Abstract
Hydrogen peroxide (H2O2) is a major component of oxygen metabolism in biological systems that, when present in high concentrations, can lead to oxidative stress in cells. Non-invasive molecular imaging of H2O2 using fluorogenic systems represents an effective way to detect and measure the accumulation of this metabolite. Herein, we detail the development of robust H2O2-sensitive fluorescent probes using a boronic ester trigger appended to the fluorophore via a benzyl ether linkage. A major advantage of the probes presented here is their synthetic accessibility, with only one step needed to generate the probes on the gram scale. The sensitivity of the probes was evaluated in simulated physiological conditions, showing micromolar sensitivity to H2O2. The probes were tested in biological model systems, demonstrating effective imaging of unstimulated, endogenous H2O2 levels in RAW 264.7 cells and murine brain tissue.
Keywords: hydrogen peroxide, fluorescent probes, reactive oxygen species
Introduction
Reactive oxygen species (ROS) encompass a wide array of endogenously produced intermediates that directly result from oxygen metabolism in biological systems.[1] The chemical biology of ROS, especially hydrogen peroxide (H2O2), is rather complex, as recent studies show that controlled generation of H2O2 is necessary to maintain cellular functions such as growth, proliferation, and immune system function.[2] However, misregulation in the production of ROS can lead to significant oxidative damage due to the inability of cells to effectively manage oxidation-reduction equilibrium.[1, 3] It is the specific cellular localization and concentration that alter the role of ROS from one of cell signaling to that of oxidative stress and disease.[2a, 2e] Indeed, H2O2 is a major ROS byproduct and has been studied as a common indicator for oxidative stress in a number of pathologies including cancer,[4] cardiovascular[5] and neurodegenerative[6] diseases, and diabetes.[7] It is therefore crucial to understand the roles and implications of H2O2 generation in biological systems.
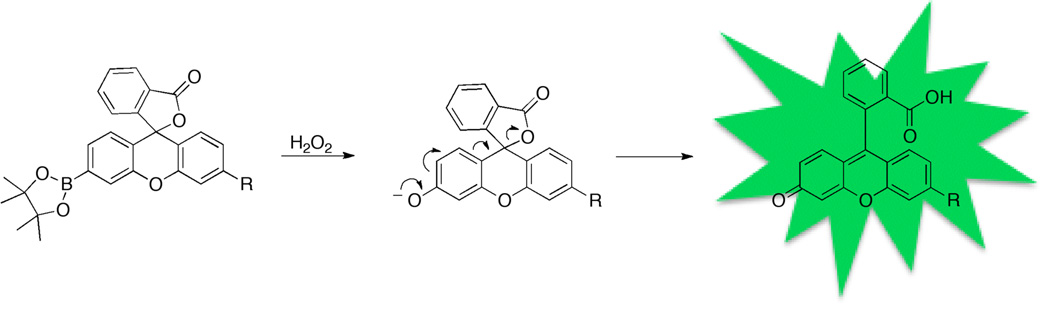
Molecular imaging of H2O2 with reaction-based fluorescent probes is a non-invasive approach used to monitor the chemistry of this particular ROS in living systems. In this way, the specific spatial and temporal distribution of H2O2 can be elucidated within cells and tissues. Several selective probes have been reported for the detection of a number of ROS including nitric oxide,[8] peroxynitrite,[9] superoxide,[10] singlet oxygen,[11] and others. The Chang laboratory has pioneered the development of H2O2 sensitive and selective fluorophores (Figure 1). In an elegant series of studies, aryl boronic ester-derivatized fluorophores were prepared that are selectively cleaved by H2O2 to generate the corresponding phenol, leading to a fluorescent turn-on event.[12] A similar study demonstrated that the direct installation of a boronic ester trigger on a coumarin-based molecule can be effective for H2O2 imaging, further illustrating the generality of this approach.[13] Collectively, these reports have established boronic esters as the triggering motif of choice for H2O2 over other biologically relevant ROS.[12, 14]
Figure 1.
General design and activation of boronic ester-based H2O2-sensitive fluorescent probes pioneered by the Chang laboratory.
When designing a fluorescent probe for biological imaging, several key factors must be considered including the following: synthetic ease, aqueous solubility and stability, kinetic rates of deprotection, and fluorescent turn-on response. Ideally, a probe should also have the ability to image biologically relevant levels of H2O2 with and without external stimulation. The seminal studies by Chang and coworkers have inspired other groups, including ours, to address the following remaining challenges: a) visualization of unstimulated, basal levels of endogenous H2O2;[12, 14–15] b) developing readily synthesized probes, making them more accessible to the broader research community for biological studies. With respect to the first point above, many of the reported probes image H2O2 after adding exogenous H2O2, or by adding H2O2 stimulants such as phorbol myristate acetate (PMA) or epidermal growth factor receptor (EGFr). Hence, these probes can visualize exogenous or upregulated levels of H2O2 (such as in oxidative-mediated cell signaling), but have not been shown to visualize nominal biological levels of H2O2.
Inspired by these boronic ester based H2O2 fluorophores, our group reported the first example of H2O2 activated prodrugs using a related protecting group strategy.[16] The boronic ester trigger was appended to the phenolic moiety of the metal-binding group (MBG) of a known metalloenzyme inhibitor via a benzyl ether linkage. In the presence of H2O2, the boronic ester is cleaved by nucleophilic attack of H2O2, leading to a spontaneous 1,6-benzyl elimination reaction to generate the active MBG, thus turning on metalloenzyme inhibition.[16] Similar carbonate and carbamate linkage strategies have been applied to dendritic self-immolative systems[17] as well as some prodrugs[18] to link various triggers to the desired reporter or drug. Carbonate and carbamate linkages are much more common in the literature due to the thermodynamic driving force of the release of CO2 in the elimination reaction, leading to fast rates of reaction.[19] Studies on our boronic ester-benzyl ether linkage indicated superior aqueous stability to that of the corresponding carbonate ester, with similar cleavage kinetics.[20] Our initial studies have led to the development of biocompatible polymeric nanoparticles that undergo desired cargo release in response to H2O2 [21] as well as new H2O2 activated prochelators.[22] Shortly after our report, Chang and coworkers reported an H2O2-responsive bioluminescent probe for the detection of hydrogen peroxide in a murine tumor model using the benzyl ether linkage to couple a boronic acid trigger to a fluorophore.[23] All of these systems demonstrate that the benzyl ether linked boronic ester system has advantages when compared to other linkage approaches. Therefore, in this report, the boronic ester-benzyl ether linkage strategy is used to prepare synthetically accessible fluorescent probes for H2O2 imaging. These probes overcome both of the aforementioned limitations, namely, the detection of endogenous H2O2 and a one-step, highly accessible synthetic procedure that allows for gram scale preparation using routine synthetic techniques.
Results and Discussion
Development of H2O2-Sensitive Probes
Having elucidated the benefits of the benzyl ether linked system with respect to kinetics and hydrolytic stability, fluorescein was selected as an inexpensive and widely used dye for cellular imaging due to its high quantum yield, low toxicity, and excellent water solubility.[24]
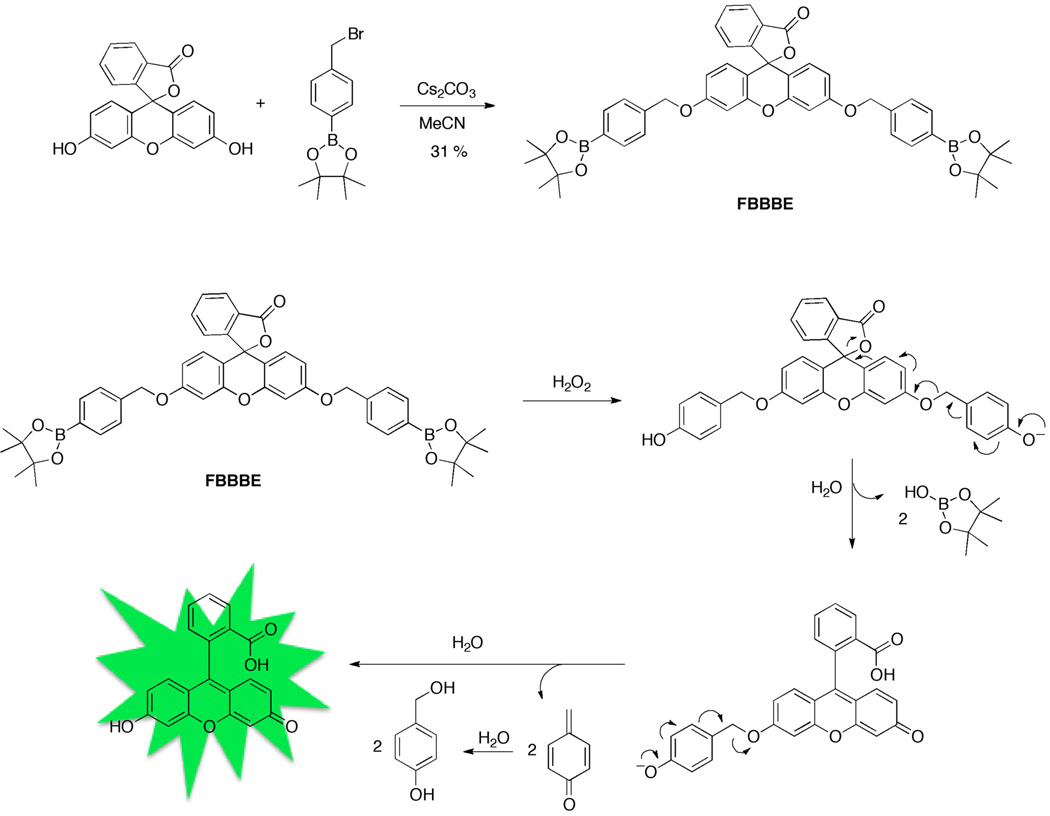
A protected fluorescein compound, FBBBE (fluorescein bis(benzyl boronic ester)), was synthesized in one step from commercially available starting materials as shown in Figure 2. Many of the previously reported H2O2 probes employing the boronic ester trigger required 3–4 challenging and relatively low yielding synthetic steps.[14–15] Additionally, the synthesis of FBBBE can be conducted on the gram scale (ESI), while most other reports describe quantities of probes in the low milligram range. FBBBE has pinacol boronic esters installed via a benzyl ether linkage to the fluorophore at both the 3′ and 6′ positions. The boronic ester moiety was chosen over the boronic acid in an attempt to enhance lipophilicity and thus cellular permeability. In order to evaluate the sensitivity of these probes to H2O2, a control compound was synthesized (FBn, Figure 3).
Figure 2.
Synthesis of FBBBE, a derivatized fluorecein compound with a H2O2-sensitive boronic ester trigger appended via a benzyl ether linkage (top). Scheme of deprotection and fluorescence activation of FBBBE in the presence of H2O2 (bottom).
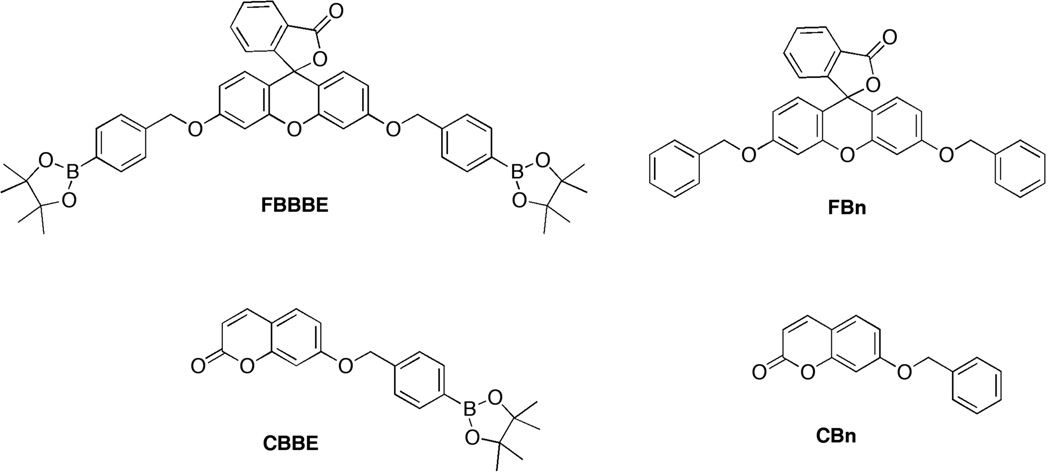
Figure 3.
Structures of latent, H2O2-sensitive fluorophores FBBBE and CBBE (left), along with their corresponding controls, FBn and CBn (right).
To investigate the synthetic generality of this approach, a coumarin-based molecule (umbelliferone or 7-hydroxycoumarin) was protected with the boronic ester motif attached via the benzyl ether linkage in a similar way (Figure 3, CBBE). The corresponding control compound lacking the boronic ester trigger was also synthesized for evaluation (Figure 3, CBn). It should be noted that a similar benzyl ether linked boronic acid to 7-hydroxycoumarin was reported by Shabat et al. for H2O2 detection involving dendritic chain reactions; however, no details on this probe were provided.[25] Both CBBE and FBBBE were quite soluble in aqueous buffer, using only ~1% DMSO (by volume) when preparing samples at micromolar concentrations.
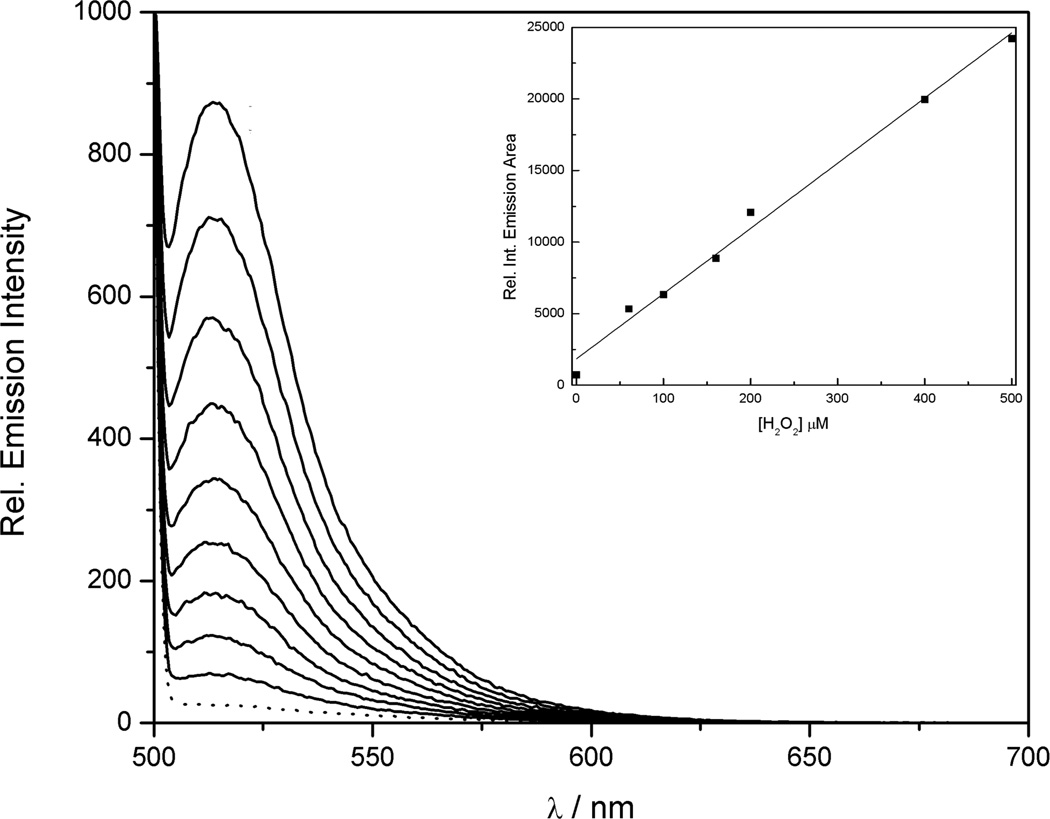
FBBBE and CBBE were evaluated for H2O2 sensitivity under simulated physiological conditions (50 mM HEPES, pH 7.5). For FBBBE, the installation of the benzyl protecting group forces the compound to remain in the closed lactone form, rendering FBBBE (and FBn) nonfluorescent. UV-Vis spectroscopy reveals a baseline absorption profile in the visible region for FBBBE (Figure S1). Upon addition of H2O2 to FBBBE, a large increase in fluorescence is observed with a maximum λem = 521 nm (Figure 4). Along with the observed fluorescence increase, the absorption spectrum of FBBBE considerably changes upon H2O2 addition with a large increase in the visible absorption band (λmax = 494 nm). The final spectrum recorded is identical to that of an authentic sample of fluorescein, providing additional proof that the probe is undergoing complete deprotection to the desired product (Figure S1). The fluorescent response of FBBBE was characterized over a wide range of H2O2 concentrations to demonstrate a linear relationship in relative emission response (Figure 4). To do this, FBBBE was incubated with different concentrations of H2O2 for 15 min and then the emission spectrum was collected. The spectra obtained were integrated between 500–700 nm and the relative emission area was plotted vs. H2O2 concentration, which showed a linear correlation (Figure 4). As expected, FBn does not show any changes in the emission or absorption spectra upon treatment with excess H2O2 (Figure S2).
Figure 4.
Fluorescent response of 50 µM FBBBE to H2O2 (5 eq, 250 µM). The dotted line represents the initial spectrum and subsequent spectra were recorded every 4 min after the addition of H2O2. Fluorescent response of 10 µM FBBBE to various concentrations of added H2O2 (insert). Spectra were collected after incubation with H2O2 at room temperature for 15 min. The collected emission was integrated between 500–700 nm (λexc = 480 nm). Spectra were acquired in 50 mM HEPES (pH 7.5).
To determine whether the cleavage of one or both benzyl ether protecting groups from FBBBE is necessary to generate fluorescence, a second fluorescein derivative (in the free carboxylic acid form, FBBE), was synthesized that showed absorption in the visible region (Figure S3). Emission spectroscopy reveals that this compound is highly fluorescent with an emission profile identical to that of fluorescein (data not shown). After treating this probe with H2O2, the absorption spectra shifts to that of one matching fluorescein (Figure S3). Taken together, these data suggest only one H2O2-mediated deprotection event is necessary to turn on fluorescence for FBBBE. However, as shown by absorption spectroscopy, treatment of FBBBE with H2O2 quickly converts the compound to fluorescein (Figure S1), showing negligible accumulation of FBBE. This suggests that FBBE is a short-lived intermediate that does not contribute significantly to the sensors response.
The coumarin-based compound, CBBE, is nonfluorescent in the absence of H2O2. The quenched fluorescence of CBBE quickly turns on in the presence of H2O2, with a maximum λem = 453 nm (Figure S4). A calibration plot similar to that of FBBBE was obtained for CBBE, showing a linear correlation between H2O2 added and observed fluorescent response (Figure S5). Absorption spectroscopy also shows a shift in absorbance after H2O2 addition to CBBE, with the resulting spectrum matching an authentic sample of 7-hydroxycoumarin. (Figure S6). The control compound, CBn, shows no change in the emission or absorption spectra after treatment with excess H2O2, as expected (Figure S7).
Additional studies were performed to quantify the fluorescence turn-on for each probe to directly compare the sensitivity to H2O2. FBBBE shows a 52-fold increase in fluorescence in response to excess H2O2, while CBBE shows a 57-fold increase (Figure S8 and S9). These fluorescent turn-on responses are excellent when compared with similar fluorophores reported for endogenous imaging of H2O2 (3–50 fold turn on),[12] further demonstrating the value of these probes for sensing H2O2. To determine the sensitivity of these probes in a more quantitative fashion, pseudo first-order kinetic measurements were performed using UV-Vis absorption spectroscopy. The observed rate constants for FBBBE and CBBE activation by H2O2 were kobs = 3.1 × 10−5 s−1 and kobs = 4.6 × 10−3 s−1, respectively. It was determined that the deprotection of CBBE was much faster than FBBBE, likely due to the fact that FBBBE contains two boronic ester triggers that are required for complete release of the fluorophore. Recently, it has been shown that boronic ester based probes can be sensitive to other biologically relevant ROS, such as hypochlorite (OCl−).[26] It was confirmed that FBBBE can also react with OCl−, but is far less sensitive to OCl− when compared to H2O2 (Figure S10).
Cellular Imaging Studies
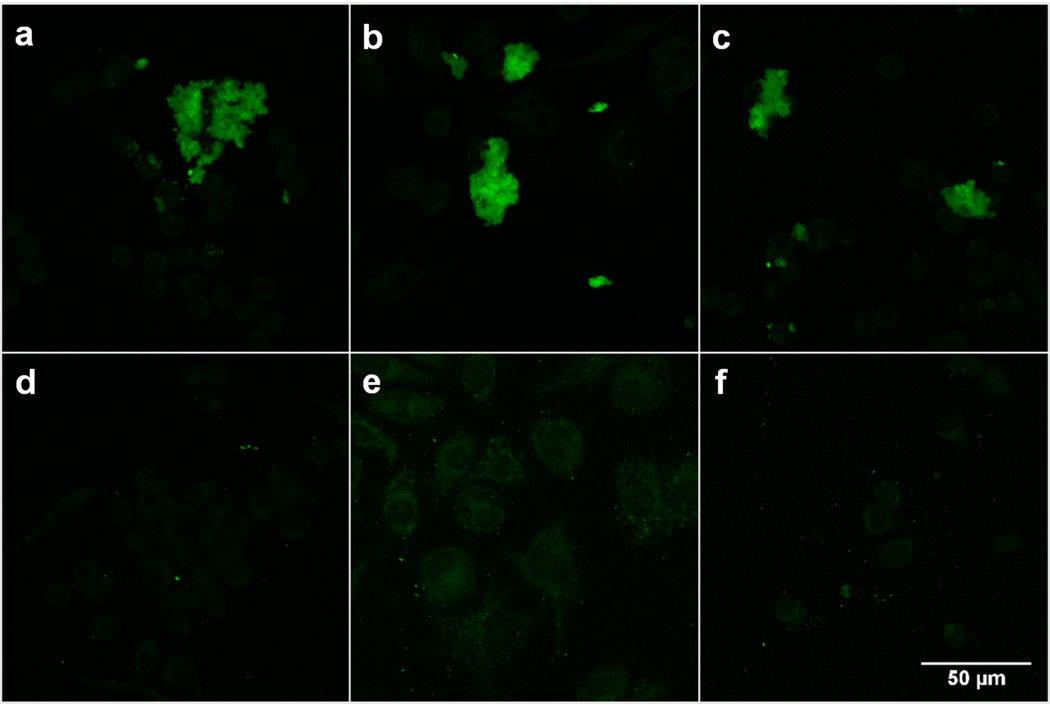
With a clear understanding of the chemistry of these probes in vitro, FBBBE was examined for H2O2 detection in biological samples via confocal microscopy. Murine macrophage RAW 264.7 cells were treated with PBS buffer (unstimulated cells), lipopolysaccharide (LPS, stimulated cells), or with exogenous H2O2, followed by incubation with either FBBBE (Fig. 5a–c) or FBn (Fig. 5d–f). The inflammatory effects of LPS in macrophages are well established and result in a burst of reactive oxygen species and reactive nitrogen intermediates among other inflammatory signatures.[27] Fluorescence from activated FBBBE was observed in all cells (Fig. 5a–c), and quantification of the signal output reveals that mean fluorescence for cells treated with PBS or H2O2 (2 equiv) is not statistically different; however, the mean fluorescence of PBS or H2O2-treated cells is both statistically different (p <0.001) than those treated with LPS (Figure S11). The observed results indicate that FBBBE is responsive to intracellular, endogenous levels of H2O2 (note: cells are washed and fixed prior to incubating with the probe). Experiments using the control probe FBn show no turn on of fluorescence in any of the cells (Fig. 5d–f). Statistical analysis also shows a significant difference between the mean fluorescence signal output between FBBBE and FBn, as expected (Figure S11).
Figure 5.
Molecular imaging of H2O2 in RAW 264.7 cells. Confocal fluorescence image of cells treated with (a) PBS and 50 μM FBBBE (b) 1 μg/mL LPS for 24 h and 50 μM FBBBE (c) 100 μM H2O2 for 1 h and 50 μM FBBBE (d) PBS and 50 μM FBn (e) 1 μg/mL LPS for 24 h and 50 μM FBn (f) 100 μM H2O2 for 1 h and 50 μM FBn.
Tissue Imaging Experiments
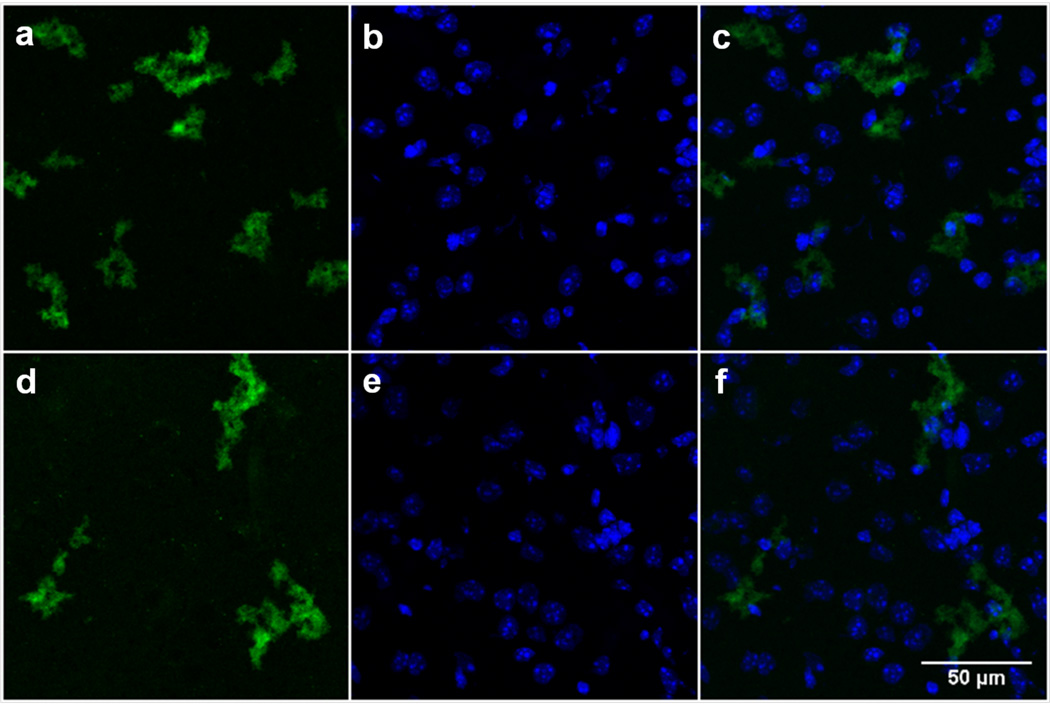
The results obtained from the cellular studies inspired us to extend our studies to a more physiologically relevant model for H2O2 detection. H2O2 is particularly active in the brain and neuronal tissue, triggered by the metabolism of neurotransmitters.[28] The brain and spinal cord from 3–4 week old female C57BL/6J mice were excised, frozen in OCT medium, and 10 µm sagittal cryosections were prepared for staining with FBBBE and FBn. At this age, the mice are undergoing extensive neuronal development and remodeling, which is associated with higher levels of neurotransmitter activity and ROS in both neurons and microglial cells in the brain. Consistent with the previous experiments, the sections were either incubated with PBS (unstimulated) or H2O2 (Figure 6). Similar to the cell studies, endogenous levels of H2O2 were sufficient to activate the probe (FBBBE), and the exogenous addition of H2O2 did not alter the fluorescence levels observed (Figure 6a, d). The probe appears to be activated intracellularly, based on its proximity to a nuclear stain (ESI, Figure 6c, f). Quantitative analysis of FBBBE in the brain tissue treatments reveals that the mean fluorescence for tissues treated with PBS and those treated with H2O2 is not statistically different, consistent with the cellular studies (Figure S12). When identical studies using the control FBn were performed, no fluorescence turn on was observed (Figure S13). Additionally, control studies in the absence of the probe showed no fluorescence.
Figure 6.
Confocal fluorescence images of H2O2 in mouse brain treated with FBBBE for 1 h. Brain tissue treated with PBS (a–c) or 100 μM H2O2 (d–f). Images on the left (a, d) are with 50 μM FBBBE; images in the middle (b, e) are nuclear staining with DAPI (4′,6-diamidino-2-phenylindole, blue); and images on the right (c, f) are an overlay of the FBn and DAPI staining.
To further investigate whether FBBBE is activated by truly endogenous levels of H2O2, our efforts focused on evaluating the probe in the spinal cord. It is well established that the spinal cord consists primarily of white matter (myelinated axons), gray matter (axons), glial cells and some fibrous tissue with few axon terminals and activated macrophages. The presence of ROS is ubiquitous at axon terminals associated with neurotransmitter activity and metabolism.[28] Thus, in a healthy, uninjured spinal cord, the presence of ROS such as H2O2 is not typical.[27c] Spinal cord sections were either incubated with PBS (unstimulated) or H2O2, in a manner identical to the brain tissue studies. Confocal fluorescence images of the unstimulated spinal cord tissue treated with FBBBE show that the probe remains essentially inactive with a weak fluorescence signal observed (likely due to tissue autofluorescence) confirming that in areas of low ROS levels, the probe remains latent. The addition of exogenous H2O2 to the spinal cord tissue shows a minimal amount of fluorescence when compared to the brain tissue (Figure S14). The lack of fluorescence signal from the H2O2-treated samples may be due to an inability of FBBBE to penetrate the spinal cord tissues, which is then subsequently washed away before the image is taken. Alternatively, FBBBE may be getting into the spinal cord tissues, but the externally added H2O2 is impermeable to the spinal cord cells and hence unable to activate FBBBE. In both tissues it appears that the exogenous addition of H2O2 does not potentiate the fluorescence signal, implying that probe activation may be caused by endogenous H2O2 activity in the brain not present in the spinal cord. These experiments suggest that the turn-on response of FBBBE is not found indiscriminately in all tissues types.
Conclusion
In summary, we have reported the synthesis, reactivity, and imaging properties of a new class of fluorescence-based molecular probes for detecting endogenous H2O2 in biological systems. By appending the H2O2-sensitive boronic ester to a fluorophore via a benzyl ether linkage, these fluorescent probes can be accessed in a single synthetic step using two commercially available starting materials. We have demonstrated, under simulated physiological conditions, that both fluoresceinand coumarin-based probes are sensitive for the detection of H2O2 in the biologically relevant micromolar concentration range. The fluorescein-based probe was particularly effective in imaging endogenous H2O2 levels with and without stimulation in murine macrophage cells using confocal miscroscopy and ex vivo imaging of endogenous H2O2 was effectively demonstrated in mouse brain. Overall, the combined synthetic ease, stability, solubility, fast reaction kinetics, and relatively high fluorescent turn-on response of these probes in comparison to other fluorophores illustrate the potential of the compounds reported here as accessible tools for molecular imaging for a wide range of researchers interested in the chemistry and biology of H2O2.
Experimental Section
Full experimental details are described in the Supporting Information.
Supplementary Material
Acknowledgements
We thank Dr. David T. Puerta and David P. Martin (UCSD) for helpful discussions. This work was supported by a grant from the National Institutes of Health (R01 GM098435, S.M.C.; R01CA112075, M.M.), AHA 12GRNT12040467 (M.M.), and the UCSD Skaggs School of Pharmacy and Pharmaceutical Sciences. K.B.D. was supported, in part, by a National Institutes of Health Training Grant (5T32DK007233-34). The authors would like to acknowledge the UCSD Neuroscience Microscopy Core Facility funded by NIH P30 NS047101.
References
- 1.Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Nat. Rev. Mol. Cell. Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 2.a) Rhee SG. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]; b) Stone JR, Yang S. Antioxid. Redox Signaling. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]; c) D'AutrEaux B, Toledano MB. Nat. Rev. Mol. Cell. Biol. 2007;4:278. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]; d) Winterbourne CC. Nat. Chem. Biol. 2008;4 [Google Scholar]; e Dickinson BC, Chang CJ. Nat. Chem. Biol. 2011;7:504–511. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckman KB, Ames BN. Phys. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 4.a) Fruehauf JP, Meyskens FL. Clin. Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]; b) Finkel T, Serrano M, Blasco MA. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]; c) Ishikawa K. Science. 2008;321:342–342. [Google Scholar]
- 5.Sugamura K. Free Radical Biol. Med. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnham KJ, Masters CL, Bush AI. Nat. Rev. Drug Disc. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 7.a Houstis N, Rosen ED, Lander ES. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]; b Jay D, Hitomi H, Griendling K. Free Radical Biol. Med. 2006;40 doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 8.a Lim MH, Lippard SJ. Acc. Chem. Res. 2007;40:41–51. doi: 10.1021/ar950149t. [DOI] [PubMed] [Google Scholar]; b McQuade LE, Lippard SJ. Curr. Opin. Chem. Biol. 2010;14:43–49. doi: 10.1016/j.cbpa.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 9.a) Yang D, Wang HL, Sun ZN, Chung NW, Shen JG. J. Am. Chem. Soc. 2006;128:6004–6005. doi: 10.1021/ja0603756. [DOI] [PubMed] [Google Scholar]; b) Sun ZN, Wang HL, Liu FQ, Chen Y, Tam PKH, Yang D. Org. Lett. 2009;11:1887–1890. doi: 10.1021/ol900279z. [DOI] [PubMed] [Google Scholar]
- 10.a) Maeda H, Yamamoto K, Nomura Y, Kohno I, Hafsi L, Ueda N, Yoshida S, Fukuda M, Fukuyasu Y, Yamauchi Y, Itoh N. J. Am. Chem. Soc. 2005;127:68–69. doi: 10.1021/ja047018k. [DOI] [PubMed] [Google Scholar]; b) Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Proc. Natl. Acad. Sci. U.S.A. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Xu KH, Liu X, Tang B. ChemBioChem. 2007;8:453–458. doi: 10.1002/cbic.200600392. [DOI] [PubMed] [Google Scholar]; d) Xu KH, Liu X, Tang B, Yang GW, Yang Y, An LG. Chem.- Eur. J. 2007;13:1411–1416. doi: 10.1002/chem.200600497. [DOI] [PubMed] [Google Scholar]
- 11.a) Umezawa N, Tanaka K, Urano Y, Kikuchi K, Higuchi T, Nagano T. Angew. Chem. Int. Ed. 1999;38:2899–2901. doi: 10.1002/(sici)1521-3773(19991004)38:19<2899::aid-anie2899>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]; b) Song B, Wang GL, Tan MQ, Yuan JL. J. Am. Chem. Soc. 2006;128:13442–13450. doi: 10.1021/ja062990f. [DOI] [PubMed] [Google Scholar]
- 12.Lippert AR, De Bittner GCV, Chang CJ. Acc. Chem. Res. 2011;44:793–804. doi: 10.1021/ar200126t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du LP, Li MY, Zheng SL, Wang BH. Tetrahedron Lett. 2008;49:3045–3048. doi: 10.1016/j.tetlet.2008.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickinson BC, Huynh C, Chang CJ. J. Am. Chem. Soc. 2010;132:5906–5915. doi: 10.1021/ja1014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a) Miller EW, Albers AE, Pralle A, Isacoff EY, Chang CJ. J. Am. Chem. Soc. 2005;127:16652–16659. doi: 10.1021/ja054474f. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dickinson BC, Peltier J, Stone D, Schaffer DV, Chang CJ. Nat. Chem. Biol. 2011;7:106–112. doi: 10.1038/nchembio.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Major Jourden JL, Cohen SM. Angew. Chem. Int. Ed. 2010;49:6795–6797. doi: 10.1002/anie.201003819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a) Sella E, Shabat D. Chem. Commun. 2008:5701–5703. doi: 10.1039/b814855d. [DOI] [PubMed] [Google Scholar]; b) Blencowe CA, Russell AT, Greco F, Hayes W, Thornthwaite DW. Polym. Chem. 2011;2:773–790. [Google Scholar]
- 18.Kratz F, Muller IA, Ryppa C, Warnecke A. ChemMedChem. 2008;3:20–53. doi: 10.1002/cmdc.200700159. [DOI] [PubMed] [Google Scholar]
- 19.Lee HY, Jiang X, Lee DW. Org. Lett. 2009;11:2065–2068. doi: 10.1021/ol900433g. [DOI] [PubMed] [Google Scholar]
- 20.Major Jourden JL, Daniel KB, Cohen SM. Chem. Comm. 2011;47:7968–7970. doi: 10.1039/c1cc12526e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Gracia Lux C, Joshi-Barr S, Nguyen T, Mahmoud E, Schopf E, Fomina N, Almutairi A. J. Am. Chem. Soc. 2012;134:15758–15764. doi: 10.1021/ja303372u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kielar F, Helsel ME, Wang Q, Franz KJ. Metallomics. 2012;4:899–909. doi: 10.1039/c2mt20069d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van de Bittner GC, Dubikovskaya EA, Bertozzi CR, Chang CJ. Proc. Natl. Acad. Sci. U.S.A. 2010;107:21316–21321. doi: 10.1073/pnas.1012864107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goncalves MST. Chem. Rev. 2009;109:190–212. doi: 10.1021/cr0783840. [DOI] [PubMed] [Google Scholar]
- 25.Sella E, Lubelski A, Klafter J, Shabat D. J. Am. Chem. Soc. 2010;132:3945–3952. doi: 10.1021/ja910839n. [DOI] [PubMed] [Google Scholar]
- 26.Zielonka J, Sikora A, Hardy M, Joseph J, Dranka BP, Kalyanaraman B. Chem. Res. Toxicol. 2012;25:1793–1799. doi: 10.1021/tx300164j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.a) Emre Y, Hurtaud C, Nubel T, Criscuolo F, Ricquier D, Cassard-Doulcier AM. Biochem. J. 2007;402:271–278. doi: 10.1042/BJ20061430. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mantovani A, Sica A, Locati M. Eur. J. Immunol. 2007;37:14–16. doi: 10.1002/eji.200636910. [DOI] [PubMed] [Google Scholar]; c) West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. Nature. 2011;472:U476–U543. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uttara B, Singh AV, Zamboni P, Mahajan RT. Curr. Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.