies that arise from the lymphoid system [1]. The most common subtype of NHL in China is diffuse large B cell lymphoma (DLBCL). In general, long-term remission can be achieved in approximately 50% of NHL patients with conventional chemotherapy [2]. But a number of patients do not achieve complete remission or relapse after chemotherapy. Several studies have shown that the courses of NHLs are variable, and NHL survival patterns vary by subtypes, suggesting different prognostic risk factors for NHL histological subtypes [3], [4], [5]. International prognostic index (IPI) model was developed for predicting outcome in patients with aggressive NHL in 1993, which pointed out that more than 60-year-old, advanced tumor stage, poor performance score, extranodal involvement and elevated lactate dehydrogenase (LDH) were adverse prognostic factors for NHL [6]. However, some patients with the same IPI score still show different survival and prognosis. The clinical characteristics involved in IPI do not reflect any biological information about NHL, either. We need more specific and inherited factors to predict NHL survival and prognosis personally.
Pro-inflammatory cytokines regulate the immune system by controlling lymphoid cell development and differentiation, and regulating the balance between the T-helper immune responses, as well as proliferation, differentiation, movement and communication between tumor and stromal cells [7]. Some studies support the hypothesis that common genetic variants in inflammation and immune-related genes can affect both the susceptibility and prognosis of NHL [8], [9], [10].
We have studied the association between inflammation and immune-related gene polymorphisms and risk of NHL previously [11]. We genotyped six SNPs (TNF rs1799964T>C, LTA rs1800683G>A, IL–10 rs1800872T>G, LEP rs2167270G>A, LEPR rs1327118C>G, TNFAIP8 rs1045241C>T) and found that one SNP (rs1045241) in TNFAIP8 contributed to NHL susceptibility in a Chinese population. Subsequent stratification analyses by NHL subtypes showed that TNFAIP8 rs1045241 T allele was significantly associated with an increased risk of DLBCL and follicular lymphoma (FL), but not NK/T-cell lymphoma. The results raised the possibility that inflammation and immune-related gene variants may have important roles for pathogenesis of specific NHL subtypes.
To further test the hypothesis that SNPs in inflammation and immune-related genes affect the prognosis of NHL patients, we evaluated the association between six SNPs mentioned and progression free survival (PFS) of NHL cases in a Chinese population. Table S1 had a detailed description of the six SNPs.
Materials and Methods
Ethics Statement
The study was approved by the Institutional Review Board of Fudan University Shanghai Cancer Center. Participation was voluntary. All participants singed a written informed consent, and all clinical investigation was conducted according to the principles expressed in the Declaration of Helsinki consent.
Study Population
This study included 215 histologically confirmed NHL cases diagnosed between January 2008 and May 2011, 185 from Fudan University Shanghai Cancer Center and 30 from Kunshan First People’s Hospital Affiliated to Jiangsu University. The two centers are both in Eastern China and about 57 kilometers far apart. All patients were unrelated ethnic Han Chinese and had complete medical records. At recruitment, personal data from each participant regarding demographic information and clinical characteristics were collected via clinical record.
Diagnosis and Staging
All cases were classified and reviewed according to the 2008 WHO classification of tumors of haematopoietic and lymphoid tissues [12]. The tumor staging was defined with Ann Arbor system.
Genotyping
Genomic DNA was extracted from each blood sample by using the Qiagen Blood DNA Mini Kit (Qiagen Inc., Valencia, CA) according to the manufacturer’s instructions. DNA purity and concentration were determined by spectrophotometric measurement of absorbance at 260 and 280 nm by a UV spectrophotometer (Nano Drop Technologies, Inc., Wilmington, DE) and all DNA samples were suitable for genotyping.
All TaqMan assays for this study including the pre-designed SNP-genotyping assay mix containing PCR primers and probes were purchased from ABI (Applied Biosystems, Foster City, CA). Genotyping were conducted on the ABI 7900HT detection system (Applied Biosystems). To ensure the accuracy of genotyping results, four duplicated controls and four negative controls (no DNA) were included in each of the 384-well plates. The analyzed fiuorescence results were then auto-called into the genotypes using the built-in SDS2.2 software of the system.
Statistical Analysis
The last follow-up date was February 28, 2012. PFS was calculated from the initiation treatment to the time of progression, relapse, death, or the last follow-up. Patients alive on the last follow-up date were considered censored. PFS was assessed using the Kaplan–Meier method and compared between risk groups using the log-rank test. Cox regression model was used in the univariate and multivariate analyses. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were calculated. All statistical tests were two-sided, and P<0.05 was considered statistically significant. All analyses were performed using SPSS software, version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient Characteristics
The clinical characteristics of 215 NHL cases were summarized in Table 1. More males were enrolled, and the ratio was 1.69∶1. The median age was 48-year-old. In this study, the most common subtype of NHL was DLBCL (51.2%), followed by NK/T cell lymphoma (28.4%) and FL (11.1%).
Table 1. Clinical characteristics of 215 NHL cases.
| Characteristics | Number | % |
| Sex | ||
| Male | 135 | 62.8 |
| Female | 80 | 37.2 |
| Age at diagnosis | ||
| Median (range) | 48 (15–79) | |
| <60 | 163 | 75.8 |
| ≥60 | 52 | 24.2 |
| Subtype | ||
| DLBCL | 110 | 51.2 |
| NK/T-cell lymphoma | 61 | 28.4 |
| FL | 24 | 11.1 |
| Other B cell lymphomas | 8 | 3.7 |
| Other T cell lymphomas | 12 | 5.6 |
| Ann Arbor Stage | ||
| I | 60 | 27.9 |
| II | 74 | 34.4 |
| III | 46 | 21.4 |
| IV | 35 | 16.3 |
| B symptom | ||
| No | 145 | 67.4 |
| Yes | 70 | 32.6 |
| Elevated LDH level | ||
| No | 146 | 67.9 |
| Yes | 69 | 32.1 |
| Elevated β2-MG level | ||
| No | 95 | 44.2 |
| Yes | 120 | 55.8 |
| HBsAg positive | ||
| No | 171 | 79.5 |
| Yes | 44 | 20.5 |
| Recruitment site | ||
| Shanghai Cancer Center | 185 | 86.0 |
| Kunshan First People’s Hospital | 30 | 14.0 |
During follow-up, 97 cases (45.1%) appeared progression, relapse or death. The median progression free survival was 16 months (0–60 months).
Effect of SNP Genotypes on PFS by Subtypes
Frequencies of those six SNPs genotypes in NHL overall and subtypes are analyzed. One SNP in LTA (rs1800683) was significantly associated with risk of progression or relapse in NHL patients (HR = 1.63, 95%CI = 1.06–2.51; P = 0.028), but no associations were found between the other SNPs and PFS of NHL patients. Given that different subtypes may have different risk factors, we analyzed by subtypes. The quantities of some subtypes were so few that the statistical bias was inevitable, so we only analyzed PFS of DLBCL and NK/T cell lymphoma.
In DLBCL subtype, 110 cases were available for analysis. The median PFS was 15.5 months (0.5–40 months). Similar to the result of NHL overall, only the SNP LTA rs1800683G>A was slightly associated with risk of progression or relapse in DLBCL patients (P = 0.057 by log-rank test), but the other SNPs showed no association with PFS of DLBCL.
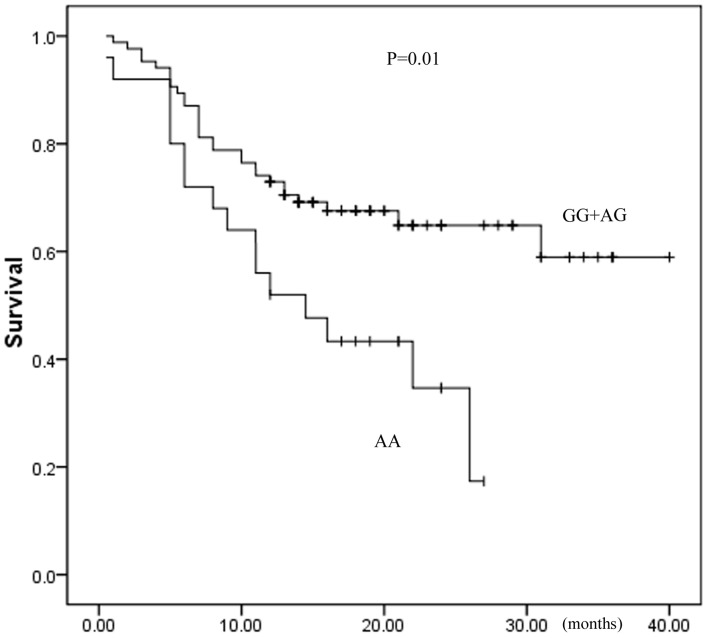
We further investigated the SNP LTA rs1800683G>A. When the LTA rs1800683 GG and AG genotype were grouped together and used as the reference group, LTA rs1800683 AA genotype was found to be associated with worse PFS of DLBCL patients, with the hazard ratios being 1.50 (95%CI: 1.10–2.04; P = 0.01), which was shown in Figure 1. The results indicated that carriers of LTA rs1800683 AA genotype among DLBCL patients have poorer prognosis, and may easier to progress or have a relapse.
Figure 1. Association between LTA rs1800683G>A genotypes and PFS in DLBCL.
There were 61 NK/T cell lymphoma cases enrolled for this part of study, with the median PFS of 16 months (0–60 months). However, no association was found between these six SNPs and risk of progression or relapse in NK/T cell lymphoma.
Effect of SNP Genotypes on Response to Chemotherapy
The cases were treated with different regimens by different subtypes, and not every subtype can benefit from chemotherapy. So we only analyzed DLBCL subtype. A total of 110 DLBCL cases received CHOP (cyclophosphamide, doxorubicin/epirubicin, vincristine, and prednisone) or CHOP-like regimen as first-line treatment. Among them, 71 cases (64.5%) received rituximab plus CHOP (R-CHOP). After 6 cycles of treatment, therapeutic outcomes were estimated. Response rates were 50.9% for complete remission (CR) or unconfirmed CR (CRu), 34.6% for partial response (PR), 0.9% for stable disease (SD) and 13.6% for progression disease (PD). The overall response (CR+PR) rate was 85.5%.
The impact of SNPs on overall response rate was assessed in 110 DLBCL cases, but no statistical differences were found for all six SNPs (The data were shown in Table S2).
Univariate and Multivariate Analysis for PFS
We also tested whether clinical characteristics contribute to progression free survival by subtypes. In DLBCL patients, Both univariate and multivariate Cox-regression model showed that Ann Arbor stage III/IV (Univariate: HR = 3.32, 95%CI = 1.81–6.10,P<0.001; Multivariate: HR = 5.04, 95%CI = 2.34–10.83,P<0.001), elevated LDH level before treatment (Univariate: HR = 3.19, 95%CI = 1.76–5.77,P<0.001; Multivariate: HR = 3.18, 95%CI = 1.48–6.83,P = 0.003) and LTA rs1800683 AA genotype carrier (Univariate: HR = 1.50, 95%CI = 1.10–2.04,P = 0.01; Multivariate: HR = 2.04, 95%CI = 1.01–4.14,P = 0.049) were independent risk factors for progression or relapse, while chemotherapy combined with rituximab may reduce the risk of progression or relapse (Univariate: HR = 0.43, 95%CI = 0.24–0.78, P = 0.005; Multivariate: HR = 0.40, 95%CI = 0.20–0.79,P = 0.008), as shown in Table 2.
Table 2. Univariate and multivariate analysis for PFS in DLBCL cases.
| Variables | Univariate analysis | multivariate analysis | ||
| HR (95%CI) | P-value | HR (95%CI) | P-value | |
| Sex (Male/Female) | 0.65(0.36–1.16) | 0.143 | 0.84(0.44–1.61) | 0.591 |
| Age (>60/≤60) | 1.09(0.55–2.15) | 0.813 | 1.18(0.55–2.58) | 0.669 |
| B symptom (Yes/No) | 1.03(0.74–1.45) | 0.853 | 0.80(0.35–1.81) | 0.588 |
| Stage (III/IV/I/II) | 3.32(1.81–6.10) | <0.001 | 5.04(2.34–10.83) | <0.001 |
| LDH (elevated/normal) | 3.19(1.76–5.77) | <0.001 | 3.18(1.48–6.83) | 0.003 |
| β2-MG (elevated/normal) | 1.61(0.88–2.94) | 0.123 | 0.63(0.29–1.38) | 0.249 |
| HBsAg (+/−) | 0.85(0.43–1.69) | 0.649 | 0.47(0.22–1.01) | 0.052 |
| Immunophenotype (ABC/GCB) | 1.22(0.60–2.47) | 0.582 | 0.68(0.31–1.51) | 0.344 |
| Rituximab plus (Yes/No) | 0.43(0.24–0.78) | 0.005 | 0.40(0.20–0.79) | 0.008 |
| rs1799964 CC/TC+TT | 1.07(0.53–2.17) | 0.857 | 4.74(0.94–23.9) | 0.059 |
| rs1800683 AA/AG+GG | 1.50(1.10–2.04) | 0.01 | 2.04(1.01–4.14) | 0.049 |
| rs1800872 GG/TG+TT | 0.68(0.41–1.13) | 0.138 | 0.89(0.09–1.92) | 0.076 |
| rs2167270 AA/AG+GG | 1.04(0.51–2.12) | 0.904 | 0.84(0.18–3.84) | 0.817 |
| rs1327118 GG/GC+CC | 4.58(0.07–9.14) | 0.479 | 6.06(0.01–12.7) | 0.967 |
| rs1045241 TT/TC+CC | 1.06(0.69–1.62) | 0.806 | 1.85(0.70–4.88) | 0.212 |
In NK/T cell lymphoma patients, we found that no characteristics except Ann Arbor stage III/IV (Univariate: HR = 5.50, 95%CI = 2.53–11.9, P<0.001; Multivariate: HR = 3.59, 95%CI = 1.45–8.87,P = 0.006) and elevated β2-MG level before treatment (Univariate: HR = 3.69, 95%CI = 1.60–8.54,P = 0.002; Multivariate: HR = 3.73, 95%CI = 1.37–10.17,P = 0.01) were the risk factors for poor prognosis when analyzed using univariate and multivariate Cox-regression model, as shown in Table 3.
Table 3. Univariate and multivariate analysis for PFS in NK/T-cell lymphoma cases.
| Variables | Univariate analysis | multivariate analysis | ||
| HR (95%CI) | P-value | HR (95%CI) | P-value | |
| Sex (Male/Female) | 1.39(0.61–3.19) | 0.439 | 1.42(0.55–3.65) | 0.468 |
| Age (>60/≤60) | 1.63(0.67–3.96) | 0.286 | 2.08(0.69–6.29) | 0.196 |
| B symptom (Yes/No) | 0.67(0.34–1.30) | 0.239 | 0.86(0.40–1.85) | 0.697 |
| Stage (III/IV/I/II) | 5.50(2.53–11.9) | <0.001 | 3.59(1.45–8.87) | 0.006 |
| LDH (elevated/normal) | 1.75(0.85–3.59) | 0.127 | 0.93(0.37–2.33) | 0.873 |
| β2-MG (elevated/normal) | 3.69(1.60–8.54) | 0.002 | 3.73(1.37–10.17) | 0.01 |
| HBsAg (+/−) | 0.81(0.31–2.10) | 0.659 | 0.36(0.12–1.15) | 0.084 |
| rs1799964 CC/TC+TT | 0.89(0.12–6.72) | 0.910 | 8.16(0.01–13.7) | 0.954 |
| rs1800683 AA/AG+GG | 1.72(0.82–3.61) | 0.151 | 0.98(0.39–2.50) | 0.973 |
| rs1800872 GG/TG+TT | 1.27(0.49–3.30) | 0.619 | 1.57(0.47–5.25) | 0.463 |
| rs2167270 AA/AG+GG | 0.29(0.04–2.09) | 0.217 | 0.01(0.00–9.61) | 0.960 |
| rs1327118 GG/GC+CC | 1.54(0.01–30.7) | 0.425 | 1.10(0.01–11.2) | 0.968 |
| rs1045241 TT/TC+CC | 2.07(0.49–8.63) | 0.319 | 3.37(0.54–21.09) | 0.193 |
Discussion
The survival patterns of different histological subtypes of NHL are highly variable. Indolent lymphomas progress slowly and have long-term survival; while aggressive lymphomas may have poor prognosis that progress in a short time [1]. International prognostic index (IPI) is still known as the effective prognostic factor for aggressive lymphomas today [3], [6]. But more and more researchers have focused on the effect of inflammation and immune-related gene polymorphisms on pathogenesis and prognosis of NHL. The cytokines coded by these genes play a key role in immune-regulating. They control lymphoid cell development and differentiation, and regulate the balance between the T-helper immune responses, as well as proliferation, differentiation, and the movement and communication between tumor and stromal cells [13], [14], [15]. Given the biologic properties, we hypothesize that the variants in cytokine genes may alter transcription and expression, and further inhibit the cellular signaling pathways, as well as the balance of immunity. Finally, the survival and prognosis of NHL patients will be influenced by the changes of microenvironment.
Our results showed that LTA rs1800683 G>A was associated with progression free survival of DLBCL patients. The carriers of LTA rs1800683 AA genotype had poorer prognosis. This is the first study to report that this polymorphism can affect PFS of DLBCL in a Chinese population. Lymphotoxin-α (LT-α) is an important member of tumor necrosis factor (TNF) family. As a key mediator of inflammation through the induction of chemokines and vascular adhesion molecular, LT-α also play crucial roles in lymphoid organ development, as well as lymphangiogenesis [16]. Scientists have been interested in whether the variants in LTA gene contribute to lymphomagenesis and prognosis of NHL. Some researchers hold the idea that polymorphisms in LTA gene may increase the synthesis and release of LT-α, which lead to deregulation of NF-κB signaling. Constitutive NF-κB activation can promote continuous lymphocyte proliferation, survival and apoptosis [16], [17]. Several studies have reported another SNP LTA G252A (rs909253) was a prognostic predictor for NHL in white populations [18], [19]. Moreover, Chae believed that LTA C804A (rs1041981) was associated with poor treatment outcome in Korean DLBCL patients [20]. The result of our study also proved that LTA rs1800683G>A influences PFS in Chinese DLBCL patients, but the biological mechanism of this polymorphism needs further functional studies to verify.
Our result also showed that after 6 cycles of CHOP or R-CHOP chemotherapy in DLBCL patients, the CR rate was 50.9%, and the overall response rate was 85.5%, which was similar to the data reported by other studies [21], [22]. But we did not find any significant association between these six SNPs and the overall response rate. While, Warzocha et al. found the serum TNF-α level was higher in carriers of TNF/LTA haplotype than other patients, and they also have poorer therapeutic outcome [17]. Another study in France reported that compared with IL-10 -1082AA genotype, patients with -1082 G allele had higher CR rate, longer five-year PFS and overall survival [23]. There are several potential explanations for the inconsistency. For one thing, ethnic and demographic differences between Chinese populations and other populations are exist, so frequencies of genotypes are different; for another, interactions among other genes and molecules may also play important roles in NHL prognosis, which means the association between a single SNP and prognosis may not be a linear relationship. The treatment outcome is affected by various factors, which should be taken into account, such as the living standard, performance status, side effects of chemotherapy and quality of caring. In our study, 62.3% cases are Ann Arbor stage I/II, and the short-term effect is better. This may explain why no difference was found for response rate. Moreover, 64.5% DLBCL patients were treated with CHOP plus rituximab, and the interaction between rituximab and polymorphisms should be considered.
The results of multivariate Cox-regression model analysis indicated that Ann Arbor stage III/IV, elevated LDH level before treatment and carriers of LTA rs1800683 AA genotype were independent prognostic risk factors for DLBCL patients, and chemotherapy combined with rituximab may reduce the risk of progression or relapse. For NK/T cell lymphoma patients, Ann Arbor stage III/IV and elevated β2-MG level before treatment were both the robust predictors of poor prognosis. It has been proved by other study that disease stage and LDH level are significantly associated with survival and prognosis of NHL patients [21]. Rituximab has also been reported to improve overall survival and progression-free survival on DLBCL patients. The result of MInT study showed after 6 cycles of CHOP or R-CHOP chemotherapy, R-CHOP group had improved time to treatment failure (TTF) than CHOP alone group (79% vs 59%), as well as overall survival [24]. Other large scale prospective studies, like GELA study and RICOVER-60 study also suggested that elder (>60 years old) DLBCL patients who received R-CHOP had longer event-free survival (EFS), PFS, disease-free survival (DFS) and OS than CHOP group [25], [26]. β2-microglobin (MG) is a non-specific tumor marker. Serum β2-MG level will elevate when the proliferation rate of lymphocyte is increased, which can be seen in multiple myeloma, malignant lymphoma and chronic lymphocytic leukemia. Previous study reported elevated serum β2-MG level could predict a relapse of DLBCL [27]. But few studies focused on the association between serum β2-MG level and prognosis of NK/T cell lymphoma. Our result suggested that serum β2-MG level was associated with the prognosis of NK/T cell lymphoma. Patients with elevated β2-MG before treatment had poorer prognosis and were easier to progress or relapse. But this conclusion should be verified with further clinical studies.
Despite of the strengths, our study also has limitations. First, we did not do the multiple testing corrections. If the p-value for LTA rs1800683 G>A was corrected by the Bonferroni method, it would no longer be significant. Second, we only discussed the effect of SNP on PFS and short-term outcome of NHL subtypes, but we did not analyze the overall survival. So our results might not comprehensively reflect the role of inflammation and immune-related gene polymorphisms. Third, the modest sized study is only powered to detect relatively large effects. We did not analyze other NHL subtypes except DLBCL and NK/T cell lymphoma due to extreme rarity. The genetic and clinical characteristics of different NHL subtypes can be quite different. Therefore, larger studies are warranted to confirm the effects of LTA rs1800683 SNP in other cohorts. In addition, we selected only one functional SNP for each candidate gene, which restricted further haplotype analysis. One SNP does not describe all the genetic variation in a gene, and optimal future studies should tag the variation in the gene and test haplotypes as well.
Conclusions
In summary, our study showed for the first time that rs1800683G>A SNP in the 5′UTR of LTA gene was associated with PFS of DLBCL in a Chinese population. The carriers of rs1800683 AA genotype had poorer prognosis and progress/relapse more easily. These results support the hypothesis that SNPs in inflammation and immune-related genes can affect the prognosis of NHL patients. It will also provide a new insight into NHL and NHL subtypes prognosis and have potential implication in clinic care of NHL patients. However, replication of our findings in other studies is warranted to confirm its significance.
Supporting Information
The information of six SNPs.
(DOC)
The association between SNP genotypes and response rate to chemotherapy.
(DOC)
Acknowledgments
We thank Jing He and Ting-Yan Shi for their help in DNA extraction, and technical support.
Funding Statement
This research was supported by a grant from National Natural Science Foundation of China (30870985). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nogai H, Dorken B, Lenz G (2011) Pathogenesis of non-Hodgkin’s lymphoma. J Clin Oncol 29: 1803–1811. [DOI] [PubMed] [Google Scholar]
- 2. Cabanillas F (2011) Non-Hodgkin’s lymphoma: the old and the new. Clin Lymphoma Myeloma Leuk 11 Suppl 1S87–90. [DOI] [PubMed] [Google Scholar]
- 3. Abdelhamid T, Samra M, Ramadan H, Mehessin M, Mokhtar N (2011) Clinical prognostic factors of diffuse large B cell non-Hodgkin lymphoma: A retrospective study. J Egypt Natl Canc Inst 23: 17–24. [DOI] [PubMed] [Google Scholar]
- 4. Chen WL, Tsai WC, Chao TY, Sheu LF, Chou JM, et al. (2010) The clinicopathological analysis of 303 cases with malignant lymphoma classified according to the World Health Organization classification system in a single institute of Taiwan. Ann Hematol 89: 553–562. [DOI] [PubMed] [Google Scholar]
- 5. Luminari S, Cesaretti M, Rashid I, Mammi C, Montanini A, et al. (2007) Incidence, clinical characteristics and survival of malignant lymphomas: a population-based study from a cancer registry in northern Italy. Hematol Oncol 25: 189–197. [DOI] [PubMed] [Google Scholar]
- 6. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project (1993) A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med 329: 987–994. [DOI] [PubMed] [Google Scholar]
- 7. Grivennikov SI, Greten FR, Karin M (2010) Immunity, Inflammation, and Cancer. Cell 140: 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernberg P, Chang ET, Duvefelt K, Hjalgrim H, Eloranta S, et al. (2010) Genetic variation in chromosomal translocation breakpoint and immune function genes and risk of non-Hodgkin lymphoma. Cancer Causes Control 21: 759–769. [DOI] [PubMed] [Google Scholar]
- 9. Lan Q, Wang SS, Menashe I, Armstrong B, Zhang Y, et al. (2011) Genetic variation in Th1/Th2 pathway genes and risk of non-Hodgkin lymphoma: a pooled analysis of three population-based case-control studies. British Journal of Haematology 153: 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Purdue MP, Lan Q, Kricker A, Grulich AE, Vajdic CM, et al. (2006) Polymorphisms in immune function genes and risk of non-Hodgkin lymphoma: findings from the New South Wales non-Hodgkin Lymphoma Study. Carcinogenesis 28: 704–712. [DOI] [PubMed] [Google Scholar]
- 11. Zhang Y, Wang MY, He J, Wang JC, Yang YJ, et al. (2012) Tumor necrosis factor-alpha induced protein 8 polymorphism and risk of non-Hodgkin’s lymphoma in a Chinese population: a case-control study. PLoS One 7: e37846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swerdlow SH, Campo E, Harris NL, Pileri S, Stein H, et al.. (2008) World Health Organisation classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC. [Google Scholar]
- 13. Lan Q (2006) Cytokine polymorphisms in the Th1/Th2 pathway and susceptibility to non-Hodgkin lymphoma. Blood 107: 4101–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aschebrook-Kilfoy B, Zheng T, Foss F, Ma S, Han X, et al. (2011) Polymorphisms in immune function genes and non-Hodgkin lymphoma survival. Journal of Cancer Survivorship 6: 102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuppers R (2004) Prognosis in follicular lymphoma–it’s in the microenvironment. N Engl J Med 351: 2152–2153. [DOI] [PubMed] [Google Scholar]
- 16. Jost PJ, Ruland J (2007) Aberrant NF-kappaB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood 109: 2700–2707. [DOI] [PubMed] [Google Scholar]
- 17. Warzocha K, Ribeiro P, Bienvenu J, Roy P, Charlot C, et al. (1998) Genetic polymorphisms in the tumor necrosis factor locus influence non-Hodgkin’s lymphoma outcome. Blood 91: 3574–3581. [PubMed] [Google Scholar]
- 18. Skibola CF, Bracci PM, Nieters A, Brooks-Wilson A, de Sanjose S, et al. (2010) Tumor necrosis factor (TNF) and lymphotoxin-alpha (LTA) polymorphisms and risk of non-Hodgkin lymphoma in the InterLymph Consortium. Am J Epidemiol 171: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seidemann K, Zimmermann M, Book M, Meyer U, Burkhardt B, et al. (2005) Tumor necrosis factor and lymphotoxin alfa genetic polymorphisms and outcome in pediatric patients with non-Hodgkin’s lymphoma: results from Berlin-Frankfurt-Munster Trial NHL-BFM 95. J Clin Oncol 23: 8414–8421. [DOI] [PubMed] [Google Scholar]
- 20. Chae YS, Kim JG, Sohn SK, Moon JH, Kim SN, et al. (2010) Lymphotoxin alfa and receptor-interacting protein kinase 1 gene polymorphisms may correlate with prognosis in patients with diffuse large B cell lymphoma treated with R-CHOP. Cancer Chemother Pharmacol 65: 571–577. [DOI] [PubMed] [Google Scholar]
- 21. Seki R, Ohshima K, Nagafuji K, Fujisaki T, Uike N, et al. (2010) Rituximab in combination with CHOP chemotherapy for the treatment of diffuse large B cell lymphoma in Japan: a retrospective analysis of 1,057 cases from Kyushu Lymphoma Study Group. Int J Hematol 91: 258–266. [DOI] [PubMed] [Google Scholar]
- 22. Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, et al. (2005) Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 23: 4117–4126. [DOI] [PubMed] [Google Scholar]
- 23. Lech-Maranda E, Baseggio L, Bienvenu J, Charlot C, Berger F, et al. (2004) Interleukin-10 gene promoter polymorphisms influence the clinical outcome of diffuse large B-cell lymphoma. Blood 103: 3529–3534. [DOI] [PubMed] [Google Scholar]
- 24. Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, et al. (2006) CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 7: 379–391. [DOI] [PubMed] [Google Scholar]
- 25. Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, et al. (2010) Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 116: 2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, et al. (2008) Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 9: 105–116. [DOI] [PubMed] [Google Scholar]
- 27. Aviles A, Narvaez BR, Diaz-Maqueo JC, Guzman R, Talavera A, et al. (1993) Value of serum beta 2 microglobulin as an indicator of early relapse in diffuse large cell lymphoma. Leuk Lymphoma 9: 377–380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The information of six SNPs.
(DOC)
The association between SNP genotypes and response rate to chemotherapy.
(DOC)