Abstract
Cognitive regulation is often used to influence behavioral outcomes. However, the computational and neurobiological mechanisms by which it affects behavior remain unknown. We studied this issue using an fMRI task in which human participants used cognitive regulation to upregulate and downregulate their cravings for foods at the time of choice. We found that activity in both ventromedial prefrontal cortex (vmPFC) and dorsolateral prefrontal cortex (dlPFC) correlated with value. We also found evidence that two distinct regulatory mechanisms were at work: value modulation, which operates by changing the values assigned to foods in vmPFC and dlPFC at the time of choice, and behavioral control modulation, which operates by changing the relative influence of the vmPFC and dlPFC value signals on the action selection process used to make choices. In particular, during downregulation, activation decreased in the value-sensitive region of dlPFC (indicating value modulation) but not in vmPFC, and the relative contribution of the two value signals to behavior shifted toward the dlPFC (indicating behavioral control modulation). The opposite pattern was observed during upregulation: activation increased in vmPFC but not dlPFC, and the relative contribution to behavior shifted toward the vmPFC. Finally, ventrolateral PFC and posterior parietal cortex were more active during both upregulation and downregulation, and were functionally connected with vmPFC and dlPFC during cognitive regulation, which suggests that they help to implement the changes to the decision-making circuitry generated by cognitive regulation.
Introduction
The human brain makes rapid evaluations that lead to adaptive choices (Lebreton et al., 2009; Milosavljevic et al., 2010), but good decisions often require further processing. Cognitive regulation of decision making uses attention, language, and executive control to modulate decisions, and features prominently in therapeutic treatments for disorders like obesity and addiction (Beck et al., 1993). While understanding of the computational and neurobiological mechanisms of unregulated choice has grown substantially (Rangel et al., 2008; Rushworth et al., 2009), we know little about how cognitive regulation affects them.
A growing consensus suggests that decision making involves two distinct processes that interact over time (Montague and Berns, 2002; Rangel et al., 2008; Kable and Glimcher, 2009; Basten et al., 2010; Rangel and Hare, 2010; Hare et al., 2011b). First, a valuation system represents the value of options under consideration. Concurrently, a comparator system receives these value representations as input to an accumulator-based action selection process (Busemeyer and Townsend, 1993; Sugrue et al., 2005; Ratcliff and McKoon, 2008; Krajbich et al., 2010; Milosavljevic et al., 2010). During this process, a relative value signal for each possible action accumulates over time based on the value of its corresponding option. Choice occurs when the accumulated signal for one action becomes sufficiently strong.
These findings suggest two mechanisms through which cognitive regulation could impact choices: value modulation, which changes the values assigned to stimuli, and behavioral control modulation, which changes the weight given to values during the action selection process. For example, a dieter considering whether to eat ice cream or broccoli could make a healthy choice by decreasing the value assigned to ice cream (i.e., value modulation) or by ignoring the value of ice cream and simply reaching for broccoli (i.e., behavioral control modulation).
Although the neural basis of cognitive regulation during emotional responding (e.g., to affective photographs) has been widely investigated (Ochsner et al., 2004; Delgado et al., 2008; Wager et al., 2008; Kober et al., 2010), the computational and neurobiological mechanisms of cognitive regulation during decision making remain largely unknown. This distinction is important. Although decision making likely shares common processes with emotional responding (e.g., subjective valuation), the need to translate values into behaviors with real consequences also likely involves unique computations, including the action selection process described above. Moreover, the evolution over time of the valuation and action selection processes, as well as their interaction (Hare et al., 2011b; Hunt et al., 2012; Sokol-Hessner et al., 2012), suggests that cognitive regulation of decision making may involve processes and time courses distinct from cognitive regulation of emotional responding.
We report the results of an fMRI task in which hungry subjects made real food purchase decisions in a natural control condition or under explicit cognitive regulation instructions. The experimental design allowed us to address three questions: (1) Does cognitive regulation during decision making operate through value modulation, behavioral control modulation, or both? (2) Do the effects of cognitive regulation change over the time course of a decision? (3) What are the neural mechanisms used to orchestrate changes in the decision-making circuitry during cognitive regulation?
Materials and Methods
Subjects
Twenty-six healthy, right-handed individuals with normal or corrected-to-normal vision (nine females; mean age, 22; range, 19–28) participated in the study. Subjects were eligible only if they reported frequent consumption of the types of foods used in the study and had no history of psychiatric or neurological conditions. Data for six additional participants were excluded from analysis: five exhibited excessive head motion during scanning, and one gave all foods the same minimum liking rating (“Not at all liked”). Subjects were paid $50 for participation and given an initial endowment of $2.50 with which to purchase foods during the study. The Institutional Review Board of Caltech approved all procedures.
Procedure
Subjects were instructed to fast for at least 4 h before the experiment and ate at most a light meal before fasting began. They were also told that, at the end of the experiment, they would remain in the laboratory for 30 min, during which time they could eat only what they had obtained during the study. The task consisted of two parts: an initial liking rating task (i.e., an unbiased measure of value) and an in-scanner bidding and regulation task (i.e., a measure of value under the influence of regulation).
During the initial liking-rating task, subjects were shown pictures of 150 different appetizing snack food items (e.g., chips, candy) and rated how much they would like to eat each food at the end of the experiment, at their own pace, using a six-point Likert scale (1, “Don't want it at all”; 6, “Want it very strongly”). These liking ratings provided a measure of the baseline value assigned to each food in the absence of explicit cognitive regulation.
Immediately afterward, subjects received instructions for the bidding and regulation task (see Fig. 1A). On each trial before the food appeared, participants saw an abstract black-and-white symbol indicating one of three trial types: DISTANCE, INDULGE, and NATURAL. Each type of trial appeared 50 times, randomly interspersed over three scanning runs. On DISTANCE trials, participants were asked to use any strategy they needed to decrease their craving for the food. On INDULGE trials, they were asked to use any strategy they needed to increase their craving. On NATURAL trials, they were asked to allow whatever thoughts and feelings came naturally. Subjects had 4 s to evaluate the item and were asked to look at the food and engage in the regulation task during this entire time. We chose this length to ensure that subjects had sufficient time to deploy cognitive regulation (Gross, 1998), and to investigate whether modulation took place during food evaluation, during response initiation, or both.
Figure 1.
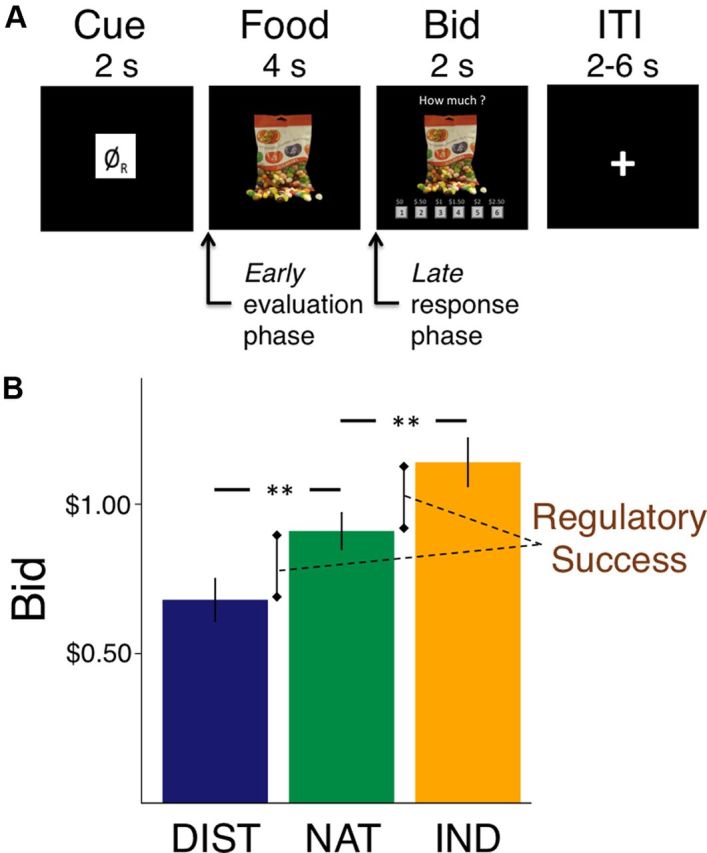
Experimental procedure and behavioral results. A, Structure and timing of an experimental trial. ITI, Intertrial interval. B, Average bids for each condition. DIST, DISTANCE; NAT, NATURAL; IND, INDULGE. Error bars show SEM. **p < 0.001.
After the 4 s delay, participants had 2 s in which to place a bid ($0–$2.50, $0.50 increments) for the right to eat that food at the end of scanning, with one trial selected randomly for implementation at the end of the experiment. The bid on that trial determined whether subjects got to eat that food and the price that they paid, according to the rules of a Becker–DeGroot–Marschak auction (Becker et al., 1964; Plassmann et al., 2007). Critically, the rules of the auction ensure that subjects' best strategy is to bid their true value for each food. For details on the auction procedure and why they induce truth telling, see Plassmann et al. (2007). These bids allowed us to measure values expressed in behavior at the time of choice. Failure to respond within 2 s resulted in a bid of $0.
MRI data acquisition
Functional imaging was conducted using a Siemens 3.0 T MRI scanner to acquire gradient echo T2*-weighted echoplanar (EPI) images. To optimize functional sensitivity in the ventromedial prefrontal cortex (vmPFC), a key region of interest, we used a tilted acquisition in an oblique orientation of 30° to the anterior commissure–posterior commissure line. In addition, we used an eight-channel phased array coil that yields a 40% signal increase in signal in the vmPFC over a standard head coil. Each volume comprised 44 axial slices. A total of 675 volumes were collected over three sessions during the experiment in an interleaved ascending manner. The first two volumes of each session were discarded to allow for scanner equilibration. The imaging parameters were as follows: echo time, 30 ms; field of view, 192 mm; in-plane resolution and slice thickness, 3 mm; repetition time, 2.75 s. Whole-brain high-resolution T1-weighted structural scans (1 × 1 × 1 mm) were acquired and coregistered with the participant's mean EPI images. These images were averaged together to permit anatomical localization of the functional activations at the group level.
MRI data preprocessing
Image analysis was performed using SPM5 (Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, UK). Images were corrected for slice acquisition time within each volume, motion corrected with realignment to the last volume, spatially normalized to the standard Montreal Neurological Institute EPI template, and spatially smoothed using a Gaussian kernel with a full width at half-maximum of 8 mm. Intensity normalization and high-pass temporal filtering (using a filter width of 128 s) were also applied to the data.
MRI data analysis
To address the questions raised in the introduction, we estimated several general linear models (GLMs) of BOLD responses with first-order autoregression. Every GLM was estimated in three steps. First, we estimated the model separately for each individual. Second, we calculated contrast statistics at the individual level. Third, we computed second-level statistics by carrying out one-sample t tests on the single-subject contrast coefficients.
Tests for value and behavioral control modulation
We applied the following logic to test for evidence of value and behavioral control modulation. First, we used GLM-1 (details below) to identify regions that might be involved in the computation of value information at the time of choice. We did this by looking for areas in which the BOLD responses were correlated with the bids during NATURAL trials, which provide a control condition without cognitive regulation. We also verified that computations during NATURAL trials were not significantly affected by the presence of regulation (GLM-1A) by identifying regions that correlated with the unbiased, prescan liking ratings during NATURAL trials. Second, we tested for value modulation by examining average responses (i.e., no parametric modulation) in these value-related regions, which should go down during DISTANCE trials and up during INDULGE trials. Third, we tested for behavioral control modulation by examining the correlation between signals in value-related regions and behavioral responses (i.e., parametric modulation with bids). If regulation reduces (or enhances) the weight that a particular value-related region receives in the action selection process, we should observe reduction (or enhancement) of the correlation between that value region and the bids.
GLM-1.
This GLM consisted of six regressors of interest: R1–R3 were indicator functions denoting NATURAL trials (R1), DISTANCE trials (R2) and INDULGE trials (R3), while R4–R6 consisted of parametric modulators of each indicator function representing the value of the participant's bids for that trial type. Indicator functions consisted of a boxcar function beginning at food onset and terminating at the bid response (average duration, ∼4.7 s). Missed response trials were modeled as a separate regressor, with duration of the average trial length (4.7 s). All regressors were convolved with the canonical form of the hemodynamic response. The model also included motion parameters and session constants as regressors of no interest.
To test for value modulation, for which we expect changes in average activation, we computed the contrasts of DISTANCE versus NATURAL trials (R2 − R1), INDULGE versus NATURAL trials (R3 − R1), and INDULGE versus DISTANCE trials (R3 − R2). To test for behavioral control modulation, for which we expect changes in the correlates of bid, we computed the contrasts of the bid parametric modulator on DISTANCE versus NATURAL trials (R5 − R4), the bid parametric modulator on INDULGE versus NATURAL trials (R6 − R4), and the bid parametric modulator on INDULGE versus DISTANCE trials (R6 − R5).
Regions are reported as significant if they passed whole-brain cluster correction (WBC) at p < 0.05 as implemented by the SPM5 data analysis software (Worsley et al., 1996), using a per-voxel threshold of p < 0.001.
We also had a strong a priori interest in the vmPFC and dorsolateral prefrontal cortex (dlPFC) because research in the decision-making and self-control literatures points to the particular importance of these regions (Kable and Glimcher, 2007; Plassmann et al., 2007; Hare et al., 2009; Sokol-Hessner et al., 2012). We thus also report results surviving small-volume correction within a combined anatomical mask of the following three regions: vmPFC, right and left dlPFC. Using WFU PickAtlas (http://fmri.wfubmc.edu/software/PickAtlas), the vmPFC was defined by a mask of the cingulate gyrus, restricted to the 234 voxels falling below the genu of the corpus callosum. This region encompasses the peak voxels related to value computation in several independent studies (McClure et al., 2004; Chib et al., 2009; Hare et al., 2009), and shares similar boundaries to a subregion of the vmPFC defined by distinct patterns of both anatomical and functional connectivity (cluster 2) (Beckmann et al., 2009). The dlPFC was defined using a mask of the middle frontal gyrus, restricted to Brodmann areas 9, 10, and 46 (using a dilation of 3 to ensure coverage of all voxels in the area, yielding 996 voxels on the right, 922 voxels on the left). This region encompasses the peak voxels reported for value computations in the dlPFC in several studies (Plassmann et al., 2007, 2010), as well as the peak dlPFC voxel reported in delayed valuation (McClure et al., 2004).
GLM-1A.
This model was identical to GLM-1, except that bids (R4–R6) were replaced by the unregulated, prescan liking ratings of each food as the parametric modulator. GLM-2 allowed us to determine the extent to which unregulated liking ratings identified the same areas as on-line bids during NATURAL trials, a measure of the extent to which the presence of regulation trials contaminated natural response.
Analysis of the time course of regulation
To test for evidence of the evolution over time of either value modulation or behavioral control modulation, we used GLM-2 to look for significant changes in average activation and/or correlation with bid that varied as a function of time (early during the evaluation process vs late during response initiation).
GLM-2.
This GLM consisted of 12 regressors of interest: R1, early period for NATURAL trials; R2, late period for NATURAL trials; R3, early period for DISTANCE trials; R4, late period for DISTANCE trials; R5, early period for INDULGE trials; R6, late period for INDULGE trials. Early period regressors (R1, R3, R5) were modeled as 0 s stick functions at food onset in each trial type. Late period regressors (R2, R4, R6) were modeled as 0 s stick functions 4 s after food onset, at the end of the evaluation period and the beginning of response elicitation. R7–R12 consisted of parametric modulators of R1–R6, representing the bid entered for each trial. Note that parametric modulators for early and late periods for a given trial type are identical (since only one bid per trial was entered), but that the correlation between these regressors when convolved with the hemodynamic response is quite low, because of the 4 s lag between them (range, r = −0.01 to −0.32; mean, r = −0.19). Nonresponse trials were modeled with two separate regressors for the early and late period.
The coefficients from these regressors were used to estimate the following interactions with time, which were calculated first at the subject level, and then submitted to group-level random-effects analysis. First, to test for effects of value modulation that varied over time, we examined the difference between conditions in unmodulated activation early versus late in the trial, looking at (1) the regulation (DISTANCE vs NATURAL) by time (Late vs Early) contrast [(R4 − R2) − (R3 − R1)], (2) the regulation (INDULGE vs NATURAL) by time (Late vs Early) contrast [(R6 − R2) − (R5 − R1)], and (3) the regulation (INDULGE vs DISTANCE) by time (Late vs Early) contrast [(R6 − R4) − (R5 − R3)]. Second, to test for effects of behavioral-control modulation that varied over time, we examined the difference between conditions in bid parametric modulator early versus late in the trial, looking at (1) the regulation (DISTANCE vs NATURAL) by time (Late vs Early) contrast [(R10 − R8) − (R9 − R7)], (2) the regulation (INDULGE vs NATURAL) by time (Late vs Early) contrast [(R12 − R8) − (R11 − R7)], and (3) the regulation (INDULGE vs DISTANCE) by time (Late vs Early) contrast [(R12 − R10) − (R11 − R9)]. Post hoc tests decomposing these interaction effects into the contribution of early and late time points separately (e.g., DISTANCE vs NATURAL at early time point only, R3 − R1) were also computed.
Reaction time analyses
To ascertain whether the effects identified in GLM-3 were accounted for by differences in reaction time between the conditions, we used a modified version of GLM-3 which was identical to that described above, with the exception that the reaction time on each trial was used as a parametric modulator of R2 (Late period for NATURAL trials), R4 (Late period for DISTANCE trials), and R6 (Late period for INDULGE trials). The bid was entered as a second parametric modulator orthogonalized to reaction times in each condition. Results of this model were not substantially different, so we report the results of the simpler model (GLM-2) in Results below.
Analysis of neural systems implementing cognitive regulation
To identify regions that may be responsible for orchestrating changes in the decision-making circuitry, we used GLM-1 to identify the conjunction of regions that were more active during both DISTANCE versus NATURAL trials (GLM-1: R2 − R1) and during INDULGE versus NATURAL trials (R3 − R1). We focus here on three regions that have also been implicated in cognitive regulation of emotional responding (Ochsner and Gross, 2005): left and right ventrolateral PFC, and left posterior parietal cortex. To determine whether and how these regions were functionally connected to the value-based decision-making circuitry during regulation, we performed six separate psychophysiological interaction (PPI) analyses, one each for the left and right vlPFC and left PPC. The vlPFC and PPC regions were defined by the set of voxels in each region active for the contrast (DISTANCE + INDULGE) − NATURAL trials, thresholded at p < 0.001, uncorrected, from GLM-1.
PPI 1.
This PPI was designed to examine whether activity in the left vlPFC was differentially correlated with vmPFC or dlPFC responses in DISTANCE compared with NATURAL trials. This was done following the procedures described by Gitelman et al. (2003) and implemented in SPM.
First, we computed individual average time series within a 4 mm sphere surrounding individual subject peaks for the contrast DISTANCE versus NATURAL (GLM-1), within the functional mask of left vlPFC as defined above. Variance associated with the six motion regressors was removed from the extracted time series. The seed time courses were then deconvolved, based on the formula for the canonical hemodynamic response, to construct a time series of neural activity in left vlPFC.
Second, we estimated a GLM with the following regressors: R1, an interaction between the neural activity in the seed region and a contrast of the indicator functions for DISTANCE trials (∼4.7 s) versus NATURAL trials (∼4.7 s), with DISTANCE weighted as +1, and NATURAL weighted as −1; R2, the contrast of DISTANCE versus NATURAL trials (+1 for DISTANCE trials, −1 for NATURAL trials, 0 elsewhere); R3, the original BOLD eigenvariate (i.e., the average time series from the 4 mm sphere). The first two regressors were convolved with a canonical form of the hemodynamic response. The model also included covariates of no interest (motion parameters and session constants).
Third, single-subject contrasts for the first regressor were calculated and submitted to a one-sample t test on the single-subject contrast coefficients to determine group activations.
PPI 2.
This model is identical with PPI 1, except that the first and second regressors used the contrast INDULGE versus NATURAL trials.
PPI 3–4.
These models are identical with PPIs 1 and 2, but are calculated using as a seed region the right vlPFC region as defined above.
PPI 5–6.
These models are identical with PPIs 1 and 2, but are calculated using as a seed region the left PPC region as defined above.
We also conducted PPI analyses analyzing changes in connectivity early versus late in the evaluation period. However, these did not reveal strong effects of time on connectivity, so we do not discuss them further.
Time course plots
To examine the time course of activation on DISTANCE, NATURAL, and INDULGE trials, we defined regions of interest (ROIs) in the vmPFC and right and left dlPFC based on the set of voxels showing a significant 2 (condition: INDULGE vs DISTANCE) by 2 (time: Late vs Early) interaction in the correlation between neural response and bid at p < 0.001 (see Fig. 2). We also defined ROIs in the vlPFC and PPC based on the set of voxels showing a conjunction between DISTANCE and INDULGE trials compared with NATURAL at p < 0.001 (see Fig. 4). From each of these ROIs, we extracted the average raw BOLD time signal at each time point, removing both the mean and the variance associated with the motion regressors using standard SPM functions. The resulting time course was then upsampled using spline interpolation into 10 time bins per TR (275 ms per sample) and subjected to finite impulse response (FIR) analysis for each ROI. This FIR model included three regressors for the main effects of condition (DISTANCE, NATURAL, INDULGE) and three regressors for the bid parametric modulator at each time bin falling within 20 s of the onset of a trial (73 time bins total). Coefficients for the bid parametric modulator were used to make the time course plots shown in Figure 3, and coefficients for the main effect of condition were used to make the time course plots in Figure 5.
Figure 2.
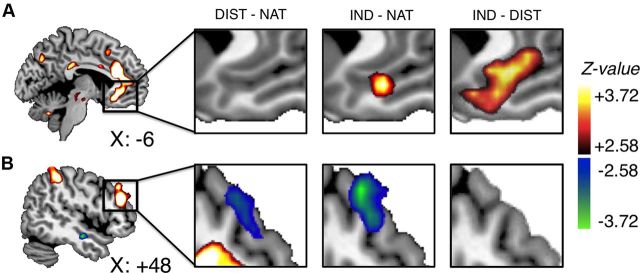
Evidence for value modulation during the full evaluation period. A, B, vmPFC (A) and right dlPFC (B) regions that correlated with bids during NATURAL trials, and (from left to right) changes in activation in these regions in DISTANCE − NATURAL trials, INDULGE − NATURAL trials, and INDULGE − DISTANCE trials.
Figure 4.
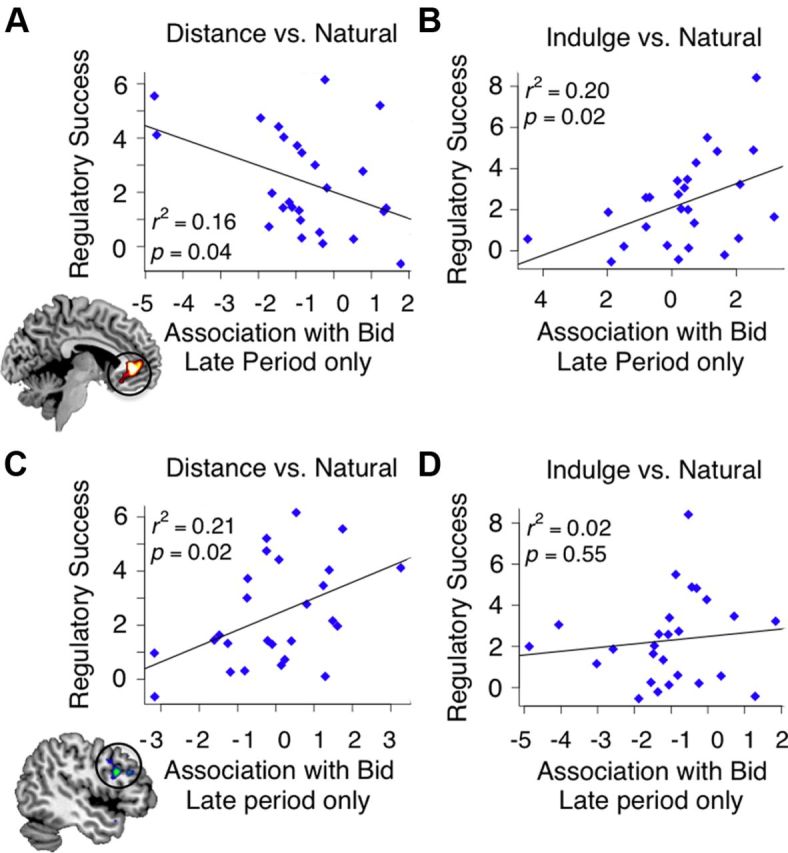
Correlation between individual differences in regulatory success and differences in correlation with bid at the end of the choice period for vmPFC in DISTANCE − NATURAL trials (A), vmPFC in INDULGE − NATURAL trials (B), left dlPFC in DISTANCE − NATURAL trials (C), and left dlPFC in INDULGE − NATURAL trials (D).
Figure 3.
Evidence for behavioral control modulation that varies as a function of time during cognitive regulation. A–C, vmPFC (A), left dlPFC (B), and right dlPFC (C) regions in which a linear contrast of correlation with behavior in each condition (DISTANCE = −1; NATURAL = 0; INDULGE = +1) showed a significant interaction with time (early evaluation vs late response initiation). The hot colors indicate greater correlation with behavior in INDULGE compared with DISTANCE. The cool colors indicate the opposite. D–F, Time course of activation in each condition (blue, DISTANCE; green, NATURAL; yellow, INDULGE). Parameter estimates indicate the average strength of association with bid at each time point, and encompass a 20 s window beginning at onset of instruction (see Materials and Methods for details). The dashed vertical lines demarcate trial events and are shifted forward 5 s to account for hemodynamic lag. The horizontal double lines at the top of the plots indicate time points at which two conditions differ (p < 0.05). The line colors indicate the conditions being compared. Transparent areas indicate SEM.
Figure 5.
Regions implementing cognitive regulation. Regions more activated during DISTANCE − NATURAL trials (A), and INDULGE − NATURAL trials (B). C, Overlap between DISTANCE (blue) and INDULGE (red) in left vlPFC and PPC. Time courses of BOLD activation are displayed for left PPC (right) and left vlPFC (left). See Materials and Methods for details.
Results
Behavioral results
The cognitive regulation instructions had a significant effect on average bidding behavior (F(2,50) = 43.03; p < 0.001; Fig. 1B). Post hoc t tests revealed that, compared with NATURAL trials [mean (M), $0.92; SE, 0.06], participants bid less in DISTANCE (M, $0.68; SE, 0.07; paired t(25) = 6.48; p < 0.001), and more in INDULGE trials (M, $1.15; SE, 0.08; paired t(25) = 5.51; p < 0.001). Regulation instructions also had a significant effect on the relationship between prescan liking ratings and bids (F(2,50) = 5.42; p = 0.007), although this varied by condition. Post hoc t tests on the Fisher-transformed z-scores suggested that, compared with the correlation in NATURAL trials (M, 0.73; SE, 0.02), DISTANCE trials were significantly less correlated with prescan ratings (M, 0.65; SE, 0.03; paired t(25) = 3.07; p = 0.005), but there was no difference for INDULGE trials (M, 0.71; SE, 0.03). Reaction time (RT) also differed among all conditions (F(2,50) = 12.08; p < 0.001; all post hoc paired t(25) > 2.36; all p < 0.03). DISTANCE trials took longest (RT, 790 ms; SE, 41 ms), followed by INDULGE trials (RT, 745 ms; SE, 38 ms), with the quickest RT in NATURAL trials (RT, 695 ms; SE, 37 ms). This suggests that both types of regulation required effort, with attempts to decrease responses being more difficult.
Regions correlating with bids in the absence of regulation
We identified candidates for regions associated with the computation of value in the absence of regulation by estimating a GLM of BOLD responses in each condition during the full evaluation period (from the appearance of the food stimulus to the bid response), with activity parametrically modulated by the bid placed on that trial (GLM-1). For NATURAL trials, activity in vmPFC and bilateral dlPFC, as well as regions of ventral striatum and parietal cortex, correlated with the bids (all p < 0.01, WBC; Table 1). We found similar results if activity in NATURAL trials was parametrically modulated by the liking ratings taken before the scanning session, instead of the bids (GLM-1A; Table 1). This suggests that the mere presence of cognitive regulation did not significantly alter value computations on NATURAL trials. For convenience, we refer in the remainder of the text to the regions of dlPFC and vmPFC correlated with bidding behavior as “value-related regions.”
Table 1.
Neural correlates of bids during the evaluation period in NATURAL trials and conjunction with correlates of liking ratings
| Region | BA | k | Z score | x | y | z |
|---|---|---|---|---|---|---|
| Correlates of NATURAL bid | ||||||
| R Dorsolateral prefrontal cortex | 10/46 | 46 | 3.42 | 42 | 51 | 0 |
| R Dorsolateral prefrontal cortex | 10/46 | 84 | 4.77 | 48 | 36 | 24 |
| L Rostral anterior cingulate cortex | 9/32 | 282 | 4.95 | −6 | 30 | 21 |
| L Ventral anterior cingulate/ventromedial prefrontal cortex | 11/32 | * | 4.13 | −3 | 27 | −12 |
| L Dorsolateral prefrontal cortex | 10/46 | 115 | 4.38 | −42 | 27 | 27 |
| L Anterior insula | 13 | 50 | 3.91 | −33 | 15 | −3 |
| R Posterior cingulate cortex | 23 | 85 | 4.55 | 3 | −30 | 27 |
| R Inferior temporal gyrus | 20 | 49 | 4.08 | 54 | −42 | −18 |
| L Precuneus | 7/19 | 236 | 4.52 | −27 | −63 | 42 |
| R Inferior parietal lobule | 40 | 327 | 3.95 | 24 | −63 | 36 |
| Conjunction with liking ratings | ||||||
| R Dorsolateral prefrontal cortex | 10/46 | 24 | 4.09 | 42 | 48 | 6 |
| R Dorsolateral prefrontal cortex | 10/46 | 48 | 4.38 | 45 | 39 | 27 |
| R Rostral anterior cingulate cortex | 9/32 | 8 | 3.98 | 9 | 39 | 21 |
| L Dorsolateral prefrontal cortex | 10/46 | 63 | 4.55 | −42 | 36 | 27 |
| L Ventral anterior cingulate/ventromedial prefrontal cortex | 11/32 | 13 | 3.89 | −3 | 36 | 3 |
| L Rostral anterior cingulate cortex | 9/32 | 5 | 3.74 | −6 | 27 | 15 |
| L Anterior insula | 13 | 23 | 3.93 | −27 | 18 | 6 |
| R Posterior cingulate cortex | 23 | 18 | 3.61 | 6 | −30 | 30 |
| R Inferior parietal lobule | 40 | 52 | 4.66 | 54 | −42 | 60 |
| R Inferior temporal gyrus | 20 | 25 | 3.93 | 63 | −45 | −15 |
| R Inferior parietal lobule | 40 | 14 | 3.62 | 45 | −81 | 30 |
Note: Clusters for bid are reported at p < 0.05, WBC (p < 0.001, uncorrected; minimum extent k = 40 voxels). Clusters for the conjunction are reported if they passed a voxel threshold of p < 0.05, WBC for the bid contrast and p < 0.001, uncorrected for the liking rating contrast, with a minimum overlap of >5 voxels.
* Indicates distinct peak within larger area.
Tests for value modulation
Value modulation predicts that, compared with NATURAL trials, the average strength of stimulus value signals in value-related regions like vmPFC and dlPFC should be lower in DISTANCE trials, and higher in INDULGE trials. To test for this, we compared average (i.e., no parametric modulation by bid) BOLD responses across conditions. We tested first for value modulation effects that were significant when averaging over the entire evaluation period (GLM-1; Table 2) and second for value modulation effects that showed a significant interaction with time (GLM-2; Table 3). We focus our discussion here on analyses in ventromedial and dorsolateral prefrontal cortices, both because these regions have previously been implicated in stimulus valuation at the time of choice (Kable and Glimcher, 2007; Plassmann et al., 2007, 2010; Basten et al., 2010; Sokol-Hessner et al., 2012), and because we were particularly interested in clarifying their respective roles in decision making during cognitive regulation. We also highlight effects in other regions when they overlapped with areas that correlated with value during NATURAL trials.
Table 2.
Changes in average response during cognitive regulation
| Region | BA | Cluster size | Z score | x | y | z |
|---|---|---|---|---|---|---|
| Regions overlapping with areas correlated with NATURAL bid | ||||||
| DISTANCE > NATURAL | ||||||
| No regions significant | ||||||
| DISTANCE < NATURAL | ||||||
| R Dorsolateral prefrontal cortex | 10/46 | 41 | 3.06* | 42 | 33 | 21 |
| INDULGE > NATURAL | ||||||
| L Ventral anterior cingulate/ventromedial prefrontal cortex | 32 | 33 | 3.71† | −3 | 39 | −12 |
| INDULGE < NATURAL | ||||||
| R Dorsolateral prefrontal cortex | 10/46 | 37 | 3.39 | 51 | 27 | 42 |
| INDULGE > DISTANCE | ||||||
| R Dorsal anterior cingulate cortex | 32 | 48 | 4.43 | 0 | 24 | 15 |
| L Ventral anterior cingulate/ventromedial prefrontal cortex | 32 | 60 | 3.73 | 0 | 36 | −6 |
| INDULGE < DISTANCE | ||||||
| No regions significant | ||||||
| Regions outside of areas correlated with NATURAL bid | ||||||
| DISTANCE > NATURAL | ||||||
| R Superior medial frontal gyrus | 8 | 1023 | 5.76 | 3 | 39 | 48 |
| R Ventrolateral prefrontal cortex | 45/47 | 476 | 4.97 | 42 | 36 | −18 |
| L Ventrolateral prefrontal cortex | 45/47 | 566 | 5.01 | −57 | 24 | 9 |
| R Temporal pole | 21 | 72 | 4.94 | 48 | 12 | −42 |
| R Posterior parietal cortex | 40 | 205 | 4.65 | 63 | −51 | 33 |
| L Posterior parietal cortex | 40 | 330 | 4.57 | −57 | −54 | 33 |
| DISTANCE < NATURAL | ||||||
| L Posterior insula | 13 | 53 | 3.99 | −36 | −18 | 15 |
| R Postcentral gyrus | 2 | 54 | 4.05 | 57 | −33 | 63 |
| L Posterior cingulate | 29 | 86 | 4.09 | −12 | −57 | 15 |
| INDULGE > NATURAL | ||||||
| R Superior frontal gyrus | 8 | 400 | 4.65 | 9 | 51 | 36 |
| R Ventrolateral prefrontal cortex | 45 | 344 | 4.60 | 30 | 24 | −24 |
| L Ventrolateral prefrontal cortex | 45 | 271 | 4.35 | −33 | 12 | −15 |
| L Posterior parietal cortex | 40 | 95 | 4.42 | −48 | −54 | 27 |
| L Supplementary motor area | 6 | 82 | 4.79 | −6 | 15 | 63 |
| R Temporal pole | 21 | 48 | 4.12 | 45 | 6 | −36 |
| INDULGE < NATURAL | ||||||
| L Occipital cortex | 19 | 87 | 3.83 | −3 | −93 | 18 |
| INDULGE > DISTANCE | ||||||
| No regions significant | ||||||
| INDULGE < DISTANCE | ||||||
| No regions significant | ||||||
| Conjunction of regions in DISTANCE > NATURAL and INDULGE > NATURAL | ||||||
| L Supplementary motor area | 6 | 392 | −3 | 15 | 63 | |
| L Ventrolateral prefrontal cortex | 45 | 219 | −27 | 18 | −24 | |
| R Ventrolateral prefrontal cortex | 45 | 227 | 57 | 15 | 9 | |
| L Superior frontal gyrus | 8 | 13 | −18 | 45 | 36 | |
| L Posterior parietal cortex | 40 | 44 | −66 | −48 | 36 | |
Note: Clusters are reported at p < 0.05, whole-brain cluster corrected (p < 0.001, uncorrected, and minimum extent k = 44 voxels) unless otherwise noted. Clusters for conjunction are reported if they passed a voxel threshold of p < 0.05, WBC, for the contrast of DISTANCE > NATURAL and INDULGE > NATURAL, with a minimum overlap of >5 voxels. For ease of interpretation, the table is divided into (1) significant regions that overlapped with value-related areas (i.e., overlap with a mask including all voxels correlated with value during NATURAL trials at p < 0.001, uncorrected) and (2) regions with no overlap of value-related regions (i.e., overlap with a mask that excluded all voxels correlated with value during NATURAL trials at p < 0.001, uncorrected).
*p < 0.05, small-volume corrected within an anatomically defined mask including vmPFC, right dlPFC, and left dlPFC.
†p < 0.005, uncorrected, reported for completeness.
Table 3.
Interaction between time and changes in average response during cognitive regulation
| Region | BA | Late versus Early |
DISTANCE versus NATURAL |
INDULGE versus NATURAL |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early time point only |
Late time period only |
Early time point only |
Late time period only |
|||||||||||
| Z score | k | x | y | z | β (SE) | p | β (SE) | p | β (SE) | p | β (SE) | p | ||
| DISTANCE versus NATURAL | ||||||||||||||
| L Ventrolateral prefrontal cortex | 45/47 | 4.54 | 119 | −45 | 36 | −3 | −0.28 (0.42) | NS | 3.33 (0.64) | <0.001 | 0.66 (0.55) | NS | 1.46 (0.43) | 0.002 |
| L Superior frontal gyrus | 8 | 3.79 | 100 | −9 | 33 | 66 | −0.15 (0.36) | NS | 3.11 (0.52) | <0.001 | 0.67 (0.50) | NS | 1.91 (0.46) | <0.001 |
| L Middle temporal gyrus | 21 | 4.45 | 114 | −57 | −27 | −3 | −0.71 (0.31) | 0.03 | 2.08 (0.44) | <0.001 | −0.79 (0.37) | 0.04 | 1.01 (0.41) | 0.02 |
| R Middle temporal gyrus | 21/22 | 3.95 | 86 | 45 | −36 | 0 | −0.43 (0.28) | NS | 2.09 (0.46) | <0.001 | −0.31 (0.35) | NS | 1.45 (0.35) | <0.001 |
| R Middle temporal gyrus | 21/22 | 3.7 | 47 | 66 | −48 | 12 | −0.87 (0.37) | 0.02 | 1.85 (0.46) | <0.001 | −0.48 (0.37) | NS | 1.57 (0.38) | <0.001 |
| DISTANCE < NATURAL | ||||||||||||||
| No regions significant | ||||||||||||||
| INDULGE > NATURAL | ||||||||||||||
| R Middle temporal gyrus | 22 | 4.07 | 121 | 63 | −66 | 12 | −0.82 (0.38) | 0.04 | −0.84 (0.36) | 0.03 | 1.75 (0.49) | 0.002 | 1.61 (0.37) | <0.001 |
| INDULGE < NATURAL | ||||||||||||||
| L Posterior cingulate cortex | 31 | −4.57 | 50 | −3 | −15 | 33 | 0.88 (0.23) | <0.001 | −0.79 (0.42) | 0.07 | 1.13 (0.31) | 0.001 | −0.92 (0.27) | 0.002 |
| INDULGE > DISTANCE | ||||||||||||||
| R Postcentral gyrus | 5 | 4.01 | 43 | 36 | −42 | 72 | −0.19 (0.71) | NS | −1.66 (0.90) | 0.07 | −0.82 (0.86) | NS | 1.65 (0.67) | 0.02 |
| INDULGE < DISTANCE | ||||||||||||||
| L Inferior parietal lobule | 40 | 3.62 | 45 | −51 | −60 | 51 | −0.34 (0.51) | NS | 2.48 (0.74) | 0.002 | 1.20 (0.59) | 0.05 | 0.52 (0.63) | NS |
Note: Clusters are reported at p < 0.05, whole-brain cluster corrected unless otherwise noted.
*p = 0.05, SVC, within an anatomically defined mask including vmPFC, right dlPFC, and left dlPFC.
As shown in Figure 2, when averaging over the full evaluation period, BOLD responses were significantly lower in DISTANCE compared with NATURAL trials in right dlPFC [p = 0.04, small-volume corrected (SVC)], but not in vmPFC. In contrast, average BOLD in INDULGE compared with NATURAL trials was marginally higher in vmPFC (41 voxels at p = 0.005, uncorrected), and lower in dlPFC (p = 0.05, SVC). A direct comparison of DISTANCE with INDULGE trials confirmed greater response in the vmPFC during INDULGE trials (p = 0.001, SVC; p = 0.03, WBC) and was not significant within the dlPFC at our omnibus threshold.
To examine whether the impact of the regulation instructions on the average BOLD signals changed over time across the trial (GLM-2; Table 3, Late vs Early contrast), we divided the evaluation period into early (immediately after food onset) and late (beginning of response initiation) phases, and estimated the strength of the 2 (Condition: DISTANCE vs NATURAL, INDULGE vs NATURAL, or INDULGE vs DISTANCE) by 2 (Time: Late vs Early) interaction on average activation. Within regions showing significant effects of this interaction (i.e., differences in the effect of regulation over time), we conducted post hoc ROI-based analyses, decomposing the same comparison (DISTANCE vs NATURAL, INDULGE vs NATURAL, or INDULGE vs DISTANCE) into effects at the early or late time points only. This allowed us to determine whether the interaction was driven primarily by early or late changes, or by both.
We observed no significant effects of time on average activation in any value-related regions. However, we did observe significant increases over time in other regions not related to value, including a region of ventrolateral prefrontal cortex associated with regulation (see below, Neural systems implementing cognitive regulation).
Finally, we asked whether the magnitude of changes in the average BOLD response correlated across subjects with regulatory success (the average decrease in bids during DISTANCE and increase in bids during INDULGE when compared with NATURAL bids). However, we did not find any such correlation for any region, either when using activity measures for the full trial or for the late phase only (all p > 0.11).
These results suggest that value modulation plays a modest role in this task. They also suggest that vmPFC activity was more easily and quickly upregulated, while dlPFC activity may be more easily and quickly downregulated.
Tests of behavioral control modulation
Behavioral control modulation predicts changes in the relative influence of the computations of a region on behavioral outcomes. This can be tested using the previous GLMs by asking whether cognitive regulation modifies the correlation between bids and BOLD responses in vmPFC or dlPFC, either for activity over the whole evaluation period, or differentially as a function of time.
Using GLM-1, we looked for differences in the correlates of bid in DISTANCE or INDULGE compared with NATURAL over the entire evaluation period, using bid responses in each condition as parametric modulators. We found no significant effects at our omnibus threshold.
We then used GLM-2 to test for differences that emerged over time. We began by assessing NATURAL trials alone for significant time-dependent changes in correlation with bid (i.e., changes in effects of the bid parametric modulator). No regions exhibited this pattern at our omnibus threshold. We then estimated the strength of the 2 (Condition: DISTANCE vs NATURAL, INDULGE vs NATURAL, or INDULGE vs DISTANCE) by 2 (Time: Late vs Early) interaction on correlates of bid (parametric modulation). Within regions showing significant interactions with time (Table 4, Late vs Early period), we decomposed the same comparison into effects at the early or late time points only (Table 4, Early period only, Late period only).
Table 4.
Interaction between time and changes in correlates of bid during cognitive regulation
| Region | BA | Late versus Early |
DISTANCE versus NATURAL |
INDULGE versus NATURAL |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early time point only |
Late time period only |
Early time point only |
Late time period only |
|||||||||||
| Z score | k | x | y | z | β (SE) | p | β (SE) | p | β (SE) | p | β (SE) | p | ||
| DISTANCE > NATURAL | ||||||||||||||
| R Dorsolateral prefrontal cortex | 10/46 | 4.26 | 45 | 30 | 39 | −1.15 (0.27) | <0.001 | 1.07 (0.31) | 0.002 | −0.20 (0.33) | NS | −0.57 (0.31) | 0.07 | |
| DISTANCE < NATURAL | ||||||||||||||
| L Ventral anterior cingulate/ventromedial prefrontal cortex | 32 | 3.00† | 25 | −9 | 48 | 0 | 0.67 (0.29) | 0.03 | −0.87 (0.29) | 0.007 | −0.37 (0.31) | NS | 0.14 (0.32) | NS |
| L Caudate | 4.95 | −3 | 9 | −6 | 1.31 (0.30) | <0.001 | −0.97 (0.27) | 0.001 | 0.82 (0.29) | 0.01 | −0.16 (0.31) | NS | ||
| INDULGE > NATURAL | ||||||||||||||
| No regions significant | ||||||||||||||
| INDULGE < NATURAL | ||||||||||||||
| L Dorsolateral prefrontal cortex | 10/46 | 4.3 | 40 | −39 | 21 | 27 | −0.09 (0.24) | NS | −0.33 (0.25) | NS | 0.38 (0.17) | 0.03 | −1.05 (0.20) | <0.001 |
| R Dorsolateral prefrontal cortex | 10/46 | 3.12* | 32 | 51 | 45 | 12 | 0.05 (0.45) | NS | −0.24 (0.34) | NS | 0.78 (0.43) | NS | −1.01 (0.31) | 0.003 |
| L Inferior parietal lobule | 7 | 4.1 | 49 | −9 | −69 | 60 | 1.22 (0.81) | NS | 1.47 (0.85) | NS | 2.06 (0.82) | 0.02 | −2.24 (0.71) | 0.004 |
| INDULGE > DISTANCE | ||||||||||||||
| L Ventral anterior cingulate/ventromedial prefrontal cortex | 32 | 4.43 | 52 | −6 | 39 | 0 | 0.51 (0.35) | NS | −0.76 (0.30) | 0.02 | −0.66 (0.31) | 0.04 | 0.34 (0.32) | NS |
| INDULGE < DISTANCE | ||||||||||||||
| R Dorsolateral prefrontal cortex | 10/46 | 3.73 | 61 | 45 | 30 | 24 | −0.85 (0.23) | 0.001 | 0.92 (0.36) | 0.02 | 0.16 (0.29) | NS | −0.95 (0.29) | 0.003 |
| L Dorsolateral prefrontal cortex | 10/46 | 3.36† | 26 | −39 | 30 | 24 | −0.61 (0.32) | 0.07 | −0.01 (0.29) | NS | 0.41 (0.25) | NS | −1.03 (0.29) | 0.001 |
| R Parietal cortex | 40 | 4.24 | 61 | 27 | −48 | 39 | −0.62 (0.27) | 0.03 | 0.71 (0.36) | 0.06 | 0.55 (0.31) | 0.08 | −0.86 (0.34) | 0.02 |
| L Precuneus | 7 | 4.13 | 149 | −12 | −69 | 57 | 0.09 (0.54) | NS | 1.21 (0.62) | 0.06 | 1.18 (0.61) | 0.06 | −1.76 (0.55) | 0.003 |
Note: Clusters are reported at p < 0.05, whole-brain cluster corrected unless otherwise noted.
*p = 0.05 SVC within an anatomically defined mask including vmPFC, right dlPFC, and left dlPFC.
†p < 0.005, uncorrected, reported for completeness.
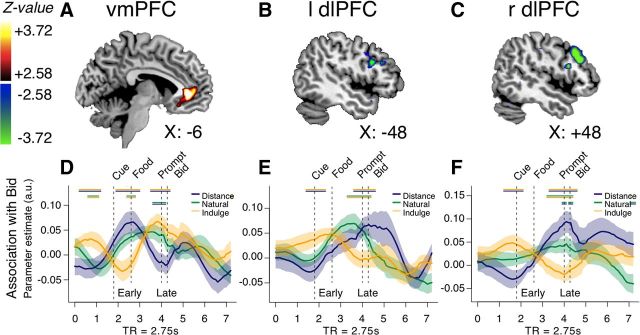
Cognitive regulation had a striking and significant time-dependent effect on the correlates of bid (Fig. 3A–C; Table 4, Late vs Early period). A linear contrast of the three conditions (DISTANCE = −1; NATURAL = 0; IND = +1) showed a significant positive interaction (i.e., increased association with bid over time) in vmPFC (p < 0.001, SVC, and p = 0.01, WBC; Fig. 3A), and a significant negative interaction (i.e., decreased association with bid over time) in right dlPFC (Fig. 3B; p = 0.001, SVC; p = 0.001, WBC), left dlPFC (Fig. 3C; p = 0.001, uncorrected; p = 0.09, SVC), and right inferior parietal cortex (p = 0.001, WBC). Interaction analyses for DISTANCE versus NATURAL and INDULGE versus NATURAL separately largely identified the same areas (for details, see Table 4).
To better understand these interactions, we performed ROI analyses to decompose them into the following components: the change in correlation with bid in DISTANCE versus NATURAL separately at the early and late time points, and the change in correlation with bid in INDULGE versus NATURAL separately at the early and late time points. For simplicity, we focus in the text on analysis of regions identified by the contrast of INDULGE versus DISTANCE, because they mostly encompassed areas identified in each regulation condition separately. Table 4 reports the results for all regions.
We found that vmPFC showed both a late-period decrease in the correlation with bid in the contrast of DISTANCE − NATURAL trials (p = 0.02), as well as an early-period decrease in correlation with bid in the contrast of INDULGE − NATURAL trials (p = 0.04). Dorsolateral and parietal areas exhibited the reverse pattern. In the contrast of DISTANCE − NATURAL bids, their association with bids was lower early in the trial (range, p = 0.07–0.001), and increased by late in the trial (range, p = 0.06-.02), while in the contrast of INDULGE − NATURAL bids, their association was not strongly different at the early period (all p > 0.06) and robustly decreased by late in the trial (all p < 0.02). Separate analyses controlling for reaction time yielded nearly identical results to all analyses reported above, suggesting that these results were not driven merely by differences in response time.
We performed two additional post hoc analyses to further explore these results. First, we plotted the association between bids and BOLD activity in the vmPFC and dlPFC regions of interest as it evolved over the trial. In NATURAL trials, bids correlated with vmPFC activity beginning almost immediately after food onset, and continued to do so up until bid placement. In contrast, in DISTANCE trials the correlation was significant only during the early phase, and in INDULGE trials only during the late phase (Fig. 3D). Bilateral dlPFC exhibited the opposite pattern (shown for left and right dlPFC in Fig. 3E,F).
We also tested for a correlation between regulatory success (as measured by the change in average bid) and the extent to which bid-BOLD correlations in valuation areas differed during either the early evaluation phase or the late response initiation phase. Note that such a shift in mean bids could result from changes in brain–behavior correlations if regions where average activation is high or unchanged (i.e., vmPFC) are prevented from driving behavior during DISTANCE, and regions where activation is low (i.e., dlPFC) are prevented from driving behavior during INDULGE. This predicts that greater vmPFC control during INDULGE trials (when vmPFC activation increased more than dlPFC) should be associated with higher bids, and that greater dlPFC control during DISTANCE trials (when dlPFC activation decreased more than vmPFC) should be associated with lower bids.
We found no correlation between regulatory success and changes during the early evaluation phase in vmPFC, dlPFC, or inferior parietal cortex association with bids. However, we found significant correlations at the late response initiation phase. Consistent with behavioral control modulation, the less vmPFC correlated with bids in DISTANCE trials, the less people bid (r = −0.4; p = 0.04; Fig. 4A), while the more vmPFC activity correlated with bids in INDULGE trials, the more they bid (r = +0.45; p = 0.02; Fig. 4B). In DISTANCE trials, we also found a significant correlation between the extent to which left dlPFC correlated with the bids and regulatory success (r = 0.46; p = 0.02; Fig. 4C), although the analogous result for INDULGE trials was not significant (Fig. 4D). Right dlPFC and parietal responses did not correlate with regulatory success.
Together, these analyses provide evidence for control modulation during decision making in our cognitive regulation task, and suggest that the vmPFC and dlPFC may make parallel but distinct contributions to value. When participants decreased their desire for food, vmPFC response to the food predicted the bid less, while dlPFC response predicted it more. When participants increased their desire for food, vmPFC response continued to predict bids, while dlPFC response predicted bids less. In addition, these shifts predicted regulatory success.
Neural systems implementing cognitive regulation
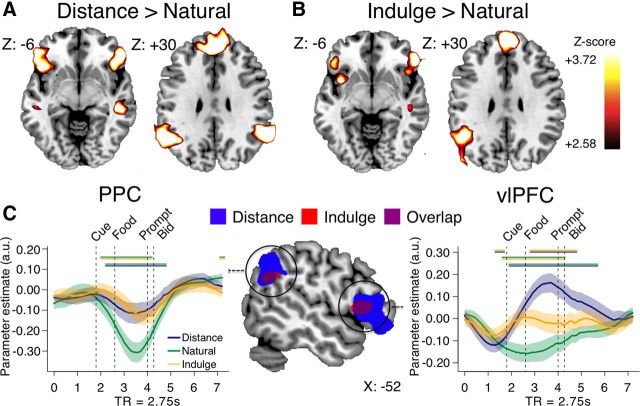
We next sought to identify regions likely to play a role in producing the changes in value-related regions during cognitive regulation. Using GLM-1, we searched for areas exhibiting stronger average activity in DISTANCE or INDULGE versus NATURAL trials, over the full time period. We found stronger activity in ventrolateral prefrontal cortex (vlPFC) and posterior parietal cortex (PPC) for both regulation conditions separately, compared with NATURAL trials (Fig. 5A,B; p < 0.05, WBC; Table 2). A conjunction analysis confirmed that vlPFC bilaterally, as well as left PPC, responded positively during both conditions (Fig. 5C). Comparing the two regulation conditions to each other revealed no differences in these regions.
Time courses of activation in vlPFC and PPC suggested somewhat different patterns of response in these two regions (Fig. 5C). Activity in vlPFC gradually increased above baseline during DISTANCE trials and peaked near the end of the evaluation period, with a similar but smaller increase in INDULGE trials (Fig. 5C, right). In the PPC, response during DISTANCE and INDULGE trials did not change from baseline. Instead, NATURAL trials showed a pronounced deactivation not present in regulation trials (Fig. 5C, left).
Finally, we tested the hypothesis that vlPFC and PPC play a role in cognitive regulation by modulating value computations in dlPFC and vmPFC. We estimated psychophysiological interaction models that allowed us to compare functional connectivity of these areas in the different conditions. We found that left vlPFC was significantly more negatively coupled with vmPFC (but not dlPFC) in DISTANCE compared with NATURAL trials (110 voxels; negative peak at 0, 45, 0; p = 0.001, SVC), but did not differ in connectivity on INDULGE trials. In addition, although changes in PPC connectivity did not meet our omnibus significance threshold for either dlPFC or vmPFC, at a slightly more liberal threshold (p < 0.005, uncorrected), the left PPC was more positively coupled with both left (53 voxels; peak at −48, 39, 24) and right dlPFC (19 voxels; peak at 51, 30, 33) on DISTANCE trials, and more negatively coupled with right dlPFC on INDULGE trials (33 voxels; negative peak at 27, 48, 42).
Given these effects, we also sought to determine whether vlPFC or PPC responses were correlated with either behavioral measures of regulatory success or with the changes in neural response that we observed in vmPFC and dlPFC. However, these analyses revealed no significant correlations.
Discussion
Our experiment provides novel insights into the computational and neurobiological mechanisms of cognitive regulation during decision making. We identified areas of both vmPFC and dlPFC where activity correlated with values expressed in behavior. We then examined how cognitive regulation affected these regions. We tested and found evidence for two distinct regulatory mechanisms: value modulation (changing the values assigned to stimuli) and behavioral control modulation (changing how value signals affect behavior). However, dlPFC and vmPFC were differentially sensitive to value modulation: attempts to distance more effectively downregulated dlPFC signals, while attempts to indulge more effectively upregulated vmPFC signals. Behavioral control modulation also affected these regions differentially: the vmPFC contribution to behavior decreased over time to near-zero in DISTANCE trials, while dlPFC contribution increased. The opposite pattern emerged in INDULGE trials. Moreover, whereas value modulation effects were consistent over time, behavioral control modulation effects varied considerably, and correlated with regulatory success only late in the trial. Finally, the pattern of vlPFC and PPC activity suggests that they play a role in coordinating the regulation-related changes in value regions: vlPFC and PPC responded more strongly during both regulatory conditions, and changes in their connectivity with vmPFC and dlPFC corresponded to changes in the correlation of each value region with behavior. These results have implications for several domains.
Value construction: single versus multiple value systems
Studies of choice in the absence of explicit regulation point to the importance of both vmPFC and dlPFC in the computation of value at the time of choice (Kable and Glimcher, 2007; Plassmann et al., 2007, 2010; Kim et al., 2008; Basten et al., 2010; Sokol-Hessner et al., 2012), but their relative roles are unclear. Some have argued that they compute parallel but distinct values, with vmPFC placing more weight on immediate, concrete outcomes (e.g., taste), and dlPFC placing more weight on long-term, abstract goals (e.g., healthiness). In this view, the quality of decisions depends on the relative weight given to the two value signals in behavior (McClure et al., 2004). Others have proposed that a single value system (usually associated with vmPFC) integrates information about all stimulus attributes, including health and taste, and then determines choice (Kable and Glimcher, 2007; Hare et al., 2009). In this view, decisions depend on how vmPFC value computations weight different stimulus attributes, with that weighting modulated by the dlPFC.
Our results inform this debate. First, vmPFC and dlPFC signals both correlated with behaviorally expressed values, suggesting that they both contribute to choice. Second, cognitive regulation more effectively decreased dlPFC activity, but more effectively increased vmPFC activity, consistent with the hypothesis that these regions represent different types of information. Third, cognitive regulation changed the relative impact that dlPFC and vmPFC had on choices. For example, when subjects tried to decrease craving, dlPFC activity showed greater correlation with behavior than vmPFC activity. Together, these findings suggest that, in our task, vmPFC and dlPFC participate in the computation of value signals with different properties. Moreover, the influence of these two signals on behavior can be dynamically adjusted according to the current goal.
Value construction: differences in vmPFC versus dlPFC value-related activity
Our results do not speak directly to the computations instantiated by vmPFC and dlPFC in this context, but together with other findings in the literature, they provide some clues. Our finding that vmPFC and dlPFC seem differentially sensitive to attempts to increase and decrease desire, respectively, suggests that these regions may weight basic and abstract attributes (e.g., taste vs health) differently. Consistent with this, an area of dlPFC close to that identified here correlated with health ratings in a study involving instructions to attend to longer-term health consequences of choice (Hare et al., 2011a).
However, other data are less consistent with this hypothesis. The dlPFC does not correlate with value consistently (Hare et al., 2008; Chib et al., 2009; Litt et al., 2011), nor have all studies of self-control found that dlPFC correlates specifically with the value of abstract attributes (Kable and Glimcher, 2007; Hare et al., 2009). We speculate that differences in the attentional requirements of the task or the relative flexibility of the dlPFC compared with vmPFC in representing different quantities may underlie these inconsistencies, but further evidence will be needed to bear this out.
Value construction: differential integration of value signals over time
Our results show that the relative influence of vmPFC and dlPFC value-related areas changes over time, particularly toward the end of the 4 s decision period. This result is consistent with models that assume the brain makes choices using a value integration and comparison process that unfolds over time (Bogacz et al., 2006; Ratcliff and McKoon, 2008; Basten et al., 2010; Milosavljevic et al., 2010; Hare et al., 2011b). In these models, valuation areas compute signals that feed into an action selection network, activating different responses until activation for one response accumulates strongly enough to cross a threshold and initiate choice. Importantly, these models suggest that both early- and late-occurring evidence can affect the comparator process and choice (Resulaj et al., 2009). Our results suggest that cognitive regulation can affect both evidence quality (via value modulation) and its ability to drive action selection (via behavioral control modulation).
Self-control: multiple contexts, multiple mechanisms
Our results present a puzzle: Why do we observe evidence for multiple value signals and behavioral control modulation in this task, but previous self-control studies have not (Kable and Glimcher, 2007; Hare et al., 2009)? For example, some (Hare et al., 2009, 2011a) have found that a single stimulus value signal, encoded in vmPFC, correlated with behavior regardless of whether subjects displayed successful self-control. However, in good dieters vmPFC reflected both health and taste, but in bad dieters it reflected only taste. Moreover, in good dieters areas of dlPFC (more posterior and dorsal than those identified here) came on-line and modulated vmPFC value representations.
Our study alone cannot resolve why these two studies differ so dramatically. We conjecture that the answer may lie in the type of self-control used. We explicitly instructed participants to do whatever necessary to modulate their craving over a 4 s period. Although this instruction could have encouraged different approaches in different people, and thus resulted in little consistency, we observed significant changes in neural responding that suggest at least some common processes across subjects, including changes in behavioral control modulation.
In contrast to our study, previous studies have not explicitly asked participants to regulate, but simply to make choices consistent with their own habits and goals. Concomitantly, responses in these studies take less time (<2 s) than many regulation effects we observed (≥4 s). We suspect that subjects used to making healthy choices (i.e., dieters) might modulate the vmPFC directly using quicker self-control mechanisms. Those unaccustomed to making healthy choices (i.e., our participants) might rely on slower, more deliberative, mechanisms that operate through different means (e.g., behavioral control). These differences may explain differences in the areas of lateral PFC activated by these two tasks, and in the speed with which self-control arises.
Self-control: decision making versus emotion regulation
The observation that some regions of dlPFC may play a role in valuation could also explain another puzzling aspect of our results: research in emotion regulation typically finds increases in dlPFC (Ochsner and Gross, 2005), whereas we found decreases. Yet most emotional regulation paradigms involve decreasing response to a negative stimulus. If dlPFC correlates positively with value, then reducing negative affect should increase activation in this region. Indeed, some studies have found decreases in similar locations of dlPFC during downregulation of positive affect (Kober et al., 2010), although these results were not highlighted. However, others find increases in more anterior regions of dlPFC when increasing negative emotion (Kim and Hamann, 2007). This suggests that the dlPFC may be a functionally heterogenous area, or may compute different signals in different contexts. We suspect that the notion that the dlPFC serves always simply to regulate affective representations in other brain areas is likely incorrect.
In contrast, the vlPFC and PPC areas we identified with cognitive regulation of decision making overlap considerably with those found during cognitive regulation of emotional experience (Ochsner et al., 2004; Wager et al., 2008) and are thought to play important roles in attentional and cognitive control (Badre and Wagner, 2005; Badre et al., 2005; Decety and Lamm, 2007). Future research should compare the roles of vlPFC and PCC in cognitive regulation of emotion versus decision making, to determine whether they implement a constant set of computations, or exhibit differences across domains.
Self-control: difficulty of regulation
Our finding that regulation effects evolve over time may explain why it often feels so difficult. If early signals in vmPFC contribute to bids but are unaffected by attempts to decrease craving, then changes to value computations in dlPFC have to overcome these early signals. Similarly, if the dlPFC responds to less arousing features when attempting to increase desire, vmPFC responses, even if increased quickly, have to counteract them. Coactivation of competing value signals during decision making may determine when regulation feels difficult.
Footnotes
This work was supported by National Science Foundation Grants SES-0851408, SES-0926544, and SES-0850840, National Institutes of Health Grant R01 AA018736, and The Gordon and Betty Moore Foundation. We thank Todd Hare for comments.
References
- Badre D, Wagner AD. Frontal lobe mechanisms that resolve proactive interference. Cereb Cortex. 2005;15:2003–2012. doi: 10.1093/cercor/bhi075. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Paré-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Basten U, Biele G, Heekeren HR, Fiebach CJ. How the brain integrates costs and benefits during decision making. Proc Natl Acad Sci U S A. 2010;107:21767–21772. doi: 10.1073/pnas.0908104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Wright FD, Newman CF, Liese BS. Cognitive therapy of substance abuse. New York: Guilford; 1993. [PubMed] [Google Scholar]
- Becker GM, DeGroot MH, Marschak J. Measuring utility by a single-response sequential method. Behav Sci. 1964;9:226–232. doi: 10.1002/bs.3830090304. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogacz R, Brown E, Moehlis J, Holmes P, Cohen JD. The physics of optimal decision making: a formal analysis of models of performance in two-alternative forced-choice tasks. Psychol Rev. 2006;113:700–765. doi: 10.1037/0033-295X.113.4.700. [DOI] [PubMed] [Google Scholar]
- Busemeyer JR, Townsend JT. Decision field theory: a dynamic-cognitive approach to decision making in an uncertain environment. Psychol Rev. 1993;100:432–459. doi: 10.1037/0033-295x.100.3.432. [DOI] [PubMed] [Google Scholar]
- Chib VS, Rangel A, Shimojo S, O'Doherty JP. Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. J Neurosci. 2009;29:12315–12320. doi: 10.1523/JNEUROSCI.2575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13:580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nat Neurosci. 2008;11:880–881. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare TA, Malmaud J, Rangel A. Focusing attention on the health aspects of foods changes calue signals in vmPFC and improves dietary choice. J Neurosci. 2011a;31:11077–11087. doi: 10.1523/JNEUROSCI.6383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Schultz W, Camerer CF, O'Doherty JP, Rangel A. Transformation of stimulus value signals into motor commands during simple choice. Proc Natl Acad Sci U S A. 2011b;108:18120–18125. doi: 10.1073/pnas.1109322108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt LT, Kolling N, Soltani A, Woolrich MW, Rushworth MF, Behrens TE. Mechanisms underlying cortical activity during value-guided choice. Nat Neurosci. 2012;15:470–476. S1–S3. doi: 10.1038/nn.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neurobiology of decision: consensus and controversy. Neuron. 2009;63:733–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Hwang J, Lee D. Prefrontal coding of temporally discounted values during intertemporal choice. Neuron. 2008;59:161–172. doi: 10.1016/j.neuron.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J Cogn Neurosci. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajbich I, Armel C, Rangel A. Visual fixations and the computation and comparison of value in simple choice. Nat Neurosci. 2010;13:1292–1298. doi: 10.1038/nn.2635. [DOI] [PubMed] [Google Scholar]
- Lebreton M, Jorge S, Michel V, Thirion B, Pessiglione M. An automatic valuation system in the human brain: evidence from functional neuroimaging. Neuron. 2009;64:431–439. doi: 10.1016/j.neuron.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Litt A, Plassmann H, Shiv B, Rangel A. Dissociating valuation and saliency signals during decision-making. Cereb Cortex. 2011;21:95–102. doi: 10.1093/cercor/bhq065. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Milosavljevic M, Malmaud J, Huth A, Koch C, Rangel A. The drift diffusion model can account for the accuracy and reaction time of value-based choices under high and low time pressure. Judgm Decis Mak. 2010;5:437–449. [Google Scholar]
- Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–284. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J Neurosci. 2007;27:9984–9988. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. J Neurosci. 2010;30:10799–10808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Hare T. Neural computations associated with goal-directed choice. Curr Opin Neurobiol. 2010;20:262–270. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, McKoon G. The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput. 2008;20:873–922. doi: 10.1162/neco.2008.12-06-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resulaj A, Kiani R, Wolpert DM, Shadlen MN. Changes of mind in decision-making. Nature. 2009;461:263–266. doi: 10.1038/nature08275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Mars RB, Summerfield C. General mechanisms for making decisions? Curr Opin Neurobiol. 2009;19:75–83. doi: 10.1016/j.conb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Sokol-Hessner P, Hutcherson C, Hare T, Rangel A. Decision value computations adjust to the available decision time. Eur J Neurosci. 2012;35:1065–1074. doi: 10.1111/j.1460-9568.2012.08076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue LP, Corrado GS, Newsome WT. Choosing the greater of two goods: neural currencies for valuation and decision making. Nat Rev Neurosci. 2005;6:363–375. doi: 10.1038/nrn1666. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]