Abstract
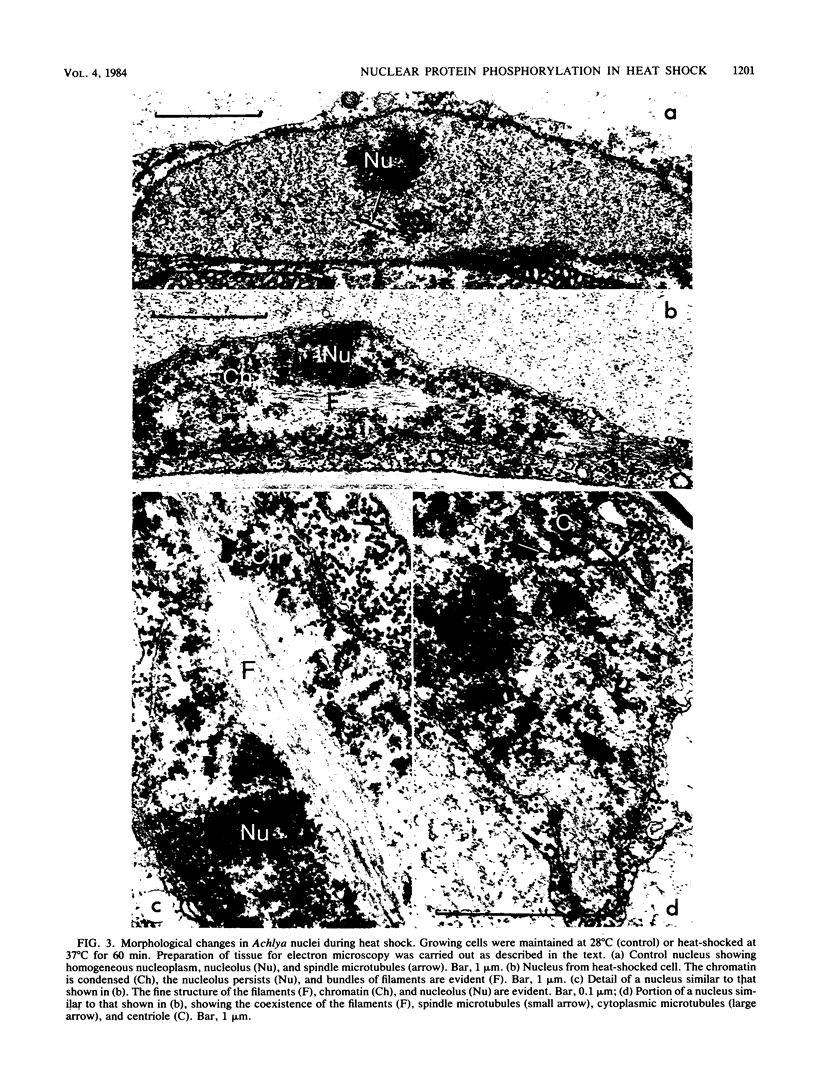
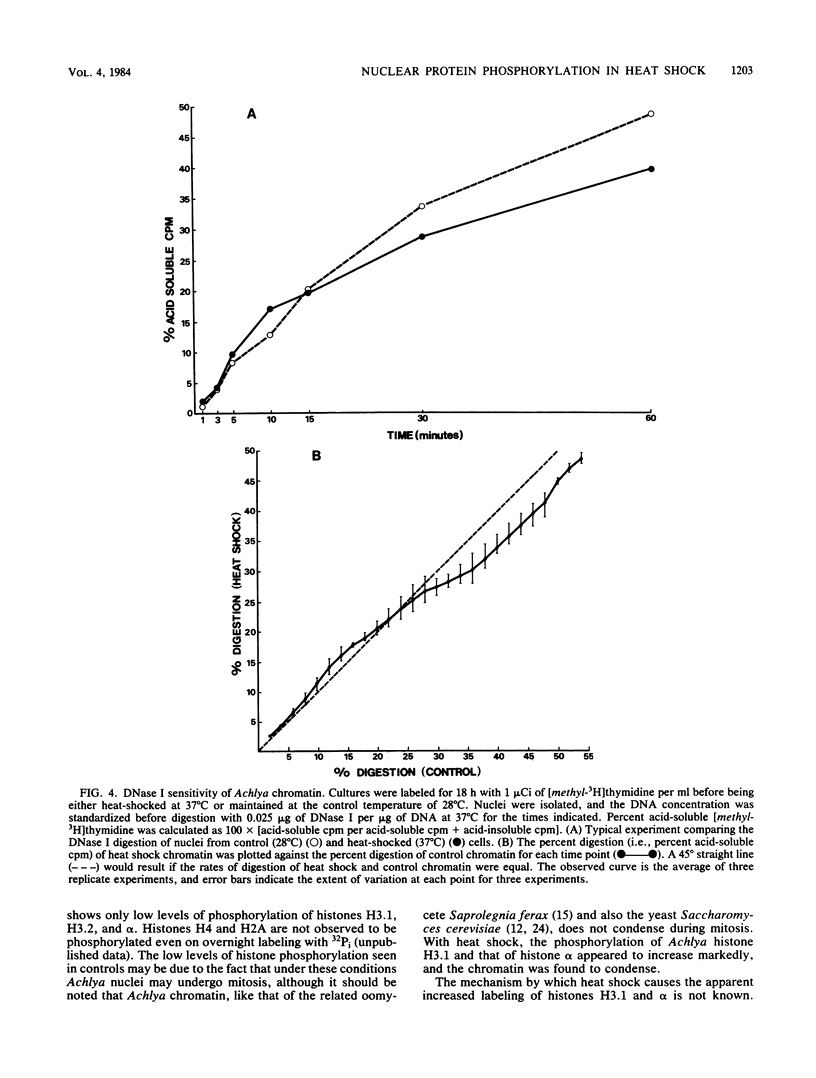
Heat shock led to marked changes in the apparent levels of phosphorylation of nuclear proteins in the fungus Achlya ambisexualis. We characterized these heat shock-induced changes in nuclear proteins on two types of two-dimensional polyacrylamide gel systems. We report here that one of two Achlya H3 histones (H3.1) and also the oomycete histone alpha appear to be highly phosphorylated with heat shock. Additional changes observed in acid-soluble nuclear proteins included an apparent increase in the 32P labeling of a 43,000-molecular-weight protein and the dephosphorylation of a major group of Achlya phosphoproteins in the 30,000-to-32,000-molecular-weight range. The changes in protein phosphorylation were accompanied by striking changes in the morphology of Achlya nuclei. Nuclei in the heat-shocked cells, but not in control cells, exhibited marked chromatin condensation and contained bundles of filaments which were approximately 4 nm in diameter. Concomitantly, the bulk of chromatin from heat-shocked nuclei showed a decreased sensitivity to digestion with the enzyme DNase I relative to chromatin from control cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P. Acetylation and methylation patterns of core histones are modified after heat or arsenite treatment of Drosophila tissue culture cells. Nucleic Acids Res. 1983 Mar 11;11(5):1389–1404. doi: 10.1093/nar/11.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R., Sarner N. Phosphorylation of very-lysine-rich histone in Physarum polycephalum. Correlation with chromosome condensation. Eur J Biochem. 1973 Feb 15;33(1):131–139. doi: 10.1111/j.1432-1033.1973.tb02664.x. [DOI] [PubMed] [Google Scholar]
- Burkholder G. D., Weaver M. G. Differential accessibility of DNA in extended and condensed chromatin to pancreatic DNase I. Exp Cell Res. 1975 May;92(2):518–522. doi: 10.1016/0014-4827(75)90412-7. [DOI] [PubMed] [Google Scholar]
- Ellzey J., Huizar E., Yanez D. Microfilament bundles in antheridial nuclei of Achlya ambisexualis E 87. Arch Microbiol. 1976 Feb;107(1):113–114. doi: 10.1007/BF00427876. [DOI] [PubMed] [Google Scholar]
- Fischer S. G., Laemmli U. K. Cell cycle changes in Physarum polycephalum histone H1 phosphate: relationship to deoxyribonucleic acid binding and chromosome condensation. Biochemistry. 1980 May 13;19(10):2240–2246. doi: 10.1021/bi00551a038. [DOI] [PubMed] [Google Scholar]
- Fukui Y. Intranuclear actin bundles induced by dimethyl sulfoxide in interphase nucleus of Dictyostelium. J Cell Biol. 1978 Jan;76(1):146–157. doi: 10.1083/jcb.76.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Katsumaru H. Nuclear actin bundles in Amoeba, Dictyostelium and human HeLa cells induced by dimethyl sulfoxide. Exp Cell Res. 1979 May;120(2):451–455. doi: 10.1016/0014-4827(79)90412-9. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover C. V. Heat shock induces rapid dephosphorylation of a ribosomal protein in Drosophila. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1781–1785. doi: 10.1073/pnas.79.6.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C. N. Chromatin behaviour during the mitotic cell cycle of Saccharomyces cerevisiae. J Cell Sci. 1977 Apr;24:81–93. doi: 10.1242/jcs.24.1.81. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., D'Anna J. A., Barham S. S., Deaven L. L., Tobey R. A. Histone phosphorylation and chromatin structure during mitosis in Chinese hamster cells. Eur J Biochem. 1978 Mar;84(1):1–15. doi: 10.1111/j.1432-1033.1978.tb12135.x. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A., Tobey R. A. Cell cycle-specific changes in histone phosphorylation associated with cell proliferation and chromosome condensation. J Cell Biol. 1974 Feb;60(2):356–364. doi: 10.1083/jcb.60.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath I. B. Behavior of kinetochores during mitosis in the fungus Saprolegnia ferax. J Cell Biol. 1980 Mar;84(3):531–546. doi: 10.1083/jcb.84.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath I. B., Greenwood A. D., Griffiths H. B. The origin of Flimmer in Saprolegnia, Dictyuchus, Synura and Cryptomonas. J Cell Sci. 1970 Sep;7(2):445–461. doi: 10.1242/jcs.7.2.445. [DOI] [PubMed] [Google Scholar]
- Heath I. B., Rethoret K. Nuclear cycle of Saprolegnia ferax. J Cell Sci. 1981 Jun;49:353–367. doi: 10.1242/jcs.49.1.353. [DOI] [PubMed] [Google Scholar]
- Kumar A., Wu R. S. Role of ribosomal RNA transcription in ribosome processing in HeLa cells. J Mol Biol. 1973 Oct 25;80(2):265–276. doi: 10.1016/0022-2836(73)90172-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Olsen A. S., Triemer D. F., Sanders M. M. Dephosphorylation of S6 and expression of the heat shock response in Drosophila melanogaster. Mol Cell Biol. 1983 Nov;3(11):2017–2027. doi: 10.1128/mcb.3.11.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Dimethylsulfoxide and the ionophore A23187 affect the arrangement of actin and induce nuclear actin paracrystals in PtK2 cells. Exp Cell Res. 1980 Sep;129(1):103–114. doi: 10.1016/0014-4827(80)90335-3. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Peterson J. B., Ris H. Electron-microscopic study of the spindle and chromosome movement in the yeast Saccharomyces cerevisiae. J Cell Sci. 1976 Nov;22(2):219–242. doi: 10.1242/jcs.22.2.219. [DOI] [PubMed] [Google Scholar]
- Ralph-Edwards A., Silver J. C. Nucleosome DNA repeat length and histone complement in a fungus exhibiting condensed chromatin. Exp Cell Res. 1983 Oct 15;148(2):363–376. doi: 10.1016/0014-4827(83)90159-3. [DOI] [PubMed] [Google Scholar]
- Scharf K. D., Nover L. Heat-shock-induced alterations of ribosomal protein phosphorylation in plant cell cultures. Cell. 1982 Sep;30(2):427–437. doi: 10.1016/0092-8674(82)90240-9. [DOI] [PubMed] [Google Scholar]
- Silver J. C., Andrews D. R., Pekkala D. Effect of heat shock on synthesis and phosphorylation of nuclear and cytoplasmic proteins in the fungus Achlya. Can J Biochem Cell Biol. 1983 Jun;61(6):447–455. doi: 10.1139/o83-060. [DOI] [PubMed] [Google Scholar]
- Silver J. C. Basic nuclear proteins in the aquatic fungus Achlya ambisexualis. Biochim Biophys Acta. 1979 Oct 25;564(3):507–516. doi: 10.1016/0005-2787(79)90040-6. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Vaughan M. H., Warner J. R., Darnell J. E. Ribosomal precursor particles in the HeLa cell nucleus. J Mol Biol. 1967 Apr 28;25(2):235–251. doi: 10.1016/0022-2836(67)90140-4. [DOI] [PubMed] [Google Scholar]
- Vavra K. J., Pederson D. S., Gorovsky M. A. Nuclease sensitivity of chromatin containing active genes: kinetic analyses utilizing continuous elution of digestion products from an ultrafiltration cell. Nucleic Acids Res. 1981 Nov 11;9(21):5825–5843. doi: 10.1093/nar/9.21.5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Wu C., Wong Y. C., Elgin S. C. The chromatin structure of specific genes: II. Disruption of chromatin structure during gene activity. Cell. 1979 Apr;16(4):807–814. doi: 10.1016/0092-8674(79)90096-5. [DOI] [PubMed] [Google Scholar]
- Zweidler A. Resolution of histones by polyacrylamide gel electrophoresis in presence of nonionic detergents. Methods Cell Biol. 1978;17:223–233. [PubMed] [Google Scholar]





