Abstract
We have investigated DNA-mediated transfer of aminopterin resistance conferred by plasmid and UV resistance conferred by genomic DNA to the Chinese hamster ovary (CHO) cell line UV-135, a UV-sensitive mutant defective in nucleotide excision repair. Plasmid pSV2gpt-CaPO4 coprecipitates induced aminopterin resistance with equal efficiency in the 6-thioguanine-resistant, aminopterin-sensitive, repair-proficient parental line AA8-4(tg-1) and in UV-135(tg-2). Genetic and molecular evidence for genomic DNA-mediated transformation of UV-135(tg-2) cells with a putative excision repair gene were obtained by demonstrating that: (i) UV resistance transformation is dependent upon and specific for genomic DNA from excision repair-competent CHO cells: (ii) UV and drug coresistant colonies are bona fide transferants as verified by hybridization and Southern blotting analysis of pSV2gpt sequences in their genomic DNAs: (iii) confirmed transferants exhibit partial to near normal UV resistances for colony formation: and (iv) UVr transferants have near normal levels of excision repair capacity. The overall frequency of drug and UV resistance cotransformation was 8 X 10(8) per cell plated. This frequency was ca. 200- to 500-fold greater than that expected from coincident but independent UVr reversion and plasmid gene transfer events. DNA transfer techniques with this CHO system will be useful for further analysis of the essential structural DNA sequences, gene cloning, and expression of functional excision repair genes.
Full text
PDF

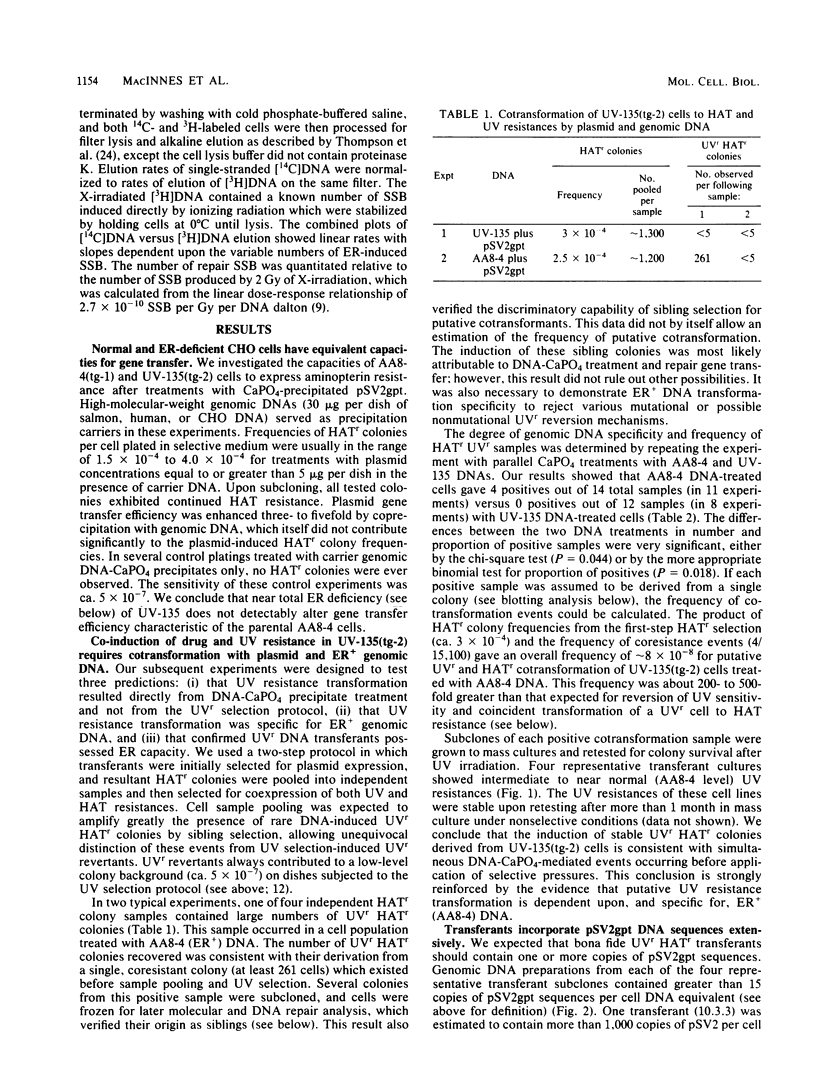
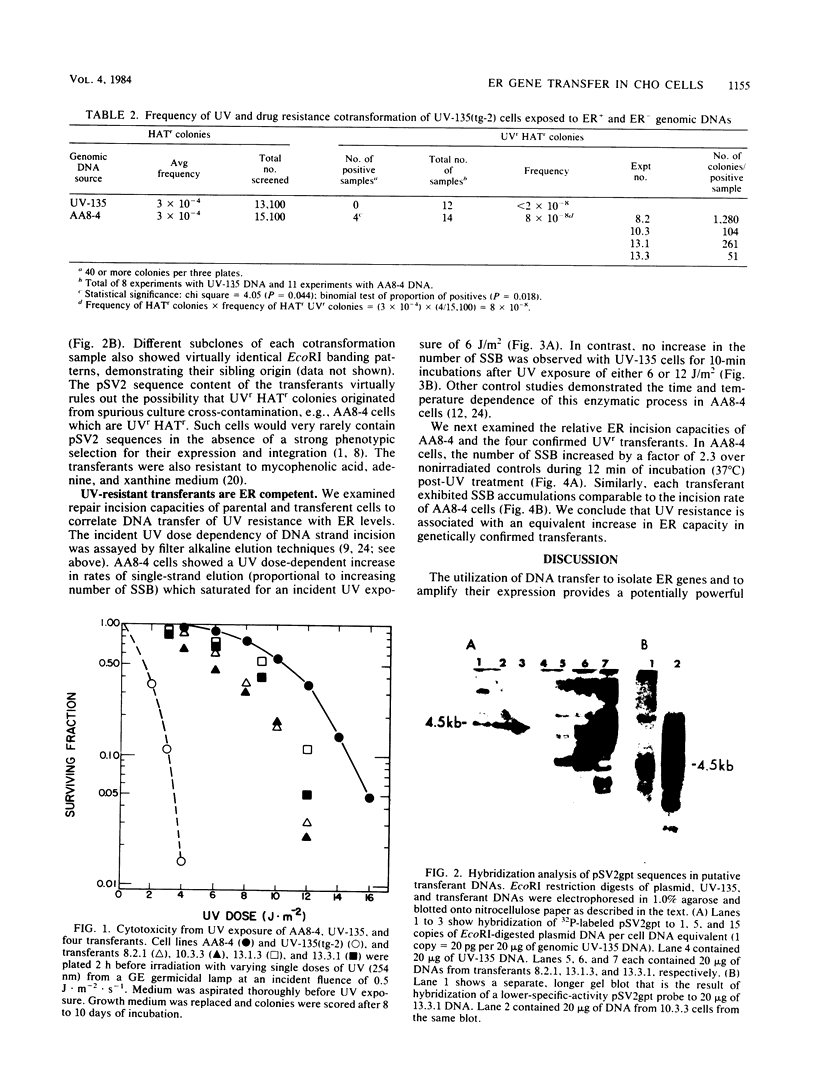
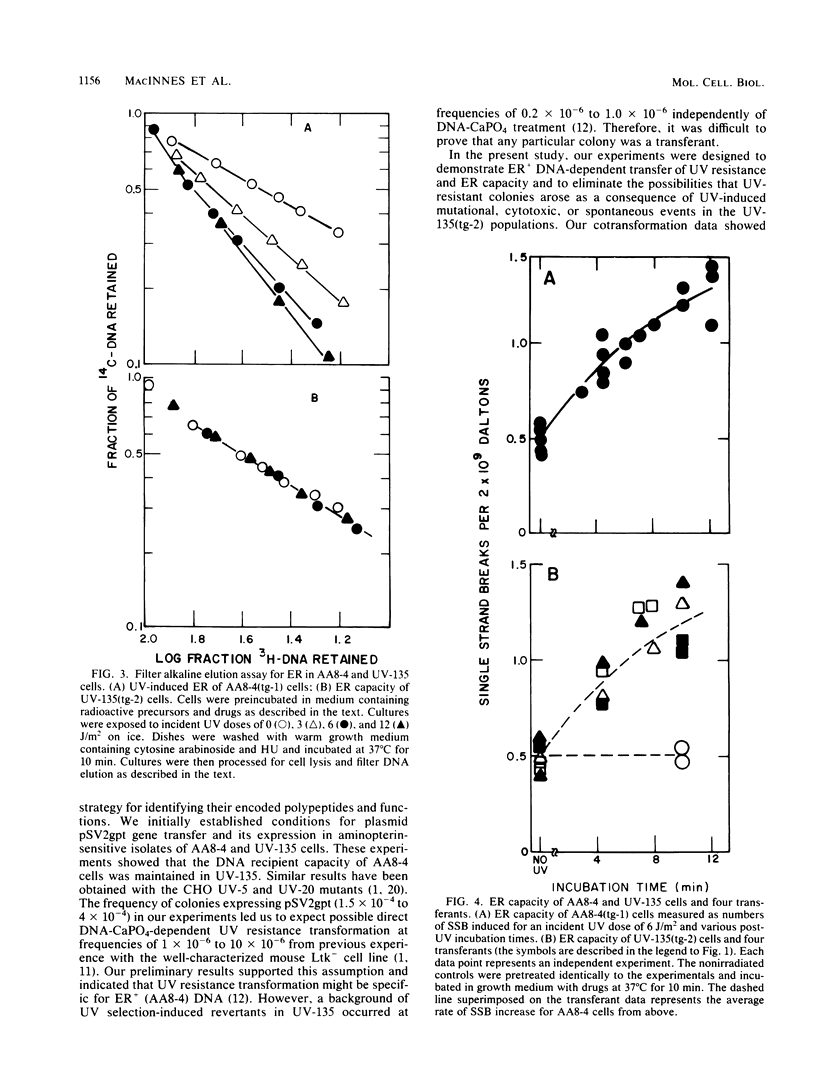


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham I., Tyagi J. S., Gottesman M. M. Transfer of genes to Chinese hamster ovary cells by DNA-mediated transformation. Somatic Cell Genet. 1982 Jan;8(1):23–39. doi: 10.1007/BF01538648. [DOI] [PubMed] [Google Scholar]
- Ayusawa D., Shimizu K., Koyama H., Takeishi K., Seno T. Unusual aspects of human thymidylate synthase in mouse cells introduced by DNA-mediated gene transfer. J Biol Chem. 1983 Jan 10;258(1):48–53. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch D. B., Cleaver J. E., Glaser D. A. Large-scale isolation of UV-sensitive clones of CHO cells. Somatic Cell Genet. 1980 May;6(3):407–418. doi: 10.1007/BF01542792. [DOI] [PubMed] [Google Scholar]
- Corsaro C. M., Pearson M. L. Enhancing the efficiency of DNA-mediated gene transfer in mammalian cells. Somatic Cell Genet. 1981 Sep;7(5):603–616. doi: 10.1007/BF01549662. [DOI] [PubMed] [Google Scholar]
- Gusella J. F., Keys C., VarsanyiBreiner A., Kao F. T., Jones C., Puck T. T., Housman D. Isolation and localization of DNA segments from specific human chromosomes. Proc Natl Acad Sci U S A. 1980 May;77(5):2829–2833. doi: 10.1073/pnas.77.5.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawalt P. C., Cooper P. K., Ganesan A. K., Smith C. A. DNA repair in bacteria and mammalian cells. Annu Rev Biochem. 1979;48:783–836. doi: 10.1146/annurev.bi.48.070179.004031. [DOI] [PubMed] [Google Scholar]
- Huttner K. M., Ruddle F. H. Analysis of a model for DNA-mediated gene transfer. Natl Cancer Inst Monogr. 1982;60:63–68. [PubMed] [Google Scholar]
- Lowy I., Pellicer A., Jackson J. F., Sim G. K., Silverstein S., Axel R. Isolation of transforming DNA: cloning the hamster aprt gene. Cell. 1980 Dec;22(3):817–823. doi: 10.1016/0092-8674(80)90558-9. [DOI] [PubMed] [Google Scholar]
- Major P. P., Egan E. M., Herrick D. J., Kufe D. W. Effect of ARA-C incorporation on deoxyribonucleic acid synthesis in cells. Biochem Pharmacol. 1982 Sep 15;31(18):2937–2940. doi: 10.1016/0006-2952(82)90266-0. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. J., Shilo B. Z., Shih C., Cowing D., Hsu H. W., Weinberg R. A. Three different human tumor cell lines contain different oncogenes. Cell. 1981 Aug;25(2):355–361. doi: 10.1016/0092-8674(81)90054-4. [DOI] [PubMed] [Google Scholar]
- Park S. D., Cleaver J. E. Inactivation of nucleotide excision repair in Chinese hamster ovary cells by exposure to an alkylating agent. Photochem Photobiol. 1982 Mar;35(3):419–421. doi: 10.1111/j.1751-1097.1982.tb02584.x. [DOI] [PubMed] [Google Scholar]
- Pero R. W., Miller D. G., Lipkin M., Markowitz M., Gupta S., Winawer S. J., Enker W., Good R. Reduced capacity for DNA repair synthesis in patients with or genetically predisposed to colorectal cancer. J Natl Cancer Inst. 1983 May;70(5):867–875. [PubMed] [Google Scholar]
- Razzaque A., Mizusawa H., Seidman M. M. Rearrangement and mutagenesis of a shuttle vector plasmid after passage in mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3010–3014. doi: 10.1073/pnas.80.10.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. S., Joyner A. L., Bernstein A., Whitmore G. F. Molecular identification of a human DNA repair gene following DNA-mediated gene transfer. Nature. 1983 Nov 10;306(5939):206–208. doi: 10.1038/306206a0. [DOI] [PubMed] [Google Scholar]
- Setlow R. B. Repair deficient human disorders and cancer. Nature. 1978 Feb 23;271(5647):713–717. doi: 10.1038/271713a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takano T., Noda M., Tamura T. Transfection of cells from a xeroderma pigmentosum patient with normal human DNA confers UV resistance. Nature. 1982 Mar 18;296(5854):269–270. doi: 10.1038/296269a0. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Dillehay L. E., Mooney C. L., Carrano A. V. Hypersensitivity to mutation and sister-chromatid-exchange induction in CHO cell mutants defective in incising DNA containing UV lesions. Somatic Cell Genet. 1982 Nov;8(6):759–773. doi: 10.1007/BF01543017. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Busch D. B., Brookman K., Mooney C. L., Glaser D. A. Genetic diversity of UV-sensitive DNA repair mutants of Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3734–3737. doi: 10.1073/pnas.78.6.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Rubin J. S., Cleaver J. E., Whitmore G. F., Brookman K. A screening method for isolating DNA repair-deficient mutants of CHO cells. Somatic Cell Genet. 1980 May;6(3):391–405. doi: 10.1007/BF01542791. [DOI] [PubMed] [Google Scholar]