Abstract
We assessed the cortical processing of self-movement stimuli in aging and Alzheimer’s disease (AD). Our goal was to identify distinguishing effects on neural mechanisms related to driving and navigation. Young (YNC) and older (ONC) normal controls, and early AD patients (EAD) viewed real-world videos and dot motion stimuli simulating self-movement scenes. We recorded visual motion event related potentials (VMERPs) to stimulus motion coherence and speed. Aging delays motion evoked N200s, whereas AD diminishes response amplitudes. EADs respond to increments in motion coherence, but they are uniquely unresponsive to increments in motion speed that simulate accelerating self-movement. AD related impairments of self-movement processing may have grave consequences for driving safety and navigational independence.
Keywords: aging, Alzheimer’s disease, vision, motion, event related potentials
INTRODUCTION
Navigational impairments limit the quality of life in aging and Alzheimer’s disease (AD). These impairments limit wayfinding, even in familiar environments, leading to the withdrawal from driving and independent living.
Autonomous navigation greatly relies on the visual processing of optic flow, the patterned visual motion that accompanies self-movement (Gibson, 1950). We previously found that AD patients, and a subset of neuropsychologically intact older adults, have selectively increased perceptual thresholds for optic flow (O’Brien, et al., 2001). The critical role of optic flow processing in the navigational impairments of aging and AD has been supported by combining the psychophysical analysis of optic flow perception with behavioral assessments of real-world navigation (Monacelli, et al., 2003) (Mapstone, et al., 2003). Those studies suggest that navigational impairments in aging and AD reflect cortical deficits of optic flow processing that block access to the visual cues about self-movement heading and speed.
Visual motion processing has been previously studied by using event related potentials (ERPs) evoked by horizontally moving dot patterns or gratings in normal subjects (Bach and Ullrich, 1994) (Kubova, et al., 1990). Such stimuli evoke a negative wave peaking at around 200 ms after motion onset (N200 response). We later modified these techniques by using random dot stimuli that simulate the radial pattern of optic flow and were able to evoke posterior N200s in older adults and AD patients, and relate them to navigational deficits in AD (Kavcic, et al., 2006). Furthermore, linking these deficits to responses evoked by the sudden presentation of a stationary dot pattern revealed cortical hyper-responsiveness in our most mildly impaired patients, raising the possibility that early hyper-responsiveness might be a precursor to more profound deficits (Fernandez, et al., 2007).
To better understand the neural mechanisms and behavioral implications of navigational impairments in aging and AD we varied the motion coherence and speed in real-world video clips of optic flow. Those pilot studies are followed by detailed analysis of responsiveness to simulated optic flow. We find that coherence and speed have different effects on motion responses highlighting behaviorally important distinctions between aging and early AD.
METHODS
Subject Groups
We studied young normal controls (YNC), older normal controls (ONC), and patients with early Alzheimer’s disease (EAD) with normal, or corrected to normal, vision: YNCs were local undergraduates. ONCs were from programs for healthy elderly or patient’s spouses. EADs were from the University of Rochester’s clinical programs and met NINCDS-ADRDA criteria for probable AD (McKhann, et al., 1984) with functionally significant memory impairment by history and testing (Table 1), and aphasia, agnosia, apraxia, inattention, or executive incapacity. Informed consent was obtained from all subjects before enrollment. All procedures were approved by the University’s Research Subjects Review Board.
Table 1.
Demographic and neuropsychological characteristics of older normal control subjects (ONC) and early Alzheimer’s disease patients (EAD) enrolled in the dot motion dynamics neurophysiological studies. The ages of subjects in these groups were not significantly different. Their neuropsychological characteristics are consistent with group assignment.
| Older Adults, n=16 | Alzheimer’s Disease, n=15 | |||
|---|---|---|---|---|
|
| ||||
| Mean | SD | Mean | SD | |
| Age, y | 76.2 | 10 | 78.6 | 8 |
| Education, y | 16.6 | 4 | 14.7 | 3 |
| OS Visual acuity (20/x) | 31.8 | 20.1 | 33.5 | 20.6 |
| OD Visual acuity (20/x) | 27.0 | 7.7 | 28.9 | 7.8 |
| Contrast sensitivity (20/x)* | 29.7 | 14.7 | 34.7 | 19.3 |
| MMSE(Max. 30)** | 28.9 | 1.1 | 25.2 | 2.5 |
| Money Road Map (max. 32)* | 29.4 | 3.0 | 25.8 | 3.6 |
| Figural Memory (max. 10)** | 7.1 | 1.4 | 5.2 | 1.5 |
| Verbal Fluency (Normal > 12)** | 17.6 | 3.3 | 10.8 | 4.9 |
| Delayed Recall (max. 8)** | 6.8 | 1.2 | 3.6 | 1.8 |
| Categorical Naming** | 20.6 | 6.0 | 12.6 | 3.3 |
| Line Orientation (max. 30) | 23.8 | 3.9 | 21.6 | 7.1 |
| Facial Recognition (max. 54) | 46.7 | 3.5 | 45.2 | 6.1 |
p < 0.05,
p < 0.005
Neurophysiological Experiments
Visual neurophysiological studies were controlled by the REX (real-time experimental) system (Hays, et al., 1982) running on RTOS-QNX for PCs to control stimuli, displays, monitor eye position, and create data files. Subjects sat facing a rear-projection tangent screen’s 60° × 40° image while they maintained centered fixation (+/−10°) on a spot at the center of the screen under eye and head position monitored using infrared oculometry (ASL, Inc.). Subjects used their left and right index fingers to press two buttons.
Real-World Video Stimuli
Video sequences were recorded from a vehicle moving at 20 mph using a 30 frame/s digital video camera and edited Final Cut Express (Apple, Inc.) and presented using QuickTime (Apple, Inc.). Each stimulus transition was marked by the white squares on the upper left corner, outside the subject’s field of view, and detected by photocells and sent to NeuroScan (Compumedics, Inc.) as event markers.
All movies began with 1s of a gray screen with centered red fixation spot which was followed alternating cycles of 400 ms stationary images and 400 ms motion stimuli. After the last motion stimulus an additional stationary image was presented which switched to gray scale in a randomly selected 50% of the trials to prompt the subject’s button press response to maintain the subject’s sustained attention. Trials were separated by a 3 s blank screen as a fixation break.
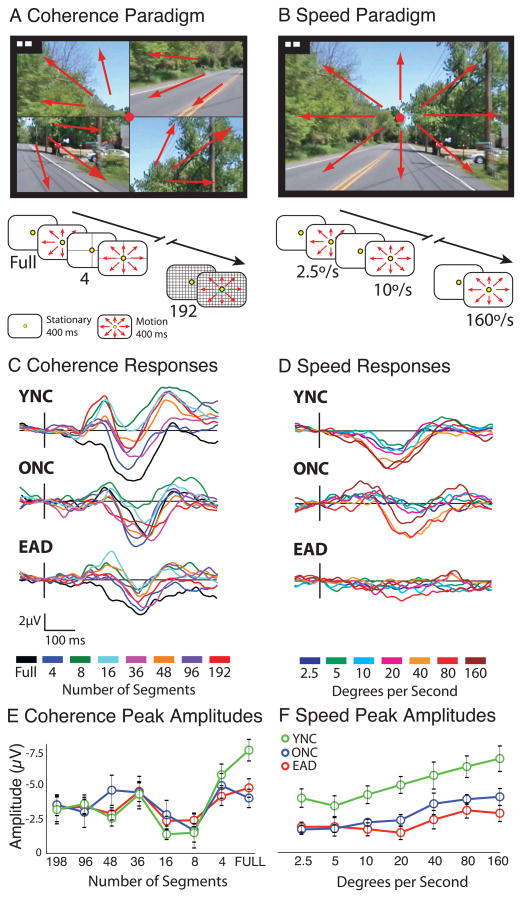
To study real-world visual motion effects we created two types of video stimuli like those seen in the random dot simulations: pattern coherence and motion speed. The motion coherence paradigm (Figure 1A) consisted of trials in which the self-movement video was presented over a period of 11 s with increasing image coherence. The coherence changes were created by importing each video frame into Image Ready (Adobe, Inc.) and dividing the frames in to a selected number of equal sized rectangular segments which were then randomly re-positioned in the image. The same segmental re-positioning was applied to all 12 frames in a 400 ms movement stimulus to maintain motion direction in each segment. A series of eight segmentation levels were presented in each trial: 192, 96, 48, 36, 16, 8, 4, and 1 (full screen).
Figure 1.
Real-world video stimulus paradigm and evoked responses. A. Coherence stimuli consisted of frames in which the image was divided into increasing numbers of equal sized, randomly repositioned, segments. A four-segment image is shown with the arrows representing the direction of local motion. B. Speed stimuli consisted of increasing speeds of simulated straight-ahead self-movement. C–D. Responses to naturalistic videos at each stimulus level (line colors) for each subject group (YNC, ONC, and EAD). C. All subject groups show clear responses at all coherence levels. D. Speed stimuli evoke clear N200s in YNCs and ONCs groups, with the fastest speeds generating the biggest amplitudes, whereas EADs show only marginal responses, regardless of stimulus speed. E–F. Line plots of mean N200 amplitudes (+/− SE) for each subject group (line color) in the coherence (E) and speed (F) stimulus paradigms. N200 response amplitudes differed across coherence, speed, and subject group. The most fragmented coherence (198–36 segments) evoked moderate responses in all groups, whereas stimuli with fewer fragments evoked a range of responses from low amplitudes (16–8 segments) to higher amplitudes (4–1 segment). The responses to speed stimuli increased in amplitude with increasing speed in all groups.
The motion speed paradigm (Figure 1B) consisted of trials in which the self-movement video was presented over a period of 10 s with increasing image speed. The speed changes were created by importing each video frame into Image Ready (Adobe, Inc.) and deleting selected frames to create a change in the apparent rate of self-movement in the re-assembled video. The resulting motion stimuli were each 500 ms in duration. Seven levels were presented having average motion speeds of: 2.5, 5, 10, 20, 40, 80, or 160 °/s.
Dot Motion Simulated Optic Flow Paradigm
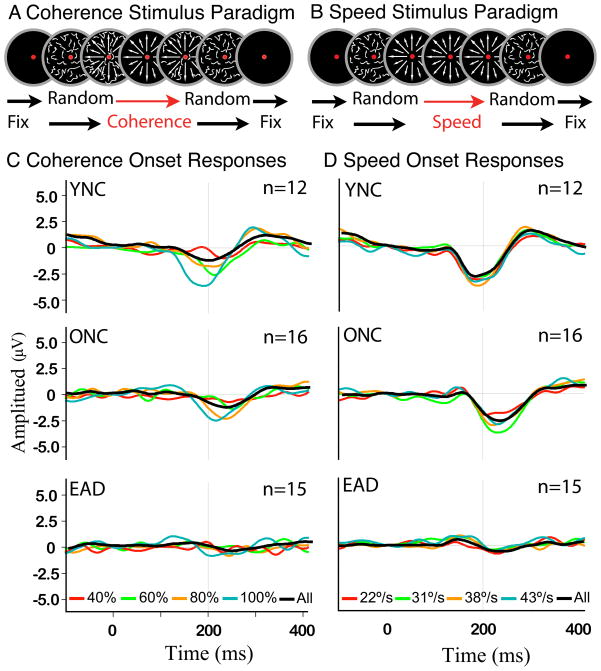
Visual motion event related potentials (VMERPs) were recorded in motion coherence and speed paradigms both with alternating radial optic flow (500 ms) and random dot motion (500 ms). Optic flow is simulated by the radial displacement of dots from the center to the periphery of the stimulus field. In the coherence paradigm (Figure 2A), optic flow was presented at five coherence values (20%, 40%, 60%, 80%, and 100%), each representing the percentage of dots forming the optic flow pattern, with the remaining dots moving in random directions. All optic flow stimuli had a centered focus of expansion (FOE) with an average dot speed of 31°/s.
Figure 2.
A–B. Animated motion sequences composed of white dots on a black background were used to simulate self-motion, while subjects maintained fixation on a red dot target placed in the center of the screen. A. Coherence trials began with zero coherence motion (fully random), followed by a series of patterned motion stimuli, and ended with vertical motion (not shown) to prompt a button press response. B. Speed trials began with zero coherence motion, followed by a series of speed stimuli, and terminated by 100% coherence vertical motion with randomly assigned upward or downward direction to prompt a button press (not shown). C–D. Group averages of optic flow responses to all stimuli of the specified motion coherence or dot speed (line color) that were immediately preceded by a 0% coherence stimulus; the black line is the grand average across all conditions in that study. Optic flow evoked large N200 responses in young subjects, somewhat smaller and delayed responses in older adults, and substantially smaller responses in AD patients for all coherence and speed conditions. C. N200 amplitudes increase and latencies decrease with higher motion coherence. D. Dot speed at onset of optic flow had no effect on N200 amplitude or latency.
In the speed paradigm (Figure 2B), each optic flow was presented at five speeds (11, 22, 31, 38, or 43°/s) with a centered focus of expansion (FOE) and 100% motion coherence. The slowest speed did not evoke clear VMERPs in any subject group and is omitted from the analysis.
Trials for both the speed and coherence paradigms began with a black screen with a red dot for central fixation, followed by 9 to 15 stimuli consisting of either optic flow or random motion, and ended with a vertical dot motion stimulus that cued a push button response as a measure of the subject’s sustained attention: Subjects pressed the upper button for upward dot motion and the lower button for downward motion (Supplementary Figure 1).
We used Kautz random sequences (Rosenfeld, 2002a, Rosenfeld, 2002b) with a repetition span of s*(s−1)(q−1), where s is the number of test stimuli and q is the sequence’s memory span. Kautz sequences combine all selected stimuli as predecessors and successors of all others to the limit of the specified memory span, providing good single and multiple-wise distributions of the stimuli.
Neurophysiological Recordings
Scalp EEG was sampled at 500 Hz with 32 bit resolution using NeuroScan Labs (Compumedics, Inc.) equipment, and electrode caps (Quik-Cap; Compumedics, Inc.) designed according to the International 10–20 System with 13 additional electrodes. Low and high pass filter settings were100 Hz and 0.1 Hz, respectively. The cutoff frequencies for these filters was set at 3 dB down; the roll off was 12 dB per octave at both sides. Impedances were maintained below 5 kΩ for each channel and balanced across all channels within a 2 kOmega; range.
Offline analysis in Scan 4.3 (Compumedics, Inc.) included ocular artifact reduction (ARTCOR) and band-pass filtering (0.1 and 50 Hz) with visual artifact rejection. Waveform components were measured by first deriving the peak latency in the grand average responses for each group across all levels (all coherences or all speeds) for each stimulus condition. We then measured the peak deflection at that latency +/− 50 ms for each subject, at each stimulus level, and in each stimulus condition. In parallel analyses, we derived group averages based-on all subjects and based-on all subjects who showed good performance in the embedded visual attentional catch trial task.
Peak amplitudes and latencies for all components from selected recording sites were entered into mixed measures ANOVAs with group (YNC, ONC, EAD) as a between subjects factor, and levels of each of the respective stimulus conditions as a within subjects factor with post-hoc Tukey’s Honestly Significant Differences (THSDs). [SPSS 2010]
RESULTS
Real World Visual Motion ERPs
Recorded scenes of vehicular self-movement were manipulated to vary motion coherence and speed and presented to a series of consecutive subjects: YNCs (n = 9; age = 29.33+/− 8.5), ONCs (n = 5, age = 70.6 +/− 6.4, MMSE = 29.4 +/− .9), and patients with EAD (n = 6, age = 73.2 +/− 6.3, MMSE = 28.0 +/− 3.1). Coherence was altered by dividing each frame into a number of image fragments (192 to 1) (Figure 1A). Speed was altered by changing the display to produce a average motion speeds (2.5, 5, 10, 20, 40, 80, and 160 °/s) (Fig. 1B). The complete set of coherence and speed stimuli were presented as ordered series of increasing motion stimulus parameters.
Figure 1C shows group average waveforms for increasing coherence. These responses are characterized by a tri-phasic morphology with a prominent negative deflection (N200), flanked by positive components. The N200 response is largest at high coherences as confirmed by a significant main effect of coherence level (F 7,159 = 4.135, p < 0.001) with no significant group effects (F 2,160= 2.43, p < 0.687) or interactions effects (F 14,160= 8.84, p < 0.176).
Increasing speeds also evoke N200s with higher speeds generating larger responses (Figure 1D). These data yield significant main effects of group (F 2,146= 28.85, p < 0.001) and stimulus speed (F 6,146 = 4.57, p < 0.001), with the YNCs showing larger responses at all speeds and ADs showing barely visible responses (THSD: YNC > ONC = EAD, p < .001) but no group-by-speed interactions (F 12,146 = 4.63, p = 0.351).
The unexpected differences between coherence and speed effects in this pilot study show that optic flow seen during real-world vehicular self-movement evokes robust N200 responses, with increasing coherence evoking large responses in all groups (Figure 1E) and increasing speeds evoking large responses in all but EADs (Fig. 1F). These results prompted studies using more well-defined random dot kinematogram stimuli in a larger number of subjects.
Stimulus Onset Responses
To better understand the effects of optic flow coherence and speed on N200 responses, we studied the effects of dot motion coherence and speed on optic flow evoked N200 responses in 12 YNCs, 16 ONCs, and 15 EADs. Comparisons of test scores from ONC and EAD subjects revealed significant (two-tailed t-test) group differences that are consistent with group assignment (Table 1). Highly significant differences between ONCs and EADs reflected the EAD’s impairment in figural and verbal memory, fluency and naming that are commonly described (Becker, et al., 1988, Cummings, 2000). Although visual acuity was similar in the ONC and EAD groups, the EADs had poorer contrast sensitivity, which is consistent with previous reports (Gilmore and Whitehouse, 1995, Lakshminarayanan, et al., 1996).
VMERP responses recorded at CPZ were the most representative of effects on optic flow N200s and we focused our analysis on those data. We first examined the effects of motion coherence on optic flow responses by presenting a series of stimuli in which a specified percentage of dots move coherently to form the radial pattern of optic flow with the remaining dots move in randomly assigned directions. The group averaged responses show larger and faster N200s with higher motion coherences and faster motion speeds (Figure 2C and D). The small N200s of EADs require a standard approach to measuring response amplitudes and latencies across groups. We used N200 peak latency from the grand average responses for each group in each condition to find the peaks for each subject at that latency +/−50ms. The amplitude and latency values for each subject were then used for quantitative analyses of the coherence and speed data.
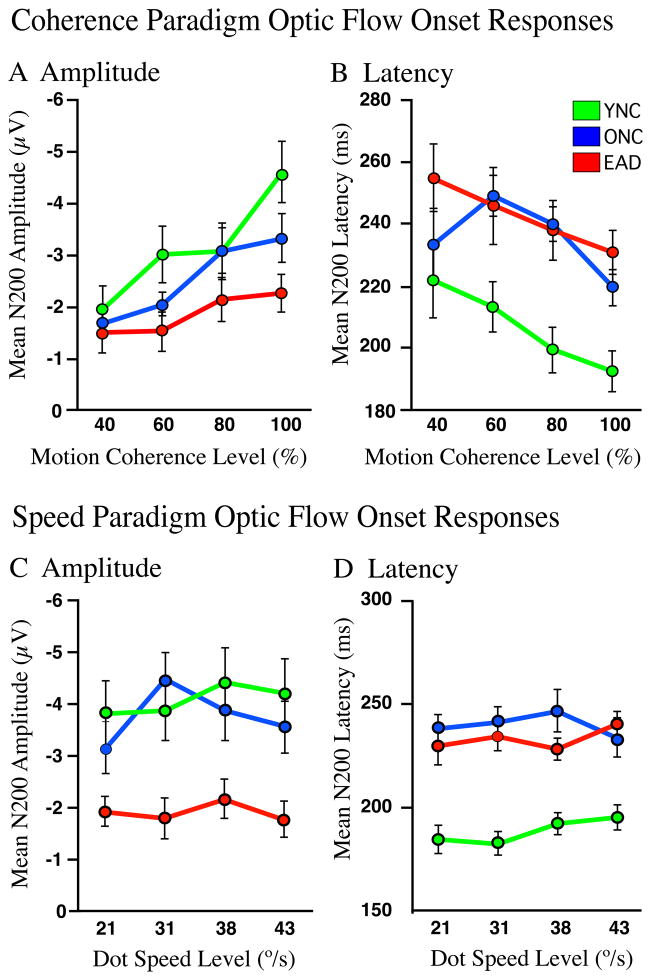
Higher motion coherences evoke larger optic flow N200s with shorter latencies in all groups (Figures 3A and B). This is confirmed by a significant main effect of coherence (F 3,171 = 12.8, p < .005) and group (F 2,171 = 6.89, p < .005) on N200 amplitude with smaller responses in EADs (THSD: YNC > AD, p < .001; ONC > EAD, p < 005). All three subject groups show the same pattern without significant interactions (F 6,171 = 1.027, p=0.410). Because of the variability, N200s latencies do not yield a significant main effect of coherence (F 3,171 = 3.85, p < .05), but there is a significant group effect (F 2,171 = 15.36, p < .005) with YNCs showing faster responses (THSD: YNC < ONC, p < .05; YNC < EAD, p < .001), again without significant interaction effects (F 6,171 = .632, p = 0.705). Thus, higher coherences yield larger N200s with the responses of EADs being smaller, and both ONCs and EADs being delayed.
Figure 3.
Mean peak amplitudes and latencies of N200 responses evoked by radial motion onset at each coherence or speed for each subject group (line color). A–B. Higher coherence levels generate larger amplitude and shorter latency responses in all groups. YNC’s responses are faster at all coherence levels compared to both ONCs and EADs. C–D. Faster speeds at motion onset did not result in significant differences in either amplitude or latency for any group. N200 amplitude clearly separated the EAD from the ONC and YNC groups, and latency separated the old from the young.
The speed paradigm evoked optic flow N200s in all groups (Figures 3C and D), with no main effect of speed on either amplitude (F 3,183 = 0.47, p < 0.704) or latency (F 3,183 = 0.375, p = 0.771). However, there are significant group effects on N200 amplitude (F 2,183 = 25.02, p < 0.001) with YNCs and ONCs yielding larger responses than EADs (THSD: YNC = ONC > EAD, p < .001) without group by speed interaction (F 6,183 = 0.58, p = 0.748). There are also significant group effects on N200 latency (F 2,183 = 48.39, p < 0.001) with YNCs generating faster responses at all speeds (THSD: YNC < ONC = EAD, p < .001) and no significant interactions (F 6,183 = 0.58, p = 0.742).
Thus, coherence and speed stimuli evoke very different response profiles: increasing optic flow coherence elicits graded increases in N200 amplitude and decreases in N200 latency. In contrast, increasing speed elicits only group differences with EADs showing smaller N200 amplitudes and both ONCs and EADs showing greater N200 latencies.
Stimulus Increment Responses
We analyzed instances in which coherence or speed was incremented across consecutive stimuli. Decremental changes in coherence and speed were also examined, but found to evoke only small N200s that did not distinguish subject groups and are not considered further.
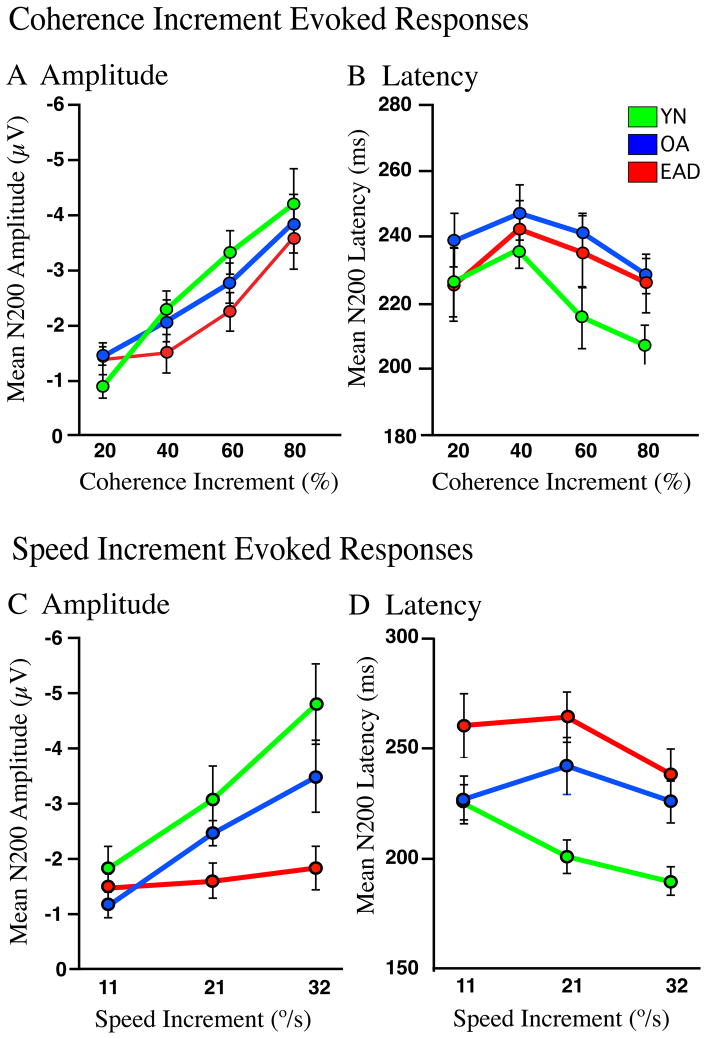
In the coherence paradigm, larger increments in stimulus coherence generate larger responses in all groups with a significant main effects of increment size (F 3,171 = 22.53, p < .005) but not of group (F 2,171 = 1.45, p = .238) and without increment by group interactions (F 6,171 = 0.693, p = .656) (Figure 4A). Larger coherence increments also evoke faster responses (F 3,171 = 3.152, p < .05), with YNCs showing the shortest latencies (THSD: YNC < EAD, p < .05) without increment by group interactions (F 6,171 = 0.350, p = .909) (Fig. 4B). Thus, larger coherence increments evoke larger and faster N200s in all groups.
Figure 4.
Mean peak amplitudes and latencies of N200 responses evoked by coherence (A–B) and speed (C–D) increments for each subject group (line color). A–B. Higher coherence increments generate larger amplitude and shorter latency responses in all groups. C–D. EADs are insensitive to the magnitude of speed increments, while YNCs and ONCs show larger responses to bigger increments. N200 latencies separate YNCs from both ONCs and EADs.
N200 amplitude and latency were also affected by speed increment size, but with a very different pattern of effects. Larger accelerations generate larger responses, with significant main effects of acceleration (F 3,131 = 13.59, p < .001) and group (F 2,131 = 9.68, p < .001) with the largest responses in YNCs (THSD: YNC > EAD, p < .001) (Figure 4C). Acceleration by group interactions are not significant across all three groups (F 6,131 = 2.353, p = .058) but are between EADs and YNCs (F 3,84 = 3.97, p = .023) or ONCs (F 3,87 = 2.159, p = .034). N200 latency is not greatly affected by acceleration size (F 4,131 = 2.95, p = .056) but there is a significant group effect (F 2,131 = 14.67, p < .005) with differences between all groups (THSD: YNC < EAD, p < .001; YNC < EAD, p < .05; ONC < EAD, p < .05) without significant interactions (F 6,131 = 1.07, p = 374) across any groups (Fig. 4D).
These results demonstrate that coherence increment stimuli can evoke near normal N200s in all subject groups, including EAD patients. In contrast, speed increment stimuli evoke very different responses across subject groups with large N200s in YNCs and ONCs but nearly no responses in EADs.
Linking Behavior and Evoked Potentials
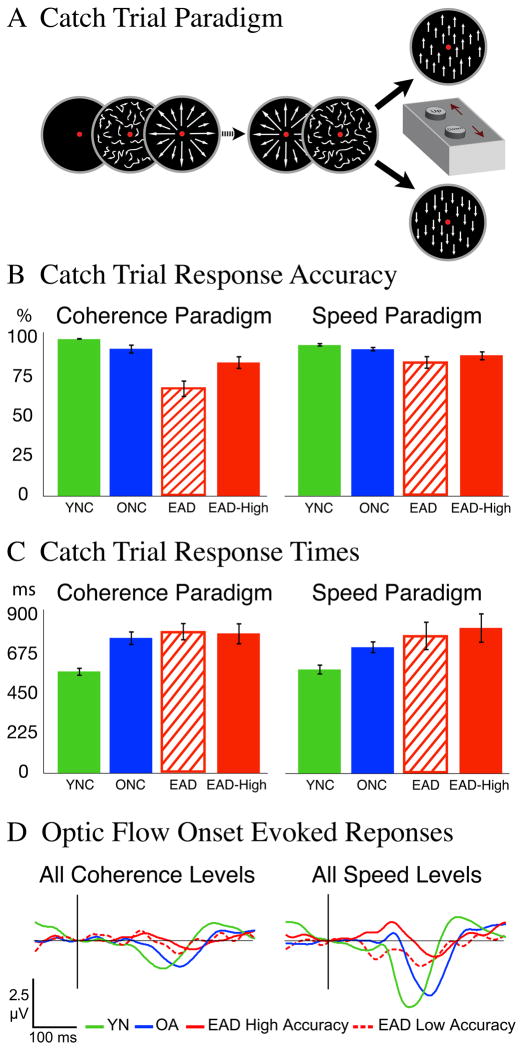
After a randomly selected number (9 to 15) of speed or coherence stimuli, each trial ended with a vertical dot motion stimulus having a duration that varied between 33 and 500 ms. Subjects were instructed to press the more distant of two buttons when they saw upward motion and the closer button when they saw downward motion (Figure 5A). This focused subjects on the visual motion display and provided measures of attention to the stimuli during each study.
Figure 5.
A behavioral catch paradigm was used to measure of sustained attention in all trials. A. Catch stimuli were presented after 9 to 15 optic flow stimuli and consisted of either upward or downward vertically moving dots with 100% coherence. Subjects pressed the far button for upward motion and the near button for downward motion. B. Response accuracy was measured as the percentage of correct responses across all coherence and speed conditions. Several EADs showed poor task performance and were omitted from a higher accuracy EAD sub-group. YNC subjects showed the greatest accuracy, with decreasing performance from ONCs and EADs. C. Response times (ms) were calculated as the interval between catch stimulus onset and button press. YNC subjects showed the shortest times, with increasing times from ONCs and EADs. D. Averaged N200s from all groups and the higher accuracy EAD sub-group show that group differences are not attributable to attentional differences.
Performance accuracy (percent correct) and response times (latency in ms) were measured. EADs were less accurate across all catch trial durations and had the slowest reaction times. A two way MANOVA of percent correct and reaction times showed a main effect of group (F4, 58 = 6.0, p<0.001), with post hoc analysis showing more accurate performance in YNC and ONC subjects compared to EAD patients (THSD: YNC = ONC; YNC > AD, p<0.001; ONC > AD, p<0.005). Reaction times were faster in YNC subjects compared to EAD patients (p = 0.02), whereas the ONC’s reaction times did not differ from either the YNCs or EADs.
There were no significant differences in performance on catch trials from the coherence and speed paradigms (group F2, 69 = 6.78, p = .002; paradigm F1, 69 = 1.24, p = .73; interaction F4, 58 = .01, p = .91). However, individual task performance records showed that four EADs did poorly in the coherence study and three did poorly in the speed study; only one subject did poorly in both. Since the task was the same in both studies, we infer that poor performance sessions constitute lapses in attention; although the EAD who did poorly in both studies may be unable to sustain attention. We separated these subjects to create two EAD sub-groups, creating a high performance EAD sub-group that was more comparable to ONCs (Figure 5B and C).
Comparison of the N200s from high performance EADs showed all of the same effects differences seen in the full group comparisons described above (Figure 5D): Coherence motion onset amplitude: group p = .004, condition p = .001, interaction p = .621; Coherence motion onset latency group p < .001, condition p = .032, interaction p = .180; Speed motion onset amplitude: group p < .001, condition p = .110, interaction p = .980; Speed motion onset latency group p < .001, condition p = .970, interaction p = .710; Coherence increment amplitude: group p = .005, condition p < .001, interaction p = .255; Coherence increment latency group p = .001, condition p = .117, interaction p = .297; Speed increment amplitude: group p < .001, condition p < .001, interaction p = .045; Speed increment latency group p < .001, condition p = .007, interaction p = .033.
Nevertheless, there are significant correlations between coherence onset N200 amplitudes and both catch trial accuracy (r2 = .48, p = .004) and response times (r2 = −.48, p = .004), with similar results for speed onset N200 amplitudes across subject groups but not within groups. There are no significant correlations between catch trial performance and the coherence or speed increment N200s.
Together, these findings suggest that inattention does not account for the smaller motion onset responses of EADs. EADs with good attentional performance yield smaller N200s than ONCs with comparable performance. For our current purposes it is more notable that attentional effects do not seem to contribute substantially to the coherence and speed increment responses.
DISCUSSION
Naturalistic Self-Movement Processing
Video clips from vehicular transit evoked N200s in all subject groups, smallest in EADs (Figure 1) much like optic flow N200s to moving dot stimuli (Kavcic, et al., 2006) (Fernandez, et al., 2007) or sinusoidal gratings (Kubova, et al.). Unlike dot motion stimuli, naturalistic self-movement evokes tri-phasic responses that may be related to the addition of object and layout cues. Importantly, our image segmentation undermines those cues as well as the global motion pattern, shifting image analysis to a finer spatial scale (Hirai and Kakigi, 2008, Kaneoke and Kakigi, 2006).
YNC subjects’ larger responses to fully coherent optic flow is consistent with their robust global pattern processing, whereas ONCs and EADs may rely more on local motion processing. Our psychophysical analyses of optic flow perception shows that removing the utility of local motion undermines performance in AD patients (O’Brien, et al., 2001). Such a shift from global to local motion processing in AD may be related to a loss of cortico-cortical interconnectivity across dorsal extrastriate motion processing areas that might undermine directional interactions across the visual field (Hof, et al.). We should emphasize that the EADs in this study are neuropsychologically typical and that they were in no way selected for exhibiting visual processing impairments. These observations prompted our further investigation of impaired processing of patterned visual motion in EAD.
Coherence and Speed Processing
Greater motion coherence evokes larger and faster optic flow N200s, especially in YNCs. These effects are consistent with greater motion coherence evoking larger planar motion N200s in young subjects (Kaneoke, et al., 2009, Niedeggen and Wist, 1999), extending that finding across aging and early AD. We also confirm that AD is associated with smaller optic flow N200s (Fernandez, et al., 2007, Kavcic, et al., 2006), with comparisons between YNCs and ONCs showing that aging has little effect on N200 amplitude but substantially increases latency. The marked response diminution in EADs distinguishes these patients from ONCs and requires the use of a grand average latency based approach to response measurement as the N200s are indistinct in some patients.
The poorer catch trial performance of some EADs suggests that inattention may contribute to their smaller and slower responses, but omitting poor responders from the analyses does not alter the effects. Thus, we conclude that aging delays optic flow N200s, whereas AD reduces their amplitude.
Optic flow speed evokes remarkable group differences, with similarly large N200s in YNCs and ONCs, and small responses in EADs. In contrast, speed evoked N200 latencies primarily reflect aging, with YNCs having short latencies, and both ONCs and EADs have long latencies (Figure 2). We find that optic flow speed does not substantially impact N200s, whereas planar motion evokes larger and faster N200s (Kaneoke and Kakigi, 2006, Kubova, et al., 1990, Nakamura, et al., 1999, Snowden, et al., 1995). The diminished contrast sensitivity of our EADs might have contributed to their smaller N200s reflecting the overlap of retino-geniculate pathways for contrast and motion processing (Gilmore and Whitehouse, 1995, Horswill and Plooy, 2008). However, contrast has generally been found to have little effect on motion ERPs (Bach and Ullrich, 1997, Kubova, et al., 1995). We consider that factors as disparate as contrast sensitivity and attention variability interact with the uniqueness of radial optic flow simulating self-movement, our use of a wide-screen 60° × 40° display, and the transition from random motion to 100% coherence to create the group differences in our studies.
A critical finding is that alternative approaches to changing the strength of visual motion, by changing its coherence versus its speed, yield very different results. While motion coherence has robust effects on N200s in all age groups, motion speed does not. This is consistent with our psychophysical evidence that coherence changes have unique effects, especially in AD. These changes are not seen with manipulations of the spatio-temporal composition of 100% coherent optic flow (Kavcic, et al., 2011). Thus, our current neurophysiological results are consistent with recent and prior psychophysical findings that highlight the apparent uniqueness of motion coherence effects on self-movement perception in aging and AD.
Responses to Stimulus Increments
Coherence and speed increments reveal further distinctions between aging and AD: In YNCs and ONCs, coherence and speed increments evoke robust N200s proportionate to increment size. In EADs, coherence increments evoke the largest responses seen in these patients, confirming that neuronal responsiveness persists, whereas speed increments evoke only minimal responses, suggesting a loss of response specificity. (Figure 3) These effects are not correlated with group differences in the behavioral catch trials and persist despite omitting EADs with poorer catch trial performance.
Coherence increment effects are consistent with the stronger responses of parieto-temporal cortical neurons to more coherent motion (Britten, et al., 1993, Heuer and Britten, 2007). The loss of responses to acceleration may reflect a loss of neuronal speed specificity in EAD (Duffy and Wurtz, 1997, Orban, et al., 1995) such that all speeds activate all neurons. Under such loss of specificity speed changes would not result in the recruitment of fresh neuronal populations that would otherwise result in the generation of larger responses such as those seen in YNCs and ONCs.
Our finding selective and contextual optic flow processing deficits in EADs has significant implications for real-world navigation. Increased risk for motor vehicle accidents is well-documented in AD (Ott, et al., 2008, Uc, et al., 2004). Age-related cognitive impairment is a strong predictor of driving cessation (Edwards, et al., 2010) with speed regulation errors correlated with crash-related injuries (Classen, et al., 2010). Optic flow is crucial for speed adjustments and braking during vehicular navigation (Bardy and Warren, 1997) (Fajen, 2007).
The EADs unresponsiveness to accelerations may reveal some of the mechanisms involved in their driving impairment and potentially help identify high-risk individuals at an earlier stage. Further work should look at possible links between neurophysiological markers and performance measures of vehicular navigation.
Acknowledgments
We gratefully acknowledge William Vaughn’s expertise in the development of the software used in these studies. We are also grateful for Teresa Steffenella’s contributions to data acquisition. This work was supported by grants from NIA (AG17596) and NEI (EY10287).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bach M, Ullrich D. Motion adaptation governs the shape of motion-evoked cortical potentials. Vision Res. 1994;34(12):1541–7. doi: 10.1016/0042-6989(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Bach M, Ullrich D. Contrast dependency of motion-onset and pattern-reversal VEPs: interaction of stimulus type, recording site and response component. Vision Res. 1997;37(13):1845–9. doi: 10.1016/s0042-6989(96)00317-3. [DOI] [PubMed] [Google Scholar]
- Bardy BG, Warren WH., Jr Visual control of braking in goal-directed action and sport. J Sports Sci. 1997;15(6):607–20. doi: 10.1080/026404197367047. [DOI] [PubMed] [Google Scholar]
- Becker JT, Huff FJ, Nebes RD, Holland A, Boller F. Neuropsychological function in alzheimer’s disease: Pattern of impairment and rates of progression. Archives of Neurology. 1988;45:263–8. doi: 10.1001/archneur.1988.00520270037018. [DOI] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. Responses of neurons in macaque MT to stochastic motion signals. Visual Neuroscience. 1993;10(6):1157–69. doi: 10.1017/s0952523800010269. [DOI] [PubMed] [Google Scholar]
- Classen S, Shechtman O, Awadzi KD, Joo Y, Lanford DN. Traffic violations versus driving errors of older adults: informing clinical practice. American Journal of Occupational Therapy. 2010;64(2):233–41. doi: 10.5014/ajot.64.2.233. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Cognitive and behavioral heterogeneity in Alzheimer’s disease: seeking the neurobiological basis. NeurobiolAging. 2000;21(6):845–61. doi: 10.1016/s0197-4580(00)00183-4. [DOI] [PubMed] [Google Scholar]
- Duffy CJ, Wurtz RH. Medial superior temporal area neurons respond to speed patterns in optic flow. Journal of Neuroscience. 1997;17(8):2839–51. doi: 10.1523/JNEUROSCI.17-08-02839.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JD, Bart E, O’Connor ML, Cissell G. Ten years down the road: predictors of driving cessation. Gerontologist. 2010;50(3):393–9. doi: 10.1093/geront/gnp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajen BR. Rapid recalibration based on optic flow in visually guided action. Exp Brain Res. 2007;183(1):61–74. doi: 10.1007/s00221-007-1021-1. [DOI] [PubMed] [Google Scholar]
- Fernandez R, Kavcic V, Duffy CJ. Neurophysiologic analyses of low- and high-level visual processing in Alzheimer disease. Neurology. 2007;68(24):2066–76. doi: 10.1212/01.wnl.0000264873.62313.81. [DOI] [PubMed] [Google Scholar]
- Gibson JJ. The Perception of the Visual World. Houghton Mifflin; Boston: 1950. [Google Scholar]
- Gilmore GC, Whitehouse PJ. Contrast sensitivity in Alzheimer’s disease: a 1-year longitudinal analysis. Optometry & Vision Science. 1995;72(2):83–91. doi: 10.1097/00006324-199502000-00007. [DOI] [PubMed] [Google Scholar]
- Hays AV, Richmond BJ, Optican LM. A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conference Proceedings. 1982;2:1–10. [Google Scholar]
- Heuer HW, Britten KH. Linear responses to stochastic motion signals in area MST. J Neurophysiol. 2007;98(3):1115–24. doi: 10.1152/jn.00083.2007. [DOI] [PubMed] [Google Scholar]
- Hirai M, Kakigi R. Differential cortical processing of local and global motion information in biological motion: an event-related potential study. J Vis. 2008;8(16):2, 1–17. doi: 10.1167/8.16.2/8/16/2/. [pii] [DOI] [PubMed] [Google Scholar]
- Hof PR, Vogt BA, Bouras C, Morrison JH. Atypical form of Alzheimer’s disease with prominent posterior cortical atrophy: a review of lesion distribution and circuit disconnection in cortical visual pathways. [Review] [105 refs] Vision Research. 1997;37(24):3609–25. doi: 10.1016/S0042-6989(96)00240-4. [DOI] [PubMed] [Google Scholar]
- Horswill MS, Plooy AM. Reducing contrast makes speeds in a video-based driving simulator harder to discriminate as well as making them appear slower. Perception. 2008;37(8):1269–75. doi: 10.1068/p5821. [DOI] [PubMed] [Google Scholar]
- Kaneoke Y, Kakigi R. Spatial integration of visual motion with separate speed and direction information. Neuroreport. 2006;17(18):1841–5. doi: 10.1097/WNR.0b013e328010a06d. 00001756-200612180-00002 [pii] [DOI] [PubMed] [Google Scholar]
- Kaneoke Y, Urakawa T, Kakigi R. Visual motion direction is represented in population-level neural response as measured by magnetoencephalography. Neuroscience. 2009;160(3):676–87. doi: 10.1016/j.neuroscience.2009.02.081. S0306-4522(09)00336-4 [pii] [DOI] [PubMed] [Google Scholar]
- Kavcic V, Fernandez R, Logan D, Duffy CJ. Neurophysiological and perceptual correlates of navigational impairment in Alzheimer’s disease. Brain. 2006;129(Pt 3):736–46. doi: 10.1093/brain/awh727. awh727 [pii] [DOI] [PubMed] [Google Scholar]
- Kavcic V, Vaughn W, Duffy CJ. Distinct visual motion processing impairments in aging and Alzheimer’s disease. Vision Res. 2011;51(3):386–95. doi: 10.1016/j.visres.2010.12.004. S0042-6989(10)00571-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubova Z, Kremlacek J, Valis M, Langrova J, Szanyi J, Vit F, Kuba M. Visual evoked potentials to pattern, motion and cognitive stimuli in Alzheimer’s disease. Doc Ophthalmol. 2010;121(1):37–49. doi: 10.1007/s10633-010-9230-5. [DOI] [PubMed] [Google Scholar]
- Kubova Z, Kuba M, Hubacek J, Vit F. Properties of visual evoked potentials to onset of movement on a television screen. Doc Ophthalmol. 1990;75(1):67–72. doi: 10.1007/BF00142595. [DOI] [PubMed] [Google Scholar]
- Kubova Z, Kuba M, Spekreijse H, Blakemore C. Contrast dependence of motion-onset and pattern-reversal evoked potentials. Vision Res. 1995;35(2):197–205. doi: 10.1016/0042-6989(94)00138-c. [DOI] [PubMed] [Google Scholar]
- Lakshminarayanan V, Lagrave J, Kean ML, Dick M, Shankle R. Vision in dementia: contrast effects. Neurological Research. 1996;18(1):9–15. doi: 10.1080/01616412.1996.11740369. [DOI] [PubMed] [Google Scholar]
- Mapstone M, Steffenella TM, Duffy CJ. A visuospatial variant of mild cognitive impairment: Getting lost between aging and AD. Neurology. 2003;60:802–8. doi: 10.1212/01.wnl.0000049471.76799.de. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS- ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Monacelli AM, Cushman LA, Kavcic V, Duffy CJ. Spatial disorientation in Alzheimer’s disease: the remembrance of things passed. Neurology. 2003;61:1491–7. doi: 10.1212/wnl.61.11.1491. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Chung HH, Graziano MS, Gross CG. Dynamic representation of eye position in the parieto-occipital sulcus. Journal of Neurophysiology. 1999;81(5):2374–85. doi: 10.1152/jn.1999.81.5.2374. [DOI] [PubMed] [Google Scholar]
- Niedeggen M, Wist ER. Characteristics of visual evoked potentials generated by motion coherence onset. Brain Res Cogn Brain Res. 1999;8(2):95–105. doi: 10.1016/s0926-6410(99)00009-9. [DOI] [PubMed] [Google Scholar]
- O’Brien HL, Tetewsky SJ, Avery LM, Cushman LA, Makous W, Duffy CJ. Visual mechanisms of spatial disorientation in Alzheimer’s disease. Cereb Cortex. 2001;11(11):1083–92. doi: 10.1093/cercor/11.11.1083. [DOI] [PubMed] [Google Scholar]
- Orban GA, Lagae L, Raiguel S, Xiao D, Maes H. The speed tuning of medial superior temporal (MST) cell responses to optic-flow components. Perception. 1995;24(3):269–85. doi: 10.1068/p240269. [DOI] [PubMed] [Google Scholar]
- Ott BR, Heindel WC, Papandonatos GD, Festa EK, Davis JD, Daiello LA, Morris JC. A longitudinal study of drivers with Alzheimer disease. Neurology. 2008;70(14):1171–8. doi: 10.1212/01.wnl.0000294469.27156.30. 01.wnl.0000294469.27156.30 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld VR. Enumerating De Bruijn sequences. Match-Communications in Mathematical and in Computer Chemistry. 2002a;(45):71–83. [Google Scholar]
- Rosenfeld VR. Enumerating Kautz sequences. Kragujevac J Math. 2002b;24:41. [Google Scholar]
- Snowden RJ, Ullrich D, Bach M. Isolation and characteristics of a steady-state visually-evoked potential in humans related to the motion of a stimulus. Vision Research. 1995;35(10):1365–73. doi: 10.1016/0042-6989(95)98716-m. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Shi Q, Dawson JD. Driver route-following and safety errors in early Alzheimer disease. Neurology. 2004;63(5):832–7. doi: 10.1212/01.wnl.0000139301.01177.35. [DOI] [PubMed] [Google Scholar]