Abstract
Purpose
We compared renal function and oncologic outcomes of parenchymal sparing ureteral resection with radical nephroureterectomy for the treatment of upper tract urothelial carcinoma confined to the ureter.
Materials and Methods
Review of a large institutional database identified 367 patients treated for primary upper tract urothelial carcinoma with radical nephroureterectomy or parenchymal sparing ureteral resection from 1994 to 2009. Patients with known renal pelvis tumors, muscle invasive urothelial carcinoma, prior cystectomy, contralateral upper tract urothelial carcinoma, metastatic disease or chemotherapy were excluded, leaving 120 patients for analysis. Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease equation. Recurrence-free, cancer specific and overall survival were estimated using Kaplan-Meier analysis.
Results
Radical nephroureterectomy was performed in 87 patients and parenchymal sparing ureteral resection in 33. Median age at surgery was 73 years in the radical nephroureterectomy group (IQR 64 –76) vs 70 years (IQR 59 –77) in the parenchymal sparing ureteral resection group (p = 0.5). The radical nephroureterectomy and parenchymal sparing ureteral resection cohorts had several disparate clinicopathological variables including preoperative hydronephrosis (80% vs 45%, p = 0.0006), stage (pT3 or greater 26% vs 9%, p = 0.01) and baseline estimated glomerular filtration rate (51 vs 63 ml/minute/1.73 m2, p = 0.009). Patients who underwent radical nephroureterectomy experienced a significantly greater decrease in estimated glomerular filtration rate after surgery (median −7 vs 0 ml/minute/1.73 m2, p <0.001). Median followup was 4.2 years. Of the patients 79 experienced cancer recurrence and 44 died (28 of upper tract urothelial carcinoma). There were no obvious differences in the rates of recurrence, cancer specific death or overall death by procedure type. However, due to the limited number of events we cannot exclude the possibility that there are large differences in oncologic outcomes by procedure type.
Conclusions
Parenchymal sparing ureteral resection is associated with superior postoperative renal function. However, the impact on cancer control cannot be determined conclusively due to the small sample size and putative selection bias.
Keywords: carcinoma, transitional cell, kidney failure, chronic, ureter
Upper tract urothelial carcinoma is a relatively uncommon malignancy that accounts for approximately 5% of all urothelial carcinomas.1 While the standard of care for the management of these tumors is radical nephroureterectomy with excision of the entire ureter and bladder cuff, various guidelines recommend the consideration of less radical interventions for patients with less aggressive UTUC.2,3
Such interventions include sparing the ipsilateral renal unit with segmental resection of the ureter and primary reanastomosis or ureteroneocystostomy, depending on the location of the lesion, called parenchymal sparing ureteral resection. Like radical nephroureterectomy, PSUR allows complete resection of the ureteral tumor with a margin of healthy ureter and periureteral tissue, and exposure for lymph node dissection. PSUR differs from RNU in that the corresponding renal unit is spared, thereby theoretically preserving renal function. The drawback to this approach is that it may result in a worse oncologic outcome. The remaining ureteral segment remains at risk for recurrence and must be surveyed regularly. Unfortunately while confronting these difficult decisions, practitioners offer guidance based on scant evidence. To summarize the functional, oncologic and survival outcomes for patients treated with PSUR and RNU we performed a retrospective review of a large institutional database.
PATIENTS AND METHODS
Patient Selection
After institutional review board approval we retrospectively reviewed prospectively collected data on 367 patients with UTUC treated with RNU or PSUR at Memorial Sloan-Kettering Cancer Center between 1994 and 2009. Of the 324 patients who underwent RNU we excluded those with known renal pelvic tumors (187) and limited our analysis to patients whose primary tumor was determined to be in the ureter, those who theoretically could have been candidates for PSUR. In addition, we excluded patients who had prior muscle invasive UC of the bladder, previous or concurrent radical cystectomy (46), contralateral or metastatic UTUC (3), or who had received prior PSUR (1), leaving 87 in the RNU group for final analysis. Similarly of the 43 patients who underwent PSUR we excluded those with prior muscle invasive UC of the bladder, previous or concurrent radical cystectomy (4), contralateral or metastatic UTUC (5) or those who had undergone prior RNU (1), leaving 33 in the PSUR group available for analysis. The type of surgical intervention chosen was based on surgeon and patient preference.
Statistical Methods
eGFR was calculated using the abbreviated Modification of Diet in Renal Disease equation, eGFR = 186 × (serum creatinine)−1.154 × (age)−0.203, multiplied by 0.742 for female patients and 1.212 for black patients.4 The eGFR measurement drawn closest to 3 months after surgery was used when comparing eGFR before and after surgery, and all postoperative measurements were determined between postoperative days 10 and 147. This timing was selected to best approximate eGFR, which would affect the delivery of adjuvant chemotherapy. Comparison of the change in renal function in the RNU and PSUR groups was performed using the Wilcoxon rank sum test.
Disease recurrence was defined as any documented radiographic or pathological failure in the remnant ureter (PSUR only), bladder, contralateral kidney, operative site or regional lymph nodes, or distant metastasis. We used Kaplan-Meier methods to estimate survival probabilities for patients treated with RNU and PSUR. Confidence intervals of differences in survival estimates were obtained using bootstrap methods. Due to the limited number of events we could not formally model these outcomes or adjust for potential confounders. Variables in the tables were compared with Fisher’s exact test (categorical variables) and the Wilcoxon rank sum test (continuous variables). All statistical analyses were conducted using STATA® 11.0.
RESULTS
Patient characteristics and preoperative tumor characteristics are shown in table 1. Patients who underwent RNU were more likely than those who underwent PSUR to have preoperative radiographic evidence of hydronephrosis (p = 0.0006) and less likely to have had a history of transitional cell carcinoma in the bladder (p = 0.008). Both groups contained a sizable number of patients with incomplete data on biopsy grade.
Table 1.
Patient characteristics and preoperative tumor characteristics
| No. RNU (%) | No. PSUR (%) | p Value | |
|---|---|---|---|
| Gender: | 0.7 | ||
| M | 58 (67) | 24 (73) | |
| F | 29 (33) | 9 (27) | |
| White race | 79 (91) | 32 (97) | 0.4 |
| American Society of Anesthesiologists score: | <0.0005 | ||
| 2 or Less | 49 (56) | 11 (33) | |
| 3 | 38 (44) | 14 (42) | |
| Missing | 0 (0) | 8 (24) | |
| Side of tumor: | 0.066 | ||
| Rt | 38 (44) | 21 (64) | |
| Lt | 49 (56) | 12 (36) | |
| History of bladder transitional cell Ca | 36 (41) | 23 (70) | 0.008 |
| Hydronephrosis on computerized tomography | 69 (80) | 13 (45) | 0.0006 |
| Biopsy grade: | <0.0005 | ||
| Low | 42 (48) | 4 (12) | |
| High | 25 (29) | 11 (33) | |
| Missing | 20 (23) | 18 (55) |
The pathological features of patients who underwent RNU and PSUR are presented in table 2. Patients who underwent RNU tended to have higher pathological stage (p = 0.01), similar pathological grade (p = 0.9) and a greater frequency of multifocal tumors (p <0.0005). Patients treated with RNU had a higher proportion of positive surgical margins (17% vs 3%) but equivalent rates of positive soft tissue margins (RNU 3% vs PSUR 3%). Most patients underwent concurrent lymph node dissection regardless of surgical technique (RNU 67% vs PSUR 82%). In addition, nodal stage (p = 0.3) and number of nodes removed (median [IQR] RNU 3 [0 –10] vs PSUR 5 [1– 8], p = 0.5) were similar between the groups.
Table 2.
Pathological features
| No. RNU (%) | No. PSUR (%) | p Value | |
|---|---|---|---|
| Pathological stage: | 0.01 | ||
| pT1 or Less | 39 (45) | 25 (76) | |
| pT2 | 25 (29) | 5 (15) | |
| pT3 or Greater | 23 (26) | 3 (9) | |
| Pathological grade: | 0.9 | ||
| Low | 18 (21) | 8 (24) | |
| High | 68 (78) | 25 (76) | |
| Missing | 1 (1) | 0 (0) | |
| Nodal stage: | 0.3 | ||
| N0 | 49 (56) | 24 (73) | |
| N1 | 10 (11) | 3 (9) | |
| NX | 28 (32) | 6 (18) | |
| Focality: | <0.0005 | ||
| Not applicable | 0 (0) | 8 (24) | |
| Unifocal | 56 (64) | 22 (67) | |
| Multifocal | 31 (36) | 3 (9) | |
| Pos surgical margins | 15 (17) | 1 (3) | 0.067 |
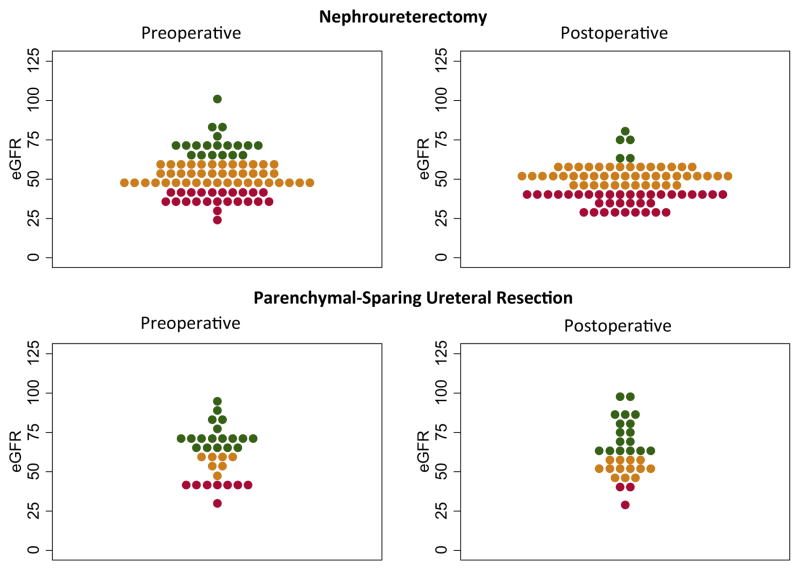
Patients who underwent RNU tended to have worse baseline renal function than those treated with PSUR (eGFR 51 vs 63 ml/minute/1.73 m2, p = 0.009, table 3). Patients who underwent RNU on average experienced a greater decrease in eGFR (median 7 vs 0 ml/minute/1.73 m2 for PSUR, p <0.001). After RNU 93% of patients had an eGFR of less than 60 ml/minute/1.73 m2 compared to 72% before surgery, while among those treated with PSUR only 48% had a postoperative eGFR less than 60 ml/minute/1.73 m2 compared to 39% preoperatively. The number of patients with an eGFR greater than 45 ml/minute/1.73 m2, a threshold below which even reduced dose cisplatin based chemotherapy is rarely administered, decreased in the RNU group from 74% to 55% while it increased in the PSUR group from 73% to 88% (fig. 1).
Table 3.
Renal function characteristics before and after surgery
| RNU | PSUR | p Value | |
|---|---|---|---|
| Median mg/dl creatinine (IQR): | |||
| Preop | 1.2 (1.1–1.5) | 1.1 (1.0–1.4) | 0.065 |
| Postop | 1.4 (1.3–1.6) | 1.1 (1.0–1.3) | <0.0005 |
| Median ml/min/1.73 m2 preop GFR (IQR) | 51 (44–61) | 63 (45–72) | 0.009 |
| No. ml/min/1.73 m2 preop GFR (%): | |||
| 45 or Less | 23 (26) | 9 (27) | 1 |
| 60 or Less | 63 (72) | 13 (39) | 0.001 |
| Median ml/min/1.73 m2 postop GFR (IQR) | 47 (39–54) | 61 (52–72) | 0.009 |
| No. ml/min/1.73 m2 postop GFR (%): | |||
| 45 or Less | 39 (45) | 4 (12) | 0.0007 |
| 60 or Less | 81 (93) | 16 (48) | <0.0005 |
| Median ml/min/1.73 m2 change in GFR (IQR) | −7 (−15–0) | 0 (−4–9) | 0.0007 |
Figure 1.
Comparative scatterplot of eGFR before and after surgery in patients treated with RNU or PSUR. Green represents values greater than 60 ml/minute/1.73 m2, orange represents values between 45 and 60 ml/minute/1.73 m2, and red represents values less than 45 ml/minute/1.73 m2. Each dot represents 1 patient, ie wider bands represent regions with more patients.
Median followup for survivors was 4.2 years. Of the patients 79 (66%) experienced disease recurrence and 44 (37%) died (28 [23%] of UTUC). Figure 2 shows the probability of experiencing recurrence, dying of bladder cancer and dying of any cause. The overall rate of recurrence was high and there were no obvious differences between patients treated with PSUR or RNU (2-year recurrence-free survival of 31% and 43%, respectively, with an absolute difference of 11% [95% CI −9, 34]). The development of metastatic disease was more frequent in patients who underwent RNU (20 patients, 23%) than in those who underwent PSUR (4 patients, 12%). The unadjusted 2-year recurrence rates could have been as much as 9% lower or 34% higher in patients treated with PSUR vs RNU. Rates of death from UTUC followed similar trends. The 2-year cancer specific survival was 87% and 89% for RNU and PSUR, respectively, with an absolute difference of 2% (95% CI −13, 16). Overall survival rates were 85% and 80% for RNU and PSUR, respectively, with an absolute difference of −5% (95% CI −22, 12). While these differences were not statistically significant, the limited number of events resulted in wide estimates around the confidence intervals. Therefore, we cannot exclude the possibility that there are large differences in oncologic outcomes by procedure type. In the PSUR group 3 patients experienced ureteral recurrence at the site of the prior resection and none experienced recurrence in the ipsilateral renal pelvis. Of these patients 2 were treated with repeat PSUR and 1 underwent RNU.
Figure 2.
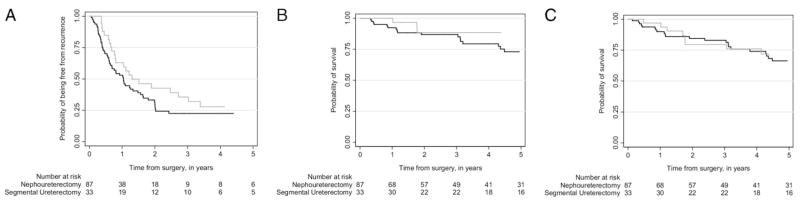
Kaplan-Meier probability of freedom from recurrence (A), death from cancer (B) and death from any cause (C) stratified by patients who underwent RNU (black line) and PSUR (gray line).
DISCUSSION
Resection of the isolated segment of tumor from the whole organ has gained greater acceptance as evidenced by its increased use in the treatment of breast, kidney and penile cancer.5–7 The goal of such interventions is curative treatment with concurrent organ preservation for improved quality of life or improved functional outcome. Because urothelial malignancy is a field defect and the entire urothelium is believed to be at risk, organ preserving intervention is the exception rather than the rule. With respect to UTUC the narrow caliber of the ureter is an additional challenge in accurate preoperative staging and conservative treatment errs toward more radical intervention. For example, in a multi-institutional series of more than 1,300 patients with UTUC who underwent RNU, 44% had pT1 or lower disease and 34% of index lesions were in the ureter,8 demonstrating that many patients who may be candidates for parenchymal preserving surgery instead undergo RNU.
Chemotherapeutic agents, particularly cisplatin, a known nephrotoxic agent, have been demonstrated to be efficacious in the treatment of urothelial malignancy.9 Extrapolating data from lower tract UC suggests that such therapy might be more ideally provided preoperatively. However, accurate preoperative staging for UTUC is difficult and it may not be until after pathological stage is obtained that a clear indication for chemotherapy is demonstrated. Eligibility for cisplatin based therapy is based heavily on kidney function. Traditionally an eGFR of 60 ml/minute/1.73 m2 or greater is required for full dose therapy whereas lower values may be reserved for reduced doses or alternate treatments.10,11 A high proportion of patients with lower tract UC are ineligible for cisplatin therapy because of poor baseline renal function. This issue is compounded in patients with UTUC because RNU, the intervention that provides proper staging and indications for chemotherapeutic agents, often results in substantial loss of renal function.11
We previously demonstrated that RNU results in decreases in eGFR that are likely to impact the receipt of cisplatin based chemotherapy.12 In this study we showed that preservation of the ipsilateral renal unit results in significantly lower decreases in renal function than RNU. Furthermore, patients who underwent PSUR were more likely to be candidates for adjuvant chemotherapy because of the preservation of eGFR. In this analysis there were 18 patients (21%) whose renal function decreased to less than 60 ml/minute/1.73 m2 after RNU vs 3 (9%) who underwent PSUR. The proportion of patients with an eGFR of 45 ml/minute/1.73 m2 or less increased by 19% after RNU but decreased 15% after PSUR. The finding of improved renal function after PSUR may be due to the resection of a partially or completely obstructing ureteral tumor, allowing improved ipsilateral renal function. However, it is unlikely that resection of a portion of the unobstructed ureter has an important beneficial or detrimental impact on renal function. While creatinine levels were similar between the groups, eGFR was lower in the RNU group (51 vs 63 ml/minute/1.73 m2). Several factors may have contributed to this difference. The RNU group tended to be older (73 vs 70 years), to have a greater incidence of hydronephrosis (80% vs 45%) and to be female (33% vs 27%). These trends underscore the importance of calculating eGFR before any surgical intervention that may impact renal function. While patients who underwent RNU experienced a greater decline in renal function than those treated with PSUR, the overall decrease in eGFR associated with RNU of −7 was less than might be anticipated with uninephrectomy.13 This may reflect a selection bias. Split renal function studies were not routinely obtained before surgical intervention. However, poor ipsilateral renal parenchyma on preoperative imaging likely influenced the decision to offer RNU rather than PSUR.
Decreases in eGFR after radical nephrectomy have been strongly associated with an increased risk of cardiac related death.14 Nephrectomy has also been associated with an increased risk of cardiovascular related events and overall mortality compared with nephron sparing surgery for malignant and benign conditions.15,16 We hypothesized that preservation of renal function may be associated with a decreased rate of death in those undergoing PSUR vs RNU. Although we found no differences in the risk of death between the groups, due to the limited number of events we cannot exclude this possibility.
Hall et al reported that preservation of the ipsilateral renal unit resulted in improved renal function, but potentially at the cost of increased cancer recurrence rates and decreased cancer specific survival.17 In our cohort 3 patients experienced ipsilateral ureteral recurrence, consistent with previous reports demonstrating rates of 7.5% to 10% in a similar period.18,19 This event by definition cannot occur after RNU because it involves complete resection of the ureter with bladder cuff, underscoring the necessity of strict adherence to surveillance regimens for patients electing PSUR and requiring frequent ureteroscopy.
It is generally believed that patients with more aggressive disease should be excluded from consideration for PSUR because it may result in worse oncologic outcomes. However, there are few data to support this assumption. In a single center series by Lehmann et al 145 consecutive patients underwent PSUR or RNU for upper tract urothelial carcinoma.19 With a median followup of 96 months no differences in survival were found among surgical approaches for pTa or pT1 disease. A recent analysis of Surveillance, Epidemiology, and End Results data on 2,044 patients with UTUC demonstrated that PSUR did not have a negative impact on cancer specific survival even in those with pT3 or greater disease.20
The primary limitations of this study are the important differences in the characteristics of patients who underwent RNU vs PSUR. These differences likely reflect the selection bias that we attempted to mitigate by excluding patients who would have been ineligible for PSUR due to the presence of disease in the pelvis and a variety of other strict exclusion criteria. However, differences remain and, as a result, conclusions with respect to oncologic outcomes are limited.
CONCLUSIONS
The 2 surgical groups we studied revealed important differences that reflect our historical practice patterns. PSUR has generally been reserved for patients with lower risk, unifocal disease with significant ipsilateral renal function or imperative indications. However, because of the small number of patients in this study we were not able to formally adjust for potential confounders. Furthermore, given the wide confidence intervals and the putative selection bias, we were not able to determine the impact of surgical approach on oncologic outcomes. While it remains possible that PSUR results in worse oncologic outcomes, we demonstrated that it is associated with better preservation of renal function. Additionally, for patients with poor pathological features, treatment with cisplatin based chemotherapy, which is critically tied to renal function, may have a greater impact on oncologic outcome than surgical approach. Larger, randomized or prospective multi-institutional studies are needed to investigate the impact of surgical approach and cisplatin based chemotherapy on cancer control in patients with UTUC.
Acknowledgments
Supported by the Sidney Kimmel Center for Prostate and Urologic Cancers, by David H. Koch through the Prostate Cancer Foundation and by the National Cancer Institute (Grants U54CA137788 and U54CA132378).
Abbreviations and Acronyms
- eGFR
estimated glomerular filtration rate
- GFR
glomerular filtration rate
- PSUR
parenchymal sparing ureteral resection
- RNU
radical nephroureterectomy
- UC
urothelial carcinoma
- UTUC
upper tract urothelial carcinoma
Footnotes
Study received institutional review board approval.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Scher H, Bahnson R, Cohen S, et al. NCCN urothelial cancer practice guidelines. National Comprehensive Cancer Network. Oncology (Williston Park) 1998;12:225. [PubMed] [Google Scholar]
- 3.Roupret M, Zigeuner R, Palou J, et al. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol. 2011;59:584. doi: 10.1016/j.eururo.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 5.Habermann EB, Abbott A, Parsons HM, et al. Are mastectomy rates really increasing in the United States? J Clin Oncol. 2010;28:3437. doi: 10.1200/JCO.2009.27.6774. [DOI] [PubMed] [Google Scholar]
- 6.Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614. doi: 10.1245/s10434-007-9563-9. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Agarwal N, Beard C, et al. NCCN clinical practice guidelines in oncology: kidney cancer. J Natl Compr Canc Netw. 2009;7:618. doi: 10.6004/jnccn.2009.0043. [DOI] [PubMed] [Google Scholar]
- 8.Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115:1224. doi: 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]
- 9.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 10.Hussain SA, Stocken DD, Riley P, et al. A phase I/II study of gemcitabine and fractionated cisplatin in an outpatient setting using a 21-day schedule in patients with advanced and metastatic bladder cancer. Br J Cancer. 2004;91:844. doi: 10.1038/sj.bjc.6602112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506. doi: 10.1002/cncr.22031. [DOI] [PubMed] [Google Scholar]
- 12.Kaag MG, O’Malley RL, O’Malley P, et al. Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol. 2010;58:581. doi: 10.1016/j.eururo.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barri YM, Parker T, 3rd, Daoud Y, et al. Definition of chronic kidney disease after uninephrectomy in living donors: what are the implications? Transplantation. 2010;90:575. doi: 10.1097/TP.0b013e3181e64237. [DOI] [PubMed] [Google Scholar]
- 14.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 15.Huang WC, Elkin EB, Levey AS, et al. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors–is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55. doi: 10.1016/j.juro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weight CJ, Lieser G, Larson BT, et al. Partial nephrectomy is associated with improved overall survival compared to radical nephrectomy in patients with unanticipated benign renal tumours. Eur Urol. 2010;58:293. doi: 10.1016/j.eururo.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 17.Hall MC, Womack S, Sagalowsky AI, et al. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52:594. doi: 10.1016/s0090-4295(98)00295-7. [DOI] [PubMed] [Google Scholar]
- 18.Leitenberger A, Beyer A, Altwein JE. Organ-sparing treatment for ureteral carcinoma? Eur Urol. 1996;29:272. doi: 10.1159/000473759. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann J, Suttmann H, Kovac I, et al. Transitional cell carcinoma of the ureter: prognostic factors influencing progression and survival. Eur Urol. 2007;51:1281. doi: 10.1016/j.eururo.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Jeldres C, Lughezzani G, Sun M, et al. Segmental ureterectomy can safely be performed in patients with transitional cell carcinoma of the ureter. J Urol. 2010;183:1324. doi: 10.1016/j.juro.2009.12.018. [DOI] [PubMed] [Google Scholar]