Abstract
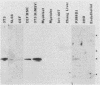
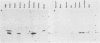
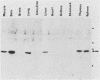
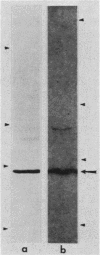
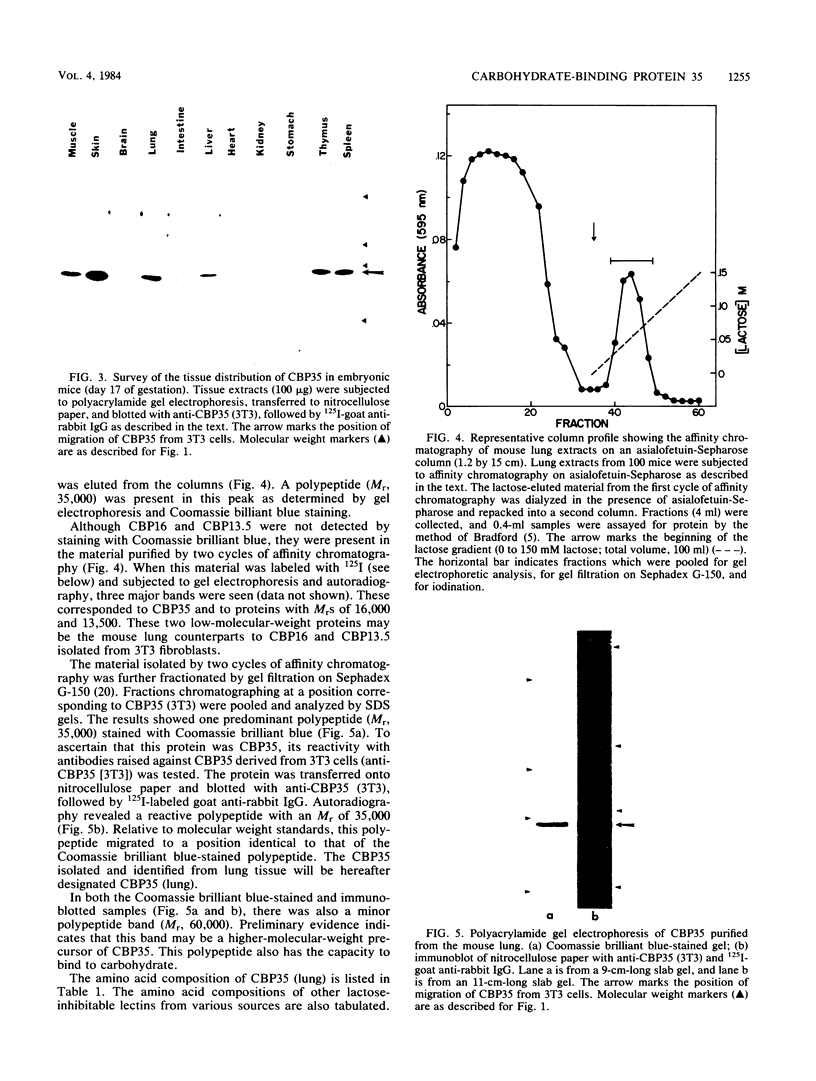
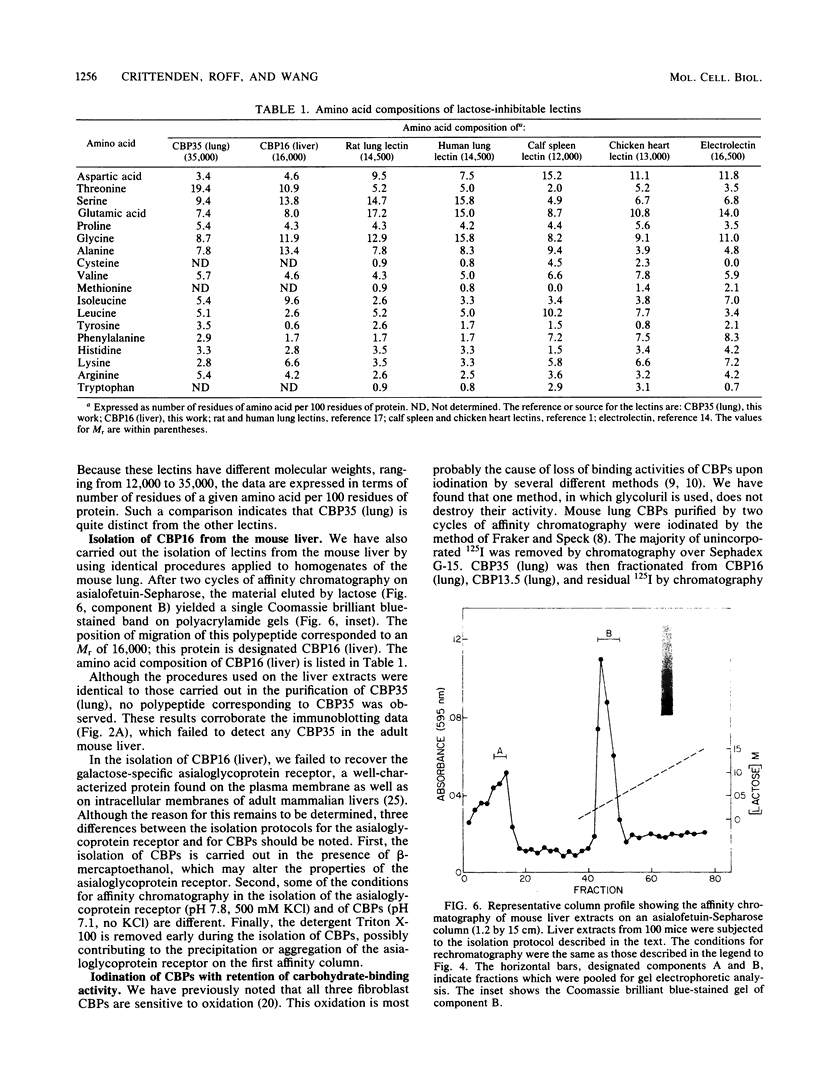
In previous studies, a lectin designated as carbohydrate-binding protein 35 (CBP35) has been isolated from cultured mouse 3T3 fibroblasts. In this study, antibodies directed against CBP35 were used to screen for cross-reactive proteins in various cultured cells and in various organs and tissues of mice. Cross-reactive proteins of the same molecular weight (Mr, 35,000) were found in human, mouse, and chicken fibroblasts and in a macrophage-like cell line, P388D1. Similarly, cross-reactive proteins were also found in the embryonic liver, lung, spleen, thymus, skin, and muscle tissue and in the lung, artery, thymus, and spleen of the adult mouse. Fractionation of extracts of mouse lung on affinity columns of asialofetuin-Sepharose yielded a protein whose molecular weight, carbohydrate-binding specificity, and immunological properties suggest that it is CBP35 derived from the lung, hereafter designated CBP35 (lung). The binding of 125I-labeled CBP35 (lung) to rabbit erythrocytes was quantitated in the presence and absence of various carbohydrates. It was found that only carbohydrates containing galactose were inhibitors of the binding; the disaccharide lactose was 100-fold more potent as an inhibitor than was the monosaccharide galactose. When extracts of the adult mouse liver were fractionated by asialofetuin-Sepharose chromatography, only a protein corresponding to CBP16 was isolated; no CBP35 was found. These results corroborate the immunoblotting data, which indicated that CBP35 was not detectable in the adult mouse liver.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer E. C., Barondes S. H. Quantitation of two endogenous lactose-inhibitable lectins in embryonic and adult chicken tissues. J Cell Biol. 1982 Jan;92(1):23–27. doi: 10.1083/jcb.92.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. C., Zweig S. E., Barondes S. H. Two lactose binding lectins from chicken tissues. Purified lectin from intestine is different from those in liver and muscle. J Biol Chem. 1980 May 10;255(9):4236–4239. [PubMed] [Google Scholar]
- Booyse F. M., Sedlak B. J., Rafelson M. E., Jr Culture of arterial endothelial cells: characterization and growth of bovine aortic cells. Thromb Diath Haemorrh. 1975 Dec 15;34(3):825–839. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Briles E. B., Gregory W., Fletcher P., Kornfeld S. Vertebrate lectins, Comparison of properties of beta-galactoside-binding lectins from tissues of calf and chicken. J Cell Biol. 1979 Jun;81(3):528–537. doi: 10.1083/jcb.81.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. I. Enzymatic iodination of mouse L cells. J Cell Biol. 1975 Feb;64(2):438–460. doi: 10.1083/jcb.64.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup W., Dean R. T. Spontaneous lysosomal enzyme secretion by a murine macrophage-like cell line. Biochem J. 1980 Sep 15;190(3):847–850. doi: 10.1042/bj1900847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop D. R., Morgan E. T., Tarr G. E., Coon M. J. Purification and characterization of a unique isozyme of cytochrome P-450 from liver microsomes of ethanol-treated rabbits. J Biol Chem. 1982 Jul 25;257(14):8472–8480. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levi G., Teichberg V. I. Isolation and physicochemical characterization of electrolectin, a beta-D-galactoside binding lectin from the electric organ of Electrophorus electricus. J Biol Chem. 1981 Jun 10;256(11):5735–5740. [PubMed] [Google Scholar]
- Nowak T. P., Kobiler D., Roel L. E., Barondes S. H. Developmentally regulated lectin from embryonic chick pectoral muscle. Purification by affinity chromatography. J Biol Chem. 1977 Sep 10;252(17):6026–6030. [PubMed] [Google Scholar]
- Powell J. T. Purification and properties of lung lectin. Rat lung and human lung beta-galactoside-binding proteins. Biochem J. 1980 Apr 1;187(1):123–129. doi: 10.1042/bj1870123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff C. F., Rosevear P. R., Wang J. L., Barker R. Identification of carbohydrate-binding proteins from mouse and human fibroblasts. Biochem J. 1983 Jun 1;211(3):625–629. doi: 10.1042/bj2110625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff C. F., Wang J. L. Endogenous lectins from cultured cells. Isolation and characterization of carbohydrate-binding proteins from 3T3 fibroblasts. J Biol Chem. 1983 Sep 10;258(17):10657–10663. [PubMed] [Google Scholar]
- Roff C. F., Wozniak R. W., Blenis J., Wang J. L. The effect of mannose6-phosphate on the turnover of cell surface glycosaminoglycans. Exp Cell Res. 1983 Apr 1;144(2):333–344. doi: 10.1016/0014-4827(83)90412-3. [DOI] [PubMed] [Google Scholar]
- Rubin H. The inhibition of chick embryo cell growth by medium obtained from cultures of Rous sarcoma cells. Exp Cell Res. 1966 Jan;41(1):149–161. doi: 10.1016/0014-4827(66)90555-6. [DOI] [PubMed] [Google Scholar]
- Steck P. A., Blenis J., Voss P. G., Wang J. L. Growth control in cultured 3T3 fibroblasts II. Molecular properties of a fraction enriched in growth inhibitory activity. J Cell Biol. 1982 Feb;92(2):523–530. doi: 10.1083/jcb.92.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck P. A., Voss P. G., Wang J. L. Growth control in cultured 3T3 fibroblasts. Assays of cell proliferation and demonstration of a growth inhibitory activity. J Cell Biol. 1979 Dec;83(3):562–575. doi: 10.1083/jcb.83.3.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T., Pricer W. E., Jr, Ashwell G. Subcellular membrane topology and turnover of a rat hepatic binding protein specific for asialoglycoproteins. J Biol Chem. 1979 Feb 25;254(4):1038–1043. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waard A., Hickman S., Kornfeld S. Isolation and properties of beta-galactoside binding lectins of calf heart and lung. J Biol Chem. 1976 Dec 10;251(23):7581–7587. [PubMed] [Google Scholar]