Abstract
Nuclear factor kappaB (NF-κB) and Type 1 interferon (T1-IFN) signaling are innate immune mechanisms activated upon viral infection. However, the role of NF-κB and its interplay with TI-IFN in antiviral immunity is poorly understood. We show that NF-κB is essential for resistance to ectromelia virus (ECTV), a mouse orthopoxvirus related to the virus causing human smallpox. Additionally, an ECTV mutant lacking an NF-κB inhibitor activates NF-κB more effectively in vivo, resulting in increased pro-inflammatory molecule transcription in uninfected cells and organs and decreased viral replication. Unexpectedly, NF-κB activation compensates for genetic defects in the T1-IFN pathway, such as a deficiency in the IRF7 transcription factor, resulting in virus control. Thus, overlap between the T1-IFN and NF-κB pathways allows the host to overcome genetic or pathogen-induced deficiencies in T1-IFN and survive an otherwise lethal poxvirus infection. These findings may also explain why some pathogens target both pathways to cause disease.
Upon infection, pathogen associated molecular patterns (PAMPS) are sensed by Pattern Recognition Receptors (PRRs), resulting in the activation of various transcription factors, prominently canonical nuclear factor κB (NF-κB) and the interferon regulatory factors (IRF) 3 and 7 (Barnes et al., 2002; Brennan and Bowie, 2010; O'Neill, 2006).
Under resting conditions, the p105 and p65 (RelA) subunits of canonical NF-κB form a complex with the inhibitor of κB (IκB). Following stimulation through various receptors, p105 is phosphorylated by activated IκB kinases (ikks) and cleaved to generate the mature p50 subunit. IκB is also phosphorylated by ikks and degraded by the proteasome. This results in the release of p50-p65 which translocates to the nucleus and binds to the promoters of many genes, including pro-inflammatory cytokines such as TNF-α, IL-1α, IL-1β and various chemokines which further induce the activation of NF-κB in a positive feedback loop (Liu, 2005).
In the mouse, the Type I interferons (TI-IFNs) are represented by one IFN-β and 12 IFN-αs (α1,2,4–7, 9–14). The transcription factors IRF3 and IRF7 play an important role in the type-I interferon (TI-IFN) expression. IRF3 is constitutively expressed in most cells. Activation of IRF3 through most PRRs induces the transcription of IFN-α4 and IFN-β which are known as “early” TI-IFNs. IRF7 is expressed constitutively only in some cells such as plasmacytoid dendritic cells (pDC) (Honda et al., 2005), but can be induced in all cells by TI-IFN (Lu et al., 2000) and possibly by other stimuli as its promoter contains an NF-κB response element (Lu et al., 2002). Activation of IRF7 through the PRR TLR9 and its adaptor MyD88 results in the rapid production of IFN-β, IFN-α4 and also non-4 IFN-α which are known as “late” TI-IFNs (Fitzgerald et al., 2003; Sharma et al., 2003).
TI-IFN signaling through the IFN-α receptor (IFNAR) results in the expression of a large number of IFN stimulated genes (ISGs) with antiviral function. While in vitro some ISGs can be induced independently of TI-IFN and dependently (Basagoudanavar et al., 2011) or not (Hasan et al., 2013) on NF-κB, whether ISGs can be induced in vivo by stimuli other than TI-IFN is not known. Because IRF7 is itself an ISG, the TI-IFN pathway can also be regulated by a positive feedback loop in an autocrine and paracrine manner (Honda et al., 2005; Sato et al., 1998).
While T1-IFNs are known to be critical for the clearance of many viruses in vivo (Bogdan, 2000) the role of NF-κB is less clear due to the variety of defects in mice deficient in this pathway (Gerondakis et al., 2006; Weih and Caamano, 2003). However, many viruses developed mechanisms to evade NF-κB signaling (Bowie and Unterholzner, 2008; Seet et al., 2003), suggesting that it is required for innate virus control in vivo. Importantly, the promoters of the early T1-IFNs have NF-κB response elements which are essential for constitutive as well as early expression of IFN-β following viral infection. In contrast, they are dispensable for late expression of IFN-β in cells infected with RNA viruses (Balachandran and Beg, 2011; Basagoudanavar et al., 2011; Wang et al., 2010; Wang et al., 2007). Thus, rather than separate, the NF-κB and T1-IFN pathways appear to be part of a complex anti-viral network. How this networking improves virus control in vivo and why some viruses must subvert several of its components remains relatively unexplored.
The genus Orthopoxvirus (OPV) comprises many species including variola virus, zoonotic monkeypoxvirus, the vaccine species vaccinia virus and ectromelia virus (ECTV), a natural mouse pathogen that penetrates the body through microabrasions in the footpad and spreads through the lymphohematogenous (LH) route (Esteban and Buller, 2005; Fenner, 1949). Mousepox susceptible mouse strains such as BALB/c cannot control virus replication and LH spread dying 7–14 days post infection (dpi) with high virus loads and extensive damage to the liver and spleen. Resistant mouse strains such as C57BL/6 (B6) control virus replication and LH spread through combined innate and adaptive immune mechanisms.
OPVs encode modulators of both the NF-κB and the T1-IFN pathways (Seet et al., 2003). Recently, a protein that binds to the NF-κB subunit p105 (herein p105 binding protein, p105bp) was identified in a proteomic screening of variola virus. In transfected cells, p105bp from variola, monkeypox, cowpox and ECTV, inhibited the proteolytic processing of p105 to p50, and blocked NF-κB activation (Mohamed et al., 2009a). Moreover, a CPXV deficient in p105bp was attenuated in mice and recruited more inflammatory cells to the infected lung than the wild type (WT) virus (Mohamed et al., 2009b). However, the effect of p105bp deficiency on the activation of NF-κB and downstream effects in vivo has not studied.
Results
p105 is required for resistance to mousepox and its inhibition is essential for ECTV virulence
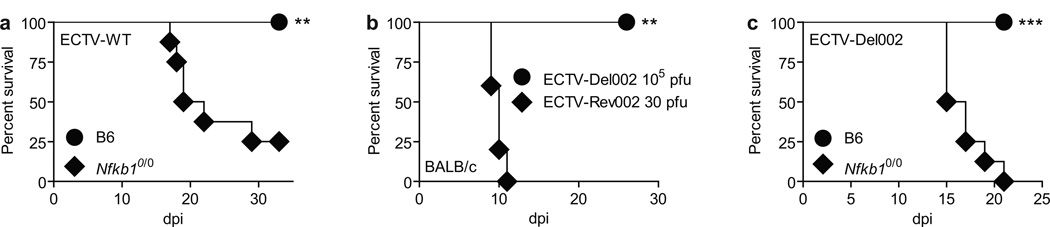
Different than wild type B6 mice, B6.Cg-Nfkb1tm1Bal/J mice (Sha et al., 1995) in a mousepox resistant B6 background (Nfkb10/0) succumbed to wild type ECTV (ECTV-WT) infection (Figure 1a) and the few that survived became very ill, indicating a crucial role for canonical NF-κB in resistance to mousepox. However, Nfkb10/0 mice have severe immune defects that make them useless for in-depth studies of viral pathogenesis (Gerondakis et al., 2006). Indeed, it has been shown that most Nfkb10/0 mice lack inguinal lymph nodes (LNs) (Lo et al., 2006) and we found that all of them lack popliteal LNs (reported as text). Because the popliteal LN participates in the dissemination as well as the control of ECTV (Fang et al., 2008; Fang et al., 2011; Fang et al., 2010; Fenner, 1949) this finding makes Nfkb10/0 mice unsuitable for in-depth studies of NF-κB in the control of ECTV.
Figure 1. Role of p105 in resistance to mousepox and of p105bp in ECTV virulence.
(a,b,c) Survival: (a) B6 (n=8, circles) and Nfkb10/0 mice (n=8, diamonds) infected with 3,000 pfu ECTV-WT, (b) BALB/c mice (n=5) infected with 30 pfu ECTV-Rev002 (diamonds) or 100,000pfu ECTV-Del002 (circles). (c) B6 (n=8, circles) and Nfkb10/0 mice (n=8, diamonds) infected with 3,000 pfu of ECTV-Del002. Data in a and c are aggregates of two experiments. See also Figure S1.
In ECTV, p105bp is encoded by Evm002 and its ability to inhibit NF-κB in cultured cells has already been demonstrated by the McFadden group using cells transfected with Evm002 (Mohamed et al., 2009a). To elude the inherent problems of NF-κB deficient mice, we generated an ECTV deficient in Evm002 (ECTV-Del002, see Figure S1a) with the purpose of using it to study the role of NF-κB in anti-viral defense in vivo. In BS-C-1 cells, ECTV-Del002 and its revertant (ECTV-Rev002, Figure S1b) replicated similarly (Figure S1c–d). In mousepox susceptible BALB/c mice, ECTV-Rev002 was 100% lethal at a dose of 30 plaque forming units (pfu), while ECTV-Del002 was non-lethal at 105 pfu (Figure 1b). However, 3000 pfu ECTV-Del002 killed all Nfkb10/0 mice (Figure 1c) indicating that NF-κB activation is essential for resistance to ECTV, and that p105bp is required for ECTV virulence.
Inhibition of p105/p50 reduces the speed and efficiency of NF-κB activation in the D-LN and liver
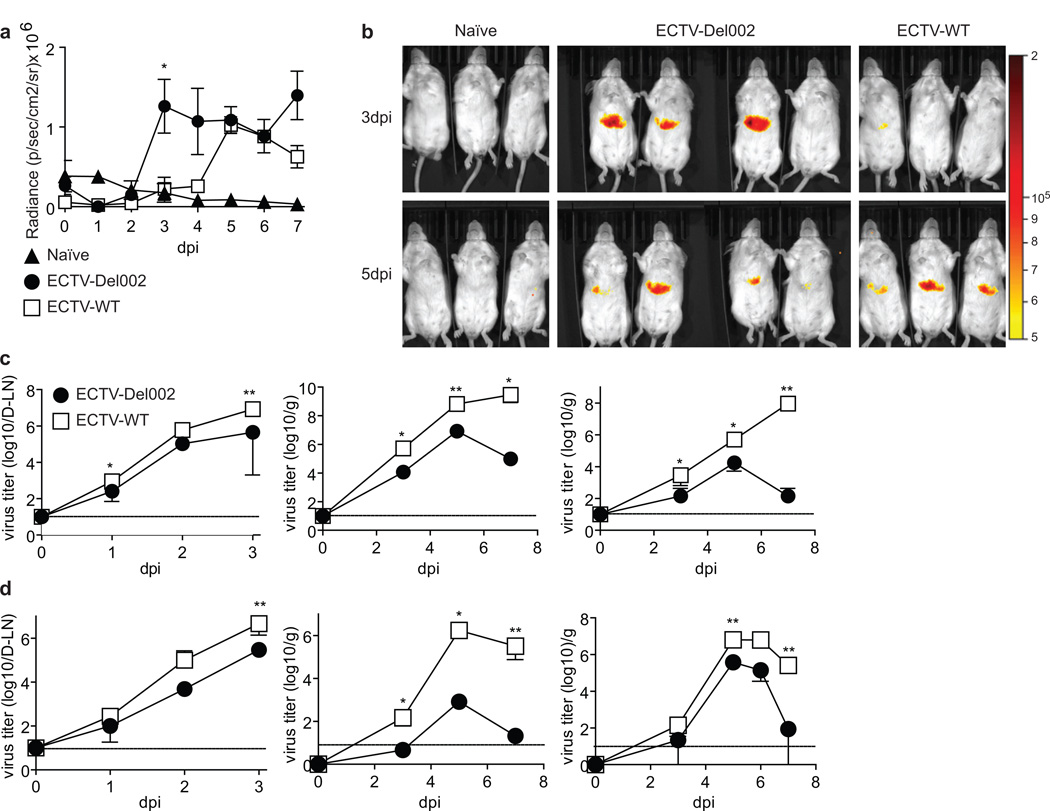
It has been shown that ECTV p105bp blocks NF-κB activation in transfected cells (Mohamed et al., 2009a). To compare NF-κB activation by ECTV in the presence or absence of p105bp in vivo, we transfected the livers of BALB/c mice with an NF-κB luciferase reporter plasmid using hydrodynamic injection (Liu et al., 1999). In these mice, ECTV-Del002 activated NF-κB in the liver earlier than ECTV-WT as determined by whole body luciferase imaging (Figure 2a–b). Further, we could observe cells with nuclear p65 in areas of infection in the D-LN at 2 dpi (Figure S2a) and in the liver at 5 dpi (Figure S2b) in mice infected with ECTV-Del-002 but not with ECTV-WT. Thus, p105bp slows and decreases the efficiency of NF-κB activation in vivo.
Figure 2. Role of p105bp in NF-kB activation and virus spread.
(a,b) In vivo NF-κB activation: The livers of BALB/c mice were transfected in vivo with an NF-κB reporter plasmid expressing firefly luciferase. 12 days post-transfection the mice were infected with 3,000 pfu ECTV-WT in the footpad and imaged daily for seven days 5 min after luciferin injection. (a) Radiance (p/sec/cm2/sr) in the liver area at the indicated dpi was determined in naïve (n=3), ECTV-Del002-(n=4) and ECTV-WT-infected mice (n=3) and plotted as mean ± SEM with significant difference indicated at 3 dpi. (b) Radiance images of the mice in d at 3 and 5 dpi. (c.d) Virus titers: BALB/c (c) or B6 mice (d) were infected in the footpad with 3,000 pfu of ECTV-WT (empty squares) or ECTV-Del002 (filled circles) viruses. Virus loads were monitored in D-LN (left panels), spleen (middle panels) and liver (right panels). N=5 mice per time point. Data are displayed as mean ± SEM. Horizontal lines indicate limit of detection. See also Figure S2.
Inhibition of p105/p50 by p105bp accelerates ECTV LH spread
At 3 dpi, virus titers in the D-LN, liver and spleen were significantly lower in BALB/c mice infected with ECTV-Del002 than with ECTV-WT suggesting that ECTV-Del002 replicated and/or disseminated less efficiently. From 5 to 7 dpi, the titers of ECTV-WT in the liver and spleen increased but those of ECTV-Del002 decreased (Figure 2c). Similar results were obtained in mousepox resistant B6 mice, except that at 7 dpi the titers of ECTV-WT were lower than at 5 dpi while ECTV-Del002 had almost been cleared (Figure 2d). Hence, inhibition of p105/p50 by p105bp promotes LH spread and slows liver clearance in mousepox sensitive and also in mousepox resistant mice. While NK cell recruitment to the D-LN at 2–3 dpi is important to control early ECTV spread (Fang et al., 2008), the decreased LH spread of ECTV-Del002 was not due to enhanced NK cell recruitment (Figure S2c).
Inhibition of p105/p50 decreases transcription of several inflammatory genes in the D-LN of BALB/c and B6 mice
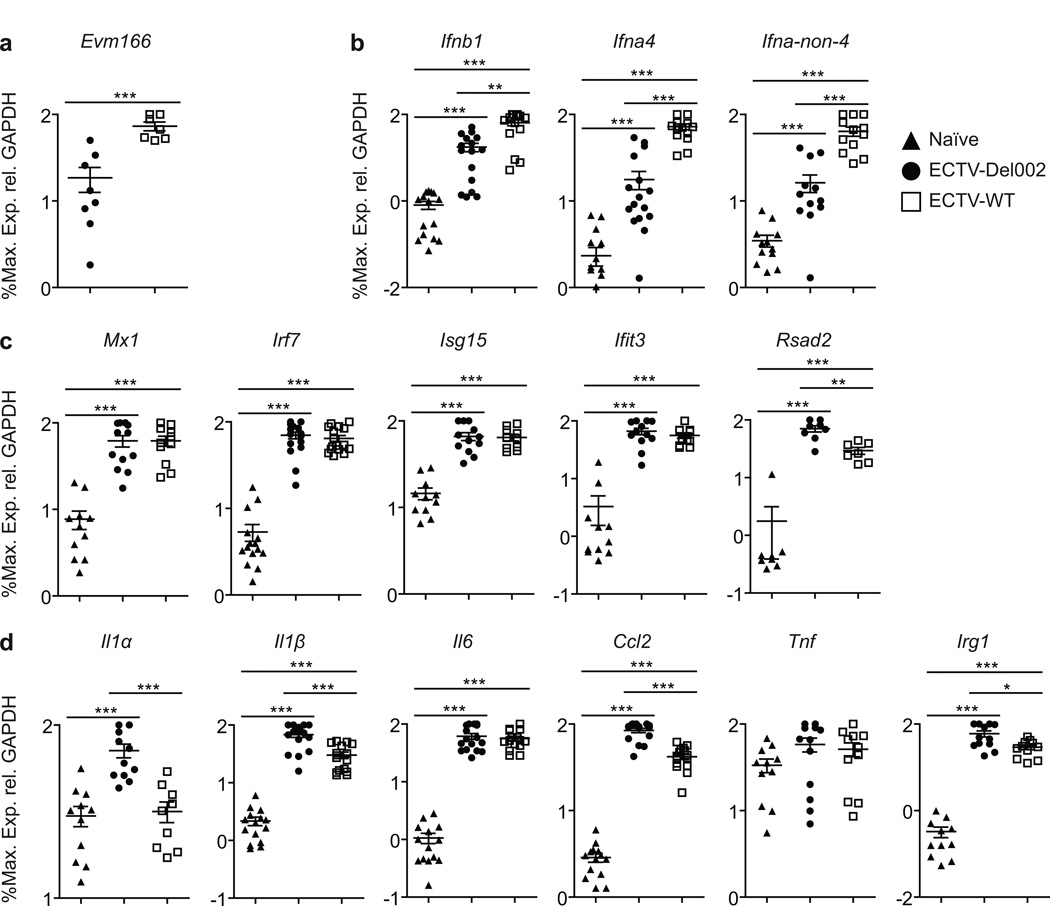
At 2.5 dpi transcription of the ECTV gene Evm166 was higher in the D-LN of ECTV-WT than in ECTV-Del002 infected BALB/c mice indicating higher virus loads in ECTV-WT infected mice (Figure 3a). ECTV-WT induced significantly higher transcription of early and late T1-IFNs (Figure 3b). However, ECTV-WT and ECTV-Del002 induced similar levels of the interferon stimulated genes (ISGs) Mx1, Irf7, Isg15 and Ifit3 (Figure 3c). Transcripts of pro-inflammatory Il1a, Il1b, Ccl2, Irg1 and Rsad2 but not Il6 were induced significantly more by ECTV-Del002 (Figure 3d). Results in B6 mice were similar (Figure S3) except that only the pro-inflammatory Il1a and Irg1 were expressed higher with ECTV-Del002 (Figure S3d). Hence, while ECTV-Del002 replicated less efficiently in vivo, it induces several pro-inflammatory genes more effectively than ECTV-WT indicating that p105bp dampens the expression of pro-inflammatory genes in the D-LN. Moreover, even though ECTV-WT replicated better and induced more T1-IFN, genes traditionally considered ISGs were equally induced by both viruses suggesting that p105bp also reduces ISG expression.
Figure 3. ECTV inhibition of p105/p50 results in decreased transcription of inflammatory genes in susceptible BALB/c mice.
BALB/c mice were infected with ECTV-Del002 or ECTV-WT. At 2.5 dpi the indicated transcripts were quantified by qPCR. Data correspond to 4 independent experiments combined. (a) Virus load, (b) T1-IFNs, (c) ISGs, (d) inflammatory genes. Triangles, naïve; circles, ECTV-Del002; squares, ECTV-WT. Data expressed as % of maximum as calculated for each individual experiment and shown in a log10 scale as mean ±SEM. See also Figure S3.
Inhibition of p105/p50 in vivo decreases the expression of pro-inflammatory mediators in uninfected cells
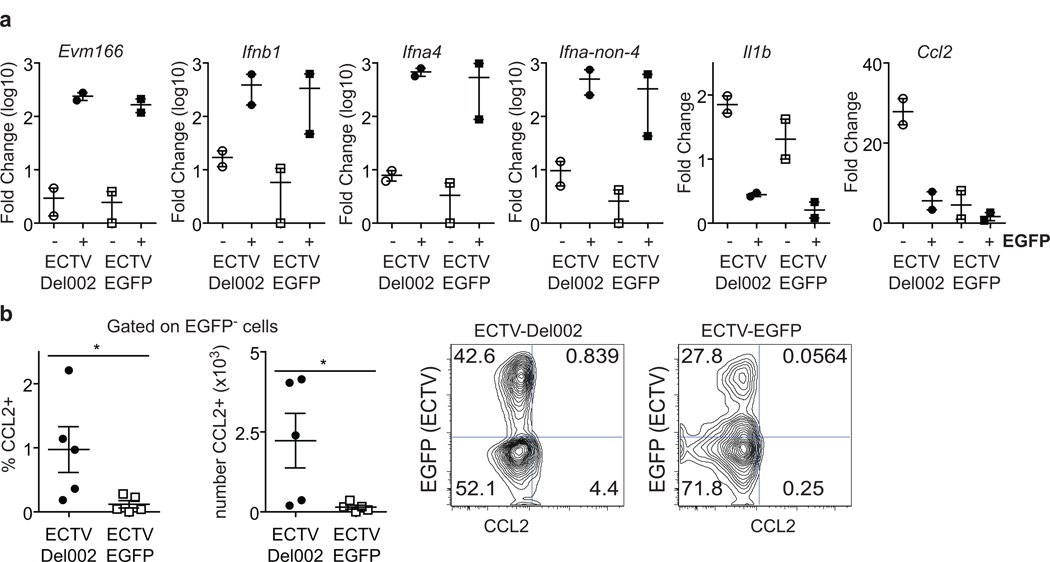
During in vivo infection, cytokines could be produced by the infected cells themselves and/or by uninfected cells receiving cues from those infected. However, to what extent infected and uninfected cells contribute to the overall cytokine response in vivo is unknown. ECTV-Del002 expresses EGFP and we have previously generated a fully virulent recombinant ECTV expressing EGFP (ECTV-EGFP) (Fang et al., 2008). Given that p105bp is a non-structural intracellular protein, and should therefore only directly affect NF-κB in infected cells, we investigated whether in vivo, ECTV inhibition of NF-κB mainly affects the ability of EGFP+ infected cells to produce cytokines, and/or indirectly affects EGFP− uninfected cells to generate those mediators. To gain easy access to infected and uninfected cells, we infected mice with ECTV-Del002 or ECTV-EGFP intraperitoneally. At 2 dpi, the cells in the peritoneal cavity were obtained by lavage and stained with Abs to various hematopoietic lineage markers. We found that for both viruses, most infected cells were CD3− DX5− B220− CD11b+ as determined by EGFP expression (reported as text). Hence, we used FACS to sort EGFP+ CD3− DX5− B220− CD11b+ and EGFP− CD3− DX5− B220− CD11b+ and gene expression was determined by RT-qPCR in the two populations. Transcription of Evm166 was strong in EGFP+ cells but not in EGFP− cells independently of the virus used for the infection (Figure 4a). This confirmed that sorting based on EGFP expression distinguished infected from uninfected cells. Independently of the virus type, early as well as late T1-IFNs were expressed almost exclusively by infected cells. Unexpectedly, the pro-inflammatory cytokines Il1b and Ccl2 were only expressed in uninfected cells. More strikingly, Ccl2 was expressed at significantly higher levels in uninfected cells from mice infected with ECTV-Del002 (Figure 4a). This was confirmed at the protein level by flow cytometry following intracellular staining with anti-CCL2 Ab. (Figure 4b).
Figure 4. Inhibition of p105/p50 in vivo decreases expression of pro-inflammatory mediators in uninfected cells.
(a) Groups of 6 mice were infected intraperitoneally with 104 pfu of ECTV-EGFP or ECTV-Del002. At 2dpi CD3− DX5− B220− CD11b+ EGFP+ and CD3− DX5− B220− CD11b+ EGFP− peritoneal cells from pools of three mice were purified by FACS and the expression of the indicated genes was determined by RT-qPCR. Each data point corresponds to a pool of three mice. The experiment was repeated with similar results. (b) Left: Frequency and calculated absolute number of CCL2+ cells in the CD3− DX5− B220− Cd11b+ F4/80+ EGFP− gate as determined by flow cytometry at 2 dpi in peritoneal cells from mice infected with the indicated viruses (left). Each dot represents an individual mouse (n=5). Mean ± SEM are shown. Representative flow cytometry plots for (right). Plots are gated on CD3− DX5− B220− Cd11b+ F4/80+ cells.
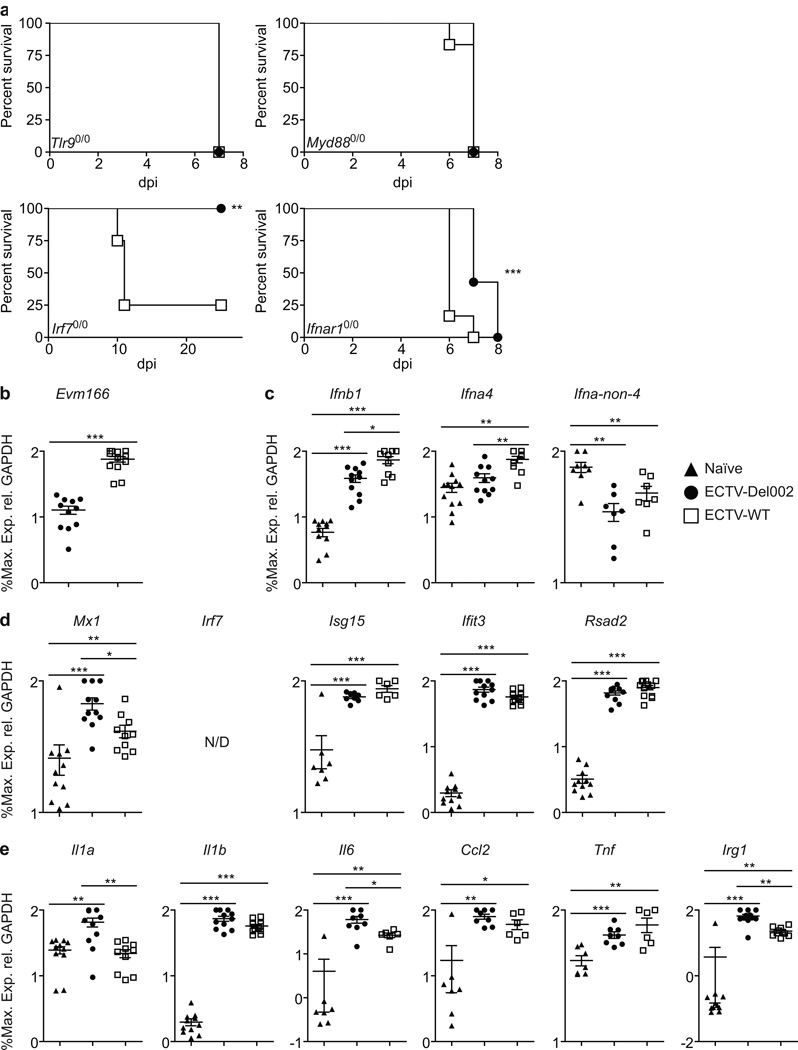
Inhibition of p105/p50 bp is required for lethality in Irf70/0 but not in Tlr90/0 or Myd880/0 mice
As reported by others (Samuelsson et al., 2008; Sutherland et al., 2011), Tlr90/0 and Myd880/0 mice succumbed to ECTV-WT. These mice were also highly sensitive to ECTV-Del002 suggesting that the TLR9/MyD88 activation of NF-κB is essential for resistance to ECTV-Del002. Remarkably, mice deficient in IRF7 (Irf70/0) which is also activated by TLR9/MyD88, succumbed to ECTV-WT but not to ECTV-Del002 (Figure 5a). This indicates that a deficiency in T1-IFN production can be compensated by increased NF-κB activation through TLR9/MyD88.
Figure 5. ECTV-Del002 is lethal to Myd880/0 and Tlr90/0 mice but not to Irf70/0 mice where it induces higher inflammatory gene expression than lethal ECTV-WT.
(a) Survival of the indicated mice: Mice (n=5/group) infected with ECTV-Del002 (filled circles) or ECTV-WT (empty squares). (b–e) Gene expression in Irf70/0 mice: At 2.5 dpi, gene transcription was determined as in Figure 2. The results are from three independent experiments combined. (b) Virus, (c) T1-IFNs, (d) ISGs, (e) inflammatory genes. Triangles, naïve; circles, ECTV-Del002; squares, ECTV-WT.; N/D, not detected. Data expressed as % of maximum as calculated for each individual experiment and shown in a log10 scale as mean ± SEM.
Inhibition of p105/p50 reduces transcription of several inflammatory genes in Irf70/0 mice
At 2.5 dpi transcription of Evm166 was higher in the D-LN of ECTV-WT than in ECTV-Del002 infected Irf70/0 mice indicating higher virus loads in the ECTV-WT infected animals (Figure 5b). ECTV-WT induced significantly more early T1-IFNs indicating that increased virus loads induce early T1-IFN independently of IRF7. In vitro, IRF7 is essential for the transcription of late T1-IFNs (Honda et al., 2005). Accordingly, neither virus induced the transcription of late T1-IFNs (Figure 5c). Both viruses induced ISGs to similar levels (Figure 5d) but pro-inflammatory Il1a, Il6 and Irg1 were induced significantly more by ECTV-Del002 (Figure 5e). Thus, the enhanced transcription of pro-inflammatory genes in the absence of p105bp compensates for the deficient production of late T1-IFN in Irf70/0 mice promoting their resistance to ECTV-Del002.
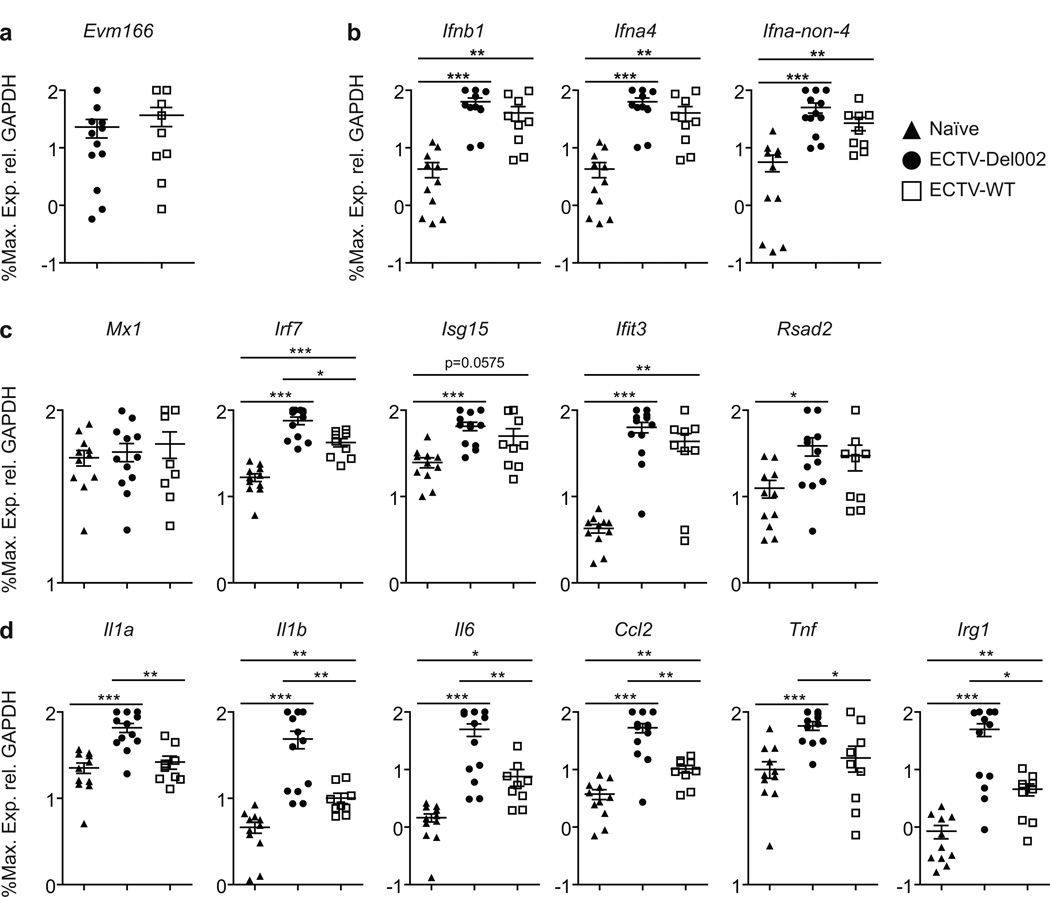
Inhibition of pro-inflammatory gene transcription by p105bp in the D-LN is independent of virus loads and T1-IFN signaling
Ifnar1 deficient (Ifnar10/0) mice are known to be highly susceptible to ECTV-WT infection (Panchanathan et al., 2005; Xu et al., 2008). Ifnar10/0 mice also succumbed to ECTV-Del002 but significantly later than to ECTV-WT (Figure 5a) suggesting that the enhanced activation of NF-κB that occurs in the absence of p105bp can moderately compensate for the absence of T1-IFN signaling. Still, at 2 dpi, transcription of Evm166 in the D-LN of Ifnar10/0 mice infected with ECTV-WT or -Del002 were indistinguishable indicating similar virus loads (Figure 6a). Thus, we took advantage of this to determine host gene transcription without any confounding differences in virus loads or T1-IFN signaling. Both viruses induced all T1-IFNs (Figure 6b) indicating that the induction of early and late T1-IFN transcription by ECTV does not require positive feed-back through IFNAR. Also, the two viruses induced the ISGs Isg15, Ifit3 and Rsad2 to similar levels indicating that many ISGs can be induced independently of IFNAR and probably also independently of NF-κB in vivo. Irf7 was induced by both viruses but expression was significantly higher in ECTV-Del002 infected mice, suggesting that the NF-κB site in Irf7 (Lu et al., 2000) plays an important role in Irf7 induction in vivo. Expression of Mx1 was not induced by either virus indicating that Mx1 is a strict ISG in the D-LN (Figure 6c). Now that virus loads were equal and T1-IFN signaling abolished, all the inflammatory genes tested (Il1a, Il1b, Il6, Ccl2, Tnf and Irg1) were induced significantly higher by ECTV-Del002 (Figure 6d). Thus, NF-κB stimulates the expression of pro-inflammatory genes, but it is not the only transcription factor that can induce them, and its effects can be obscured by high virus loads and T1-IFN signaling following infection with ECTV-WT. We also compared gene expression in the livers of Ifnar10/0 mice infected with ECTV-WT and ECTV-Del002 (Figure S4). Transcription of Evm166 was similar indicating similar virus loads (Figure S4a). The induction of T1-IFN was inconsistent and therefore difficult to evaluate (Figure S4b). However, transcripts for most of the ISGs (Figure S4c), and pro-inflammatory genes (Figure S4d) were induced significantly higher by ECTV-Del002 as compared to ECTV-WT infected mice. Thus, when virus loads are similar and IFNAR signaling is absent, increased NF-κB activation in the liver results in stronger induction not only of pro-inflammatory genes, but also of many ISGs, including Mx1.
Figure 6. Reduction of inflammatory gene transcription by p105bp is independent of virus loads and T1-IFN signaling in the D-LN.
(a) Survival of Ifnar10/0 mice: Ifnar10/0 mice (n=5/group) were infected with ECTV-Del002 (filled circles) or ECTV-WT (empty squares) viruses. (b–e) Gene transcription in Ifnar10/0 mice: At 2 dpi, gene transcription determined as in Figure 2. (b) Virus, (c) T1-IFNs, (d) ISGs, (e) inflammatory genes. Triangles, naïve; circles, ECTV-Del002; squares, ECTV-WT.; N/D, not detected. Data expressed as % of maximum as calculated for each individual experiment and shown in a log10 scale as mean ± SEM. See also Figure S4.
Discussion
It is well established that NF-κB helps control viral replication in cultured cells (Balachandran and Beg, 2011; Wang et al., 2010). However, while it is known that NF-κB plays a role in the control of reovirus replication in the heart (O'Donnell et al., 2005) and that Nfkb10/0 mice are more resistant than WT mice to encephalomyocarditis virus (Sha et al., 1995), to what extent the induction of pro-inflammatory genes by the transcription factor NF-κB contributes to virus control in vivo is less clear (Gerondakis et al., 2006). Also, whether the T1-IFN and NF-κB pathways cooperate or overlap to control an infection in vivo is unknown. Studies to understand the role of canonical NF-κB activation in the innate control of viruses using mutant mice have been hampered by the embryonic lethality of Rela0/0 mice and the multifocal innate and adaptive immune defects of Nfkb10/0 mice (Gerondakis et al., 2006). This problem became evident in our own studies where we found that Nfkb10/0 mice lack popliteal LNs, which play an important role in the normal pathogenesis of ECTV. Thus, while our experiments with Nfkb10/0 demonstrate that canonical NF-κB is essential to control ECTV; in-depth studies of the role of the pathway in vivo were not possible with these mice. Of note, Nfkb10/0 mice succumbed to WT and ECTV-Del002, but slower than other susceptible strains. This suggests that in the absence of popliteal D-LN, viral spread occurred by a slower alternative route, such as direct hematogenous spread from the footpad. Therefore, we took the alternative approach of deleting the canonical NF-κB inhibitor p105bp from ECTV and found that ECTV-Del002 was pathogenic in Nfkb10/0 mice but was severely attenuated in mousepox susceptible BALB/c mice. Hence, we used ECTV-Del002 and ECTV-WT as probes to help unravel how the NF-κB and T1-IFN pathway interact in vivo.
We found that p105bp inhibition of NF-κB accelerated the spread of the virus from the D-LN to the spleen and liver and also enhanced virus replication in the D-LN and liver. Also, p105bp decreased translocation of the NF-κB p65 subunit to the nucleus of cells in the D-LN and liver and delayed the activation of NF-κB in the liver of BALB/c mice. Thus, despite its lower loads, ECTV-Del002 induces NF-κB more efficiently at the organ level than ECTV-WT suggesting that the early activation NF-κB that occurs in the absence of p105bp takes place in the infected cell, which then releases fewer virions; and/or that in the absence of p105bp, infected cells increase the production of inflammatory factors to further enhance NF-κB activation and the anti-viral state in other cells.
We have previously shown that the recruitment of NK cells to the D-LN at 2 dpi is essential for the ability of B6 mice to resist mousepox (Fang et al., 2008; Fang et al., 2011; Fang et al., 2010). Further, we showed that a severely attenuated ECTV lacking a T1-IFN decoy receptor recruited more NK cells to the D-LN than ECTV-WT (Xu et al., 2008). However, this did not occur with ECTV-Del002. Thus, while both T1-IFN and NF-κB activation are essential to clear ECTV, their individual roles in the D-LN appear to be different.
We analyzed the transcriptional activity induced by the two viruses in mousepox susceptible and resistant mice and found that the increased virus loads in the presence of p105bp correlated with increased T1-IFN but this did not translate into a difference in the transcription levels of ISGs. Consequently, effective ISG induction in vivo can be mediated by NF-κB independently of T1-IFN. This is consistent with previous reports showing that NF-κB induces a subset of ISGs in mouse embryo fibroblasts infected with RNA virus (Basagoudanavar et al., 2011). Regardless of the lower virus loads, absence of p105bp in ECTV-Del002 resulted in stronger stimulation of inflammatory genes. Given that p105bp is intracellular (Mohamed et al., 2009a; Mohamed et al., 2009b), this finding could indicate that enhanced NF-κB activation and increased cytokine production and virus control occurred in the infected cells themselves in the absence of p105bp. Alternatively, slight changes in NF-κB activation and cytokine expression by the infected cells could provide cues to uninfected cells that amplified the response by producing pro-inflammatory cytokines. However, we found that only infected cells produced early and late T1-IFNs. This was remarkable because early T1-IFNs in infected cells are thought to stimulate the autocrine and paracrine production of late T1-IFN. The absence of a paracrine T1-IFN positive feedback loop could be due to the secreted T1-IFN decoy receptor expressed by ECTV (Smith and Alcami, 2002; Xu et al., 2008). Interestingly, there were no differences in T1-IFN production between ECTV-WT and ECTV-Del002 in infected cells suggesting that the activation of NF-κB does not play a major direct role in inducing T1-IFN expression in OPV infected cells in vivo. This is consistent with previous findings that in cultured cells, NF-κB is important for the constitutive expression of IFN-β or for its upregulation soon after infection but not for its long term expression (Balachandran and Beg, 2011; Basagoudanavar et al., 2011; Wang et al., 2010; Wang et al., 2007). More remarkable, only uninfected cells produced detectable amounts of the pro-inflammatory cytokines that we tested (we could not test more due to limitations in the amount of RNA we could obtain). Notably, Ccl2 was induced significantly more in uninfected cells from ECTV-Del002 infected mice than those of ECTV-WT infected mice. These data strongly suggests that uninfected cells have a major role in producing at least some pro-inflammatory mediators upon receiving signals induced by NF-κB activation in the infected cells. While these signals remain unidentified, they are unlikely to be T1-IFNs as cells infected with both viruses expressed similar levels of T1-IFN. Of note, in addition to p105bp, poxviruses encode multiple proteins that interfere with NF-κB activation (Mohamed and McFadden, 2009). Thus, ECTV-Del002 may still not fully activate NF-κB in infected cells and this may be the reason why cells infected with either virus do not produce detectable amounts of cytokines. Nevertheless, the amount of NF-κB induced by ECTV-Del002 appears to be sufficient to generate enough signals to stimulate paracrine cytokine production.
Despite the lower virus loads and T1-IFN transcription in the D-LN; enhanced NF-κB activation in the absence of p105bp resulted in efficient induction of ISGs and resistance of BALB/c mice to mousepox. Thus, we hypothesized that stronger NF-κB activation could overcome a defect in T1-IFN production. We compared resistance to ECTV-WT and ECTV-Del002 in mice with deficiencies in different genes involved in T1-IFN induction. As previously reported MyD880/0 and TLR90/0 deficient mice were highly susceptible to ECTV-WT (Samuelsson et al., 2008; Sutherland et al., 2011). These mice were also susceptible to ECTV-Del002. We also found that IRF7, the transcription factor responsible of T1-IFN induction downstream of TLR9/MyD88, was essential for resistance to ECTV-WT. Strikingly, Irf70/0 mice were fully resistant to ECTV-Del002 indicating that enhanced NF-κB activation can overcome suboptimal T1-IFN production. Of note, Irf30/0 mice were resistant to ECTV-WT (reported as text) suggesting that endosomal TLR9 but not cytosolic PRRs are essential for innate recognition and T1-IFN production during ECTV infection. Of interest, Irf70/0 but not Irf30/0 deficient mice also succumb to Herpes Simplex Virus 1 (HSV-1) infection (Honda et al., 2005) suggesting that IRF7 but not IRF3 may be generally required for resistance to DNA virus infections. Virus loads in the D-LN at 2.5 dpi were higher in Irf70/0 mice infected with ECTV-WT than with ECTV-Del002, correlating with early Ifna4 and Ifnb1 induction. Late T1-IFNs were not induced indicating that early T1-IFNs are sufficient to provide protection from mousepox when NF-κB is efficiently activated. As in BALB/c and B6 mice, most ISGs were induced to similar levels by both viruses and several pro-inflammatory genes were induced stronger with ECTV-Del002. Therefore, Irf7 is dispensable to induce many ISGs in vivo.
A complication when comparing transcription levels induced by virulent and attenuated viruses is that the differences in virus loads and T1-IFN levels could affect transcription. Thus, we analyzed gene expression in Ifnar10/0 mice, which are susceptible to both viruses and do not signal T1-IFN. Analysis of virus loads in the D-LN at 2 dpi by qPCR indicated that both viruses replicated similarly. In the absence of T1-IFN signaling and with similar virus loads, the ISGs Isg15, Ifit3 and Rsad2, traditionally considered T1-IFN-dependent (Chin and Cresswell, 2001; Fensterl and Sen, 2011; Okumura et al., 2008), were induced to similar levels by both viruses. Hence, in vivo most ISGs can be induced by viral infection independently of both T1-IFN and NF-κB signaling. This finding is consistent with a recent report showing IFNAR1 and NF-κB independent induction of ISGs in culture cells (Hasan et al., 2013). On the other hand, Mx1 in the D-LN was fully dependent on T1-IFN signaling. Consistent with an NF-κB site on its promoter (Lu et al., 2002), Irf7 was induced more potently in the absence of NF-κB inhibition. Moreover, all pro-inflammatory genes were induced more potently by ECTV-Del002. Analysis of the liver at 4 dpi showed similar virus loads with both viruses. However, while both viruses induced all tested ISGs (including Mx1), transcription was generally stronger with ECTV-Del002. Therefore, in the liver, ISGs can be induced independently of T1-IFN signaling but, different to the D-LN, NF-κB activation can increase their transcription. ECTV-Del002 also induced all pro-inflammatory genes more potently. Of note, Tnf was potently induced in the liver. This finding contrast with the low level of induction of this cytokine in the D-LN, indicating that the induction of Tnf by virus infection is highly tissue specific.
In summary, our results show that NF-κB induction of pro-inflammatory genes helps to control ECTV in the D-LN and reduce LH spread even when the induction of T1-IFN is defective. Furthermore, we found tissue-specific effects of NF-κB activation in the liver, because absence of p105bp resulted not only in a more potent pro-inflammatory response, but also in increased T1-IFN-independent induction of ISGs. Our data also demonstrate that both, T1-IFN and NF-κB, are essential to survive ECTV infection. However, because T1-IFN and NF-κB have overlapping functions, enhanced NF-κB activation can compensate a partial deficiency in T1-IFN production to control the virus. Our work also shows that as previously demonstrated in cultured cells (Balachandran and Beg, 2011; Basagoudanavar et al., 2011), NF-κB can induce ISGs independently of T1-IFN in vivo. Moreover, work with cultured cells has also demonstrated T1-IFN/NF-κB independent induction of ISGs (Dixit et al., 2010; Hasan et al., 2013). Our work shows that this also occurs upon viral infection in vivo. This suggests an even more complex network of gene activation during viral infection and hints that this is the reason why pathogenic viruses must target multiple components of the T1-IFN and NF-κB pathways to cause disease.
Experimental Procedures
Viruses
Virus stocks were made in BS-C-1 cells as before (Xu et al., 2012). Infections were performed with lysates clarified by centrifugation. ECTV-Del002 was generated by homologous recombination replacing Evm002 for enhanced green fluorescent protein driven by the 7.5 promoter (Figure S1a) as previously described for Evm166 (Xu et al., 2008). ECTV-Rev002 was generated using a modified transient dominant selection protocol (Falkner and Moss, 1988). Briefly, we reintroduced Evm002 to its original location by homologous recombination using a plasmid encoding E. Coli Guanine-xanthine phosphoribosyltransferase phosphotransferase (GPT) and mCherry outside the recombination site (Figure S1b). More details can be found in the legend of Figure S1.
Mice and animal experiments
All protocols involving mice were approved by the FCCC Institutional Animal Care and Use Committee. BALB/c (BALB/cAnNTac) and B6 (C57BL/6NTac) mice were purchased from Taconic. All other mice were bred at FCCC from original breeders obtained from various sources. B6.Cg-Nfkb1tm1Bal/J (Nfkb10/0) mice were from Jackson Laboratories. Ifnar1-deficient mice in a 129S2/SvPas background (Muller et al., 1994) from Dr. R. Schreiber (St Louis, MO). B6;129P2-Irf7tm1Ttg/TtgRbrc (Irf70/0) from Dr. T. Taniguchi (Honda et al., 2005). B6.129-Tlr9tm1Aki/Obs (TLR90/0) and B6.129-Myd88tm1Aki/Obs (Myd880/0) mice were originally produced by Dr. S. Akira (Adachi et al., 1998; Hemmi et al., 2000) and generously provided by Dr. R. Finberg (Worcester, MA). All mice used in experiments were 5–12 weeks old. Infections were performed in the footpad with 3,000 pfu unless otherwise indicated. For in vivo bioluminescence, the livers of BALB/c were hydrodynamically transfected (Chang et al., 2001; Liu et al., 1999) with a plasmid containing the firefly luciferase gene driven by the NF-κB promoter as described (Gross and Piwnica-Worms, 2005). Transfected mice were rested 12 days and then infected with ECTV-WT or ECTV–Del002. Mice were imaged daily for the presence of Firefly luciferase activity using an IVIS LXR system (Caliper Life Sciences, CA) 5 minutes after injection of luciferase substrate as indicated by the manufacturer (Caliper Life Sciences, CA). Cell sorting was performed using a BD Vantage sorter. Quantitative PCR (qPCR) of transcripts in organs was performed as before (Xu et al., 2012) except that we used transcript specific oligonucleotides and Probelibrary probes (Roche) (Table S1) as well as 2X FastStart Universal Probe Master with ROX (Roche). To combine independent experiments in order to increase the statistical power of the analysis, the values for each sample were normalized by GAPDH expression. The sample with the highest numerical value was considered 100% and the remaining data was calculated accordingly. Data were analyzed using Prism 5 software (GraphPad Software Inc.). We analyzed the survival curves with a Log-rank (Mantel-Cox) Test and the qPCR experiments with a non parametric T test. In all figures * = p<0.05; ** = p<0.01; *** = p<0.001. All experiments were repeated a minimum of two times and most were repeated at least three times.
Supplementary Material
Highlights.
NF-kB signaling is essential for resistance to an acute lethal poxvirus infection.
In vivo NF-κB activation in infected cells induces cytokines in uninfected cells.
Enhanced NF-kB activation overcomes suboptimal Type 1 Interferon (T1-IFN) production.
Viral infection induces Interferon Stimulated Genes independently of T1-IFN or NF-kB.
Acknowledgements
We thank Dr. Piwnica-Worms for the NF-kB luciferase reporter plasmid, Ms. Holly Gillin for secretarial assistance, Drs. Ali Alejo and Jennifer Smith-Gavin for helpful comments. James Oesterling for flow cytometry expertise, Dr. Samuel Litwin for some of the statistical analysis, and the Fox Chase Cancer Center Laboratory Animal, Cell Culture, Flow Cytometry, Biostatistics and Sequencing facilities for services. This work was supported by NIAID grants U19AI083008 and R01AI065544 to L.J. Sigal and NCI grant CA006927 to FCCC. D. R. was funded by a fellowship from Instituto de Salud Carlos III, Spanish Ministry of Health. A.A. was funded by the Spanish Ministry of Science and Innovation. The qPCR reagents and instrument were a gift from the Kirby Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran S, Beg AA. Defining emerging roles for NF-kappaB in antivirus responses: revisiting the interferon-beta enhanceosome paradigm. PLoS pathogens. 2011;7:e1002165. doi: 10.1371/journal.ppat.1002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes B, Lubyova B, Pitha PM. On the role of IRF in host defense. J Interferon Cytokine Res. 2002;22:59–71. doi: 10.1089/107999002753452665. [DOI] [PubMed] [Google Scholar]
- 4.Basagoudanavar SH, Thapa RJ, Nogusa S, Wang J, Beg AA, Balachandran S. Distinct roles for the NF-kappa B RelA subunit during antiviral innate immune responses. Journal of virology. 2011;85:2599–2610. doi: 10.1128/JVI.02213-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogdan C. The function of type I interferons in antimicrobial immunity. Curr Opin Immunol. 2000;12:419–424. doi: 10.1016/s0952-7915(00)00111-4. [DOI] [PubMed] [Google Scholar]
- 6.Bowie AG, Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol. 2008;8:911–922. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan K, Bowie AG. Activation of host pattern recognition receptors by viruses. Curr Opin Microbiol. 2010;13:503–507. doi: 10.1016/j.mib.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Chang J, Sigal LJ, Lerro A, Taylor J. Replication of the human hepatitis delta virus genome Is initiated in mouse hepatocytes following intravenous injection of naked DNA or RNA sequences. Journal of virology. 2001;75:3469–3473. doi: 10.1128/JVI.75.7.3469-3473.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin KC, Cresswell P. Viperin (cig5), an IFN-inducible antiviral protein directly induced by human cytomegalovirus. Proc Natl Acad Sci U S A. 2001;98:15125–15130. doi: 10.1073/pnas.011593298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixit E, Boulant S, Zhang Y, Lee AS, Odendall C, Shum B, Hacohen N, Chen ZJ, Whelan SP, Fransen M, et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell. 2010;141:668–681. doi: 10.1016/j.cell.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esteban DJ, Buller RM. Ectromelia virus: the causative agent of mousepox. J Gen Virol. 2005;86:2645–2659. doi: 10.1099/vir.0.81090-0. [DOI] [PubMed] [Google Scholar]
- 12.Falkner FG, Moss B. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. Journal of virology. 1988;62:1849–1854. doi: 10.1128/jvi.62.6.1849-1854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang M, Lanier LL, Sigal LJ. A role for NKG2D in NK cell-mediated resistance to poxvirus disease. PLoS pathogens. 2008;4:e30. doi: 10.1371/journal.ppat.0040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang M, Orr MT, Spee P, Egebjerg T, Lanier LL, Sigal LJ. CD94 is essential for NK cell-mediated resistance to a lethal viral disease. Immunity. 2011;34:579–589. doi: 10.1016/j.immuni.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang M, Roscoe F, Sigal LJ. Age-dependent susceptibility to a viral disease due to decreased natural killer cell numbers and trafficking. J Exp Med. 2010;207:2369–2381. doi: 10.1084/jem.20100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenner F. Mouse-pox; infectious ectromelia of mice; a review. Journal of immunology. 1949;63:341–373. [PubMed] [Google Scholar]
- 17.Fensterl V, Sen GC. The ISG56/IFIT1 gene family. J Interferon Cytokine Res. 2011;31:71–78. doi: 10.1089/jir.2010.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nature immunology. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 19.Gerondakis S, Grumont R, Gugasyan R, Wong L, Isomura I, Ho W, Banerjee A. Unravelling the complexities of the NF-kappaB signalling pathway using mouse knockout and transgenic models. Oncogene. 2006;25:6781–6799. doi: 10.1038/sj.onc.1209944. [DOI] [PubMed] [Google Scholar]
- 20.Gross S, Piwnica-Worms D. Real-time imaging of ligand-induced IKK activation in intact cells and in living mice. Nat Methods. 2005;2:607–614. doi: 10.1038/nmeth779. [DOI] [PubMed] [Google Scholar]
- 21.Hasan M, Koch J, Rakheja D, Pattnaik AK, Brugarolas J, Dozmorov I, Levine B, Wakeland EK, Lee-Kirsch MA, Yan N. Trex1 regulates lysosomal biogenesis and interferon-independent activation of antiviral genes. Nature immunology. 2013;14:61–71. doi: 10.1038/ni.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 23.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferondependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 24.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 25.Liu ZG. Molecular mechanism of TNF signaling and beyond. Cell Res. 2005;15:24–27. doi: 10.1038/sj.cr.7290259. [DOI] [PubMed] [Google Scholar]
- 26.Lo JC, Basak S, James ES, Quiambo RS, Kinsella MC, Alegre ML, Weih F, Franzoso G, Hoffmann A, Fu YX. Coordination between NF-kappaB family members p50 and p52 is essential for mediating LTbetaR signals in the development and organization of secondary lymphoid tissues. Blood. 2006;107:1048–1055. doi: 10.1182/blood-2005-06-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu R, Au WC, Yeow WS, Hageman N, Pitha PM. Regulation of the promoter activity of interferon regulatory factor-7 gene. Activation by interferon snd silencing by hypermethylation. J Biol Chem. 2000;275:31805–31812. doi: 10.1074/jbc.M005288200. [DOI] [PubMed] [Google Scholar]
- 28.Lu R, Moore PA, Pitha PM. Stimulation of IRF-7 gene expression by tumor necrosis factor alpha: requirement for NFkappa B transcription factor and gene accessibility. J Biol Chem. 2002;277:16592–16598. doi: 10.1074/jbc.M111440200. [DOI] [PubMed] [Google Scholar]
- 29.Mohamed MR, McFadden G. NFkB inhibitors: strategies from poxviruses. Cell cycle. 2009;8:3125–3132. doi: 10.4161/cc.8.19.9683. [DOI] [PubMed] [Google Scholar]
- 30.Mohamed MR, Rahman MM, Lanchbury JS, Shattuck D, Neff C, Dufford M, van Buuren N, Fagan K, Barry M, Smith S, et al. Proteomic screening of variola virus reveals a unique NF-kappaB inhibitor that is highly conserved among pathogenic orthopoxviruses. Proc Natl Acad Sci U S A. 2009a;106:9045–9050. doi: 10.1073/pnas.0900452106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohamed MR, Rahman MM, Rice A, Moyer RW, Werden SJ, McFadden G. Cowpox virus expresses a novel ankyrin repeat NF-kappaB inhibitor that controls inflammatory cell influx into virus-infected tissues and is critical for virus pathogenesis. Journal of virology. 2009b;83:9223–9236. doi: 10.1128/JVI.00861-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 33.O'Donnell SM, Hansberger MW, Connolly JL, Chappell JD, Watson MJ, Pierce JM, Wetzel JD, Han W, Barton ES, Forrest JC, et al. Organ-specific roles for transcription factor NF-kappaB in reovirus-induced apoptosis and disease. The Journal of clinical investigation. 2005;115:2341–2350. doi: 10.1172/JCI22428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Neill LA. How Toll-like receptors signal: what we know and what we don't know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Okumura A, Pitha PM, Harty RN. ISG15 inhibits Ebola VP40 VLP budding in an Ldomain- dependent manner by blocking Nedd4 ligase activity. Proc Natl Acad Sci U S A. 2008;105:3974–3979. doi: 10.1073/pnas.0710629105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panchanathan V, Chaudhri G, Karupiah G. Interferon function is not required for recovery from a secondary poxvirus infection. Proc Natl Acad Sci U S A. 2005;102:12921–12926. doi: 10.1073/pnas.0505180102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samuelsson C, Hausmann J, Lauterbach H, Schmidt M, Akira S, Wagner H, Chaplin P, Suter M, O'Keeffe M, Hochrein H. Survival of lethal poxvirus infection in mice depends on TLR9, and therapeutic vaccination provides protection. The Journal of clinical investigation. 2008;118:1776–1784. doi: 10.1172/JCI33940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato M, Hata N, Asagiri M, Nakaya T, Taniguchi T, Tanaka N. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 1998;441:106–110. doi: 10.1016/s0014-5793(98)01514-2. [DOI] [PubMed] [Google Scholar]
- 39.Seet BT, Johnston JB, Brunetti CR, Barrett JW, Everett H, Cameron C, Sypula J, Nazarian SH, Lucas A, McFadden G. Poxviruses and immune evasion. Annu Rev Immunol. 2003;21:377–423. doi: 10.1146/annurev.immunol.21.120601.141049. [DOI] [PubMed] [Google Scholar]
- 40.Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 41.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 42.Smith VP, Alcami A. Inhibition of interferons by ectromelia virus. Journal of virology. 2002;76:1124–1134. doi: 10.1128/JVI.76.3.1124-1134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutherland DB, Ranasinghe C, Regner M, Phipps S, Matthaei KI, Day SL, Ramshaw IA. Evaluating vaccinia virus cytokine co-expression in TLR GKO mice. Immunol Cell Biol. 2011;89:706–715. doi: 10.1038/icb.2010.157. [DOI] [PubMed] [Google Scholar]
- 44.Wallace GD, Buller RM. Kinetics of ectromelia virus (mousepox) transmission and clinical response in C57BL/6j, BALB/cByj and AKR/J inbred mice. Lab Anim Sci. 1985;35:41–46. [PubMed] [Google Scholar]
- 45.Wang J, Basagoudanavar SH, Wang X, Hopewell E, Albrecht R, Garcia-Sastre A, Balachandran S, Beg AA. NF-kappa B RelA subunit is crucial for early IFN-beta expression and resistance to RNA virus replication. Journal of immunology. 2010;185:1720–1729. doi: 10.4049/jimmunol.1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Hussain S, Wang EJ, Wang X, Li MO, Garcia-Sastre A, Beg AA. Lack of essential role of NF-kappa B p50, RelA, and cRel subunits in virus-induced type 1 IFN expression. Journal of immunology. 2007;178:6770–6776. doi: 10.4049/jimmunol.178.11.6770. [DOI] [PubMed] [Google Scholar]
- 47.Weih F, Caamano J. Regulation of secondary lymphoid organ development by the nuclear factor-kappaB signal transduction pathway. Immunol Rev. 2003;195:91–105. doi: 10.1034/j.1600-065x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 48.Xu RH, Cohen M, Tang Y, Lazear E, Whitbeck JC, Eisenberg RJ, Cohen GH, Sigal LJ. The orthopoxvirus type I IFN binding protein is essential for virulence and an effective target for vaccination. J Exp Med. 2008;205:981–992. doi: 10.1084/jem.20071854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu RH, Rubio D, Roscoe F, Krouse TE, Truckenmiller ME, Norbury CC, Hudson PN, Damon IK, Alcami A, Sigal LJ. Antibody inhibition of a viral type 1 interferon decoy receptor cures a viral disease by restoring interferon signaling in the liver. PLoS pathogens. 2012;8:e1002475. doi: 10.1371/journal.ppat.1002475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.