Abstract
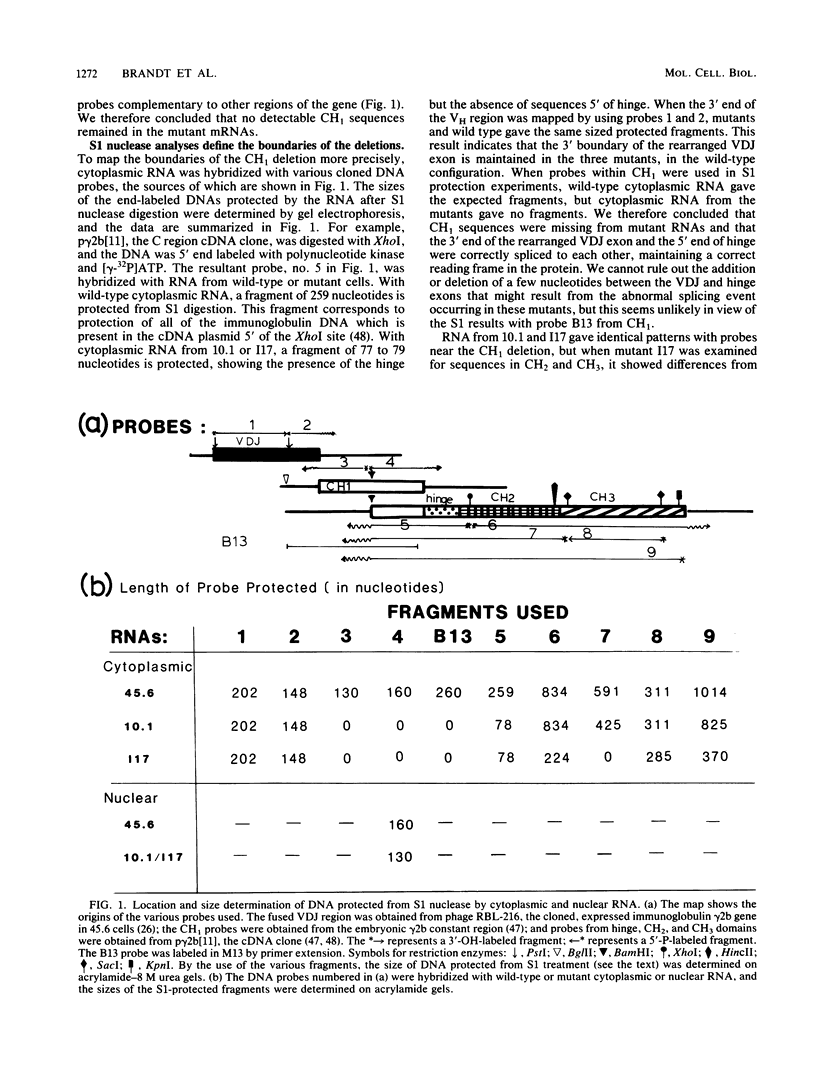
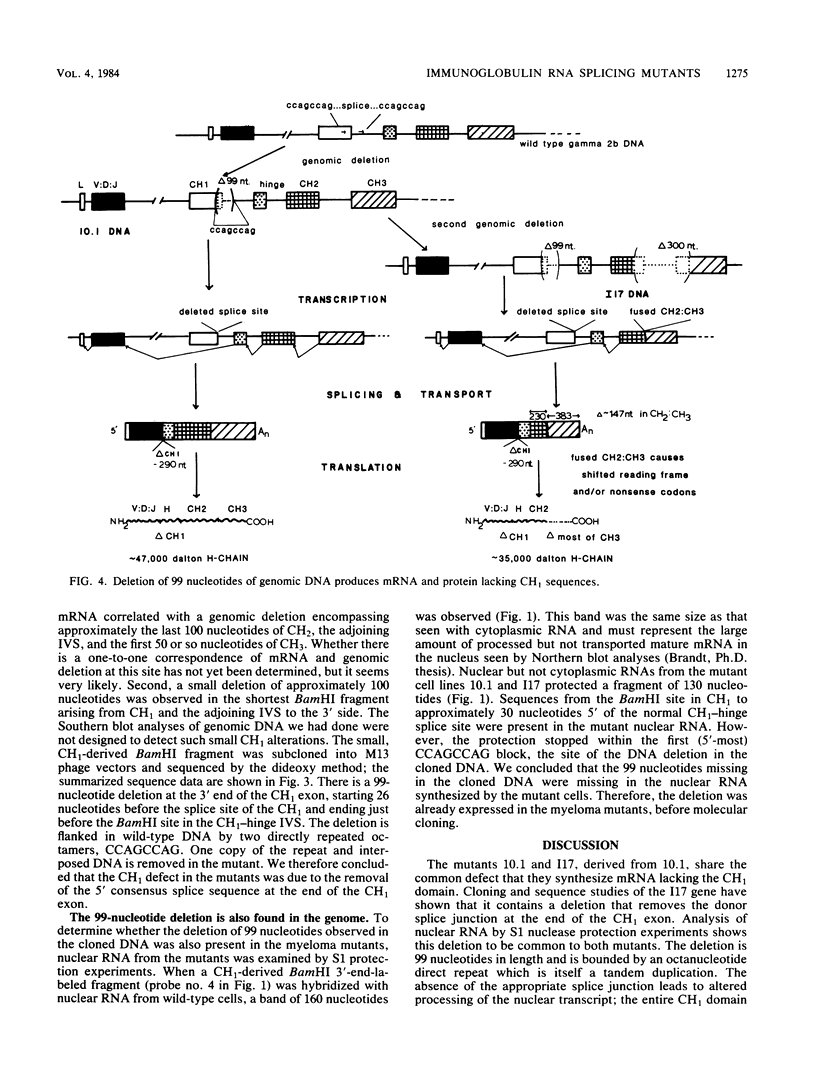
A series of mouse myeloma mutants, derived from a cell line of the murine MPC-11 tumor (gamma 2b, kappa), resemble human heavy-chain disease in their loss of an internal domain (exon). In these mutants, most of the gamma 2b CH1 exon was present in the nuclear RNA but was removed during splicing to form the mature cytoplasmic RNA. Amino acid sequence studies of one mutant (10.1) are consistent with the loss of the complete CH1 domain. A second mutant cell line (I17) derived from 10.1 and containing the same CH1 alteration was shown by S1 nuclease protection experiments to have an additional mRNA deletion spanning the CH2-CH3 domain boundary. This second deletion was shown to result from a genomic alteration that provided a marker for the isolation of the expressed H-chain allele. To determine the basis of the CH1 splicing defect, the 117 genome-expressed gamma 2b constant region DNA was cloned. Sequence studies showed a deletion of 99 nucleotides around the 3' end of the CH1 domain, which removed the splice site and flanking DNA, apparently causing the aberrant splicing of the RNA transcript. The sequence deleted in the mutant is flanked by short repeats of the octameric sequence CCAGCCAG in the wild-type gene. In the mutant, one copy of the repeat, in addition to the sequences between the repeats, has been lost.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adetugbo K., Milstein C., Secher D. S. Molecular analysis of spontaneous somatic mutants. Nature. 1977 Jan 27;265(5592):299–304. doi: 10.1038/265299a0. [DOI] [PubMed] [Google Scholar]
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Birshtein B. K., Campbell R., Greenberg M. L. A gamma 2b-gamma 2a hybrid immunoglobulin heavy chain produced by a variant of the MPC 11 mouse myeloma cell line. Biochemistry. 1980 Apr 29;19(9):1730–1737. doi: 10.1021/bi00550a002. [DOI] [PubMed] [Google Scholar]
- Birshtein B. K., Turner K. J., Cebra J. J. Structure of heavy chain from strain 13 guinea pig immunoglobulin-G(2). I. Isolation of cyanogen bromide fragments. Biochemistry. 1971 Jan 5;10(1):1–8. doi: 10.1021/bi00777a001. [DOI] [PubMed] [Google Scholar]
- Burstein Y., Breiner A. V., Brandt C. R., Milcarek C., Sweet R. W., Warszawski D., Ziv E., Schechter I. Recent duplication and germ-line diversification of rat immunoglobulin kappa chain gene joining segments. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5993–5997. doi: 10.1073/pnas.79.19.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffino P., Baumal R., Laskov R., Scharff M. D. Cloning of mouse myeloma cells and detection of rare variants. J Cell Physiol. 1972 Jun;79(3):429–440. doi: 10.1002/jcp.1040790313. [DOI] [PubMed] [Google Scholar]
- Coffino P., Scharff M. D. Rate of somatic mutation in immunoglobulin production by mouse myeloma cells. Proc Natl Acad Sci U S A. 1971 Jan;68(1):219–223. doi: 10.1073/pnas.68.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackowski W., Morrison S. L. Two alpha heavy chain disease proteins with different genomic deletions demonstrate that nonexpressed alpha heavy chain genes contain methylated bases. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7091–7095. doi: 10.1073/pnas.78.11.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Dunnick W., Rabbitts T. H., Milstein C. A mouse immunoglobulin heavy chain deletion mutant: isolation of a cDNA clone and sequence analysis of the mRNA. Nucleic Acids Res. 1980 Apr 11;8(7):1475–1484. doi: 10.1093/nar/8.7.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnick W., Rabbitts T. H., Milstein C. An immunoglobulin deletion mutant with implications for the heavy-chain switch and RNA splicing. Nature. 1980 Aug 14;286(5774):669–675. doi: 10.1038/286669a0. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Esumi H., Takahashi Y., Sato S., Nagase S., Sugimura T. A seven-base-pair deletion in an intron of the albumin gene of analbuminemic rats. Proc Natl Acad Sci U S A. 1983 Jan;80(1):95–99. doi: 10.1073/pnas.80.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esumi H., Takahashi Y., Sekiya T., Sato S., Nagase S., Sugimura T. Presence of albumin mRNA precursors in nuclei of analbuminemic rat liver lacking cytoplasmic albumin mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(3):734–738. doi: 10.1073/pnas.79.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh P. J., Schmeissner U., Hofer M., Miller J. H. Genetic studies of the lac repressor. VII. On the molecular nature of spontaneous hotspots in the lacI gene of Escherichia coli. J Mol Biol. 1978 Dec 25;126(4):847–857. doi: 10.1016/0022-2836(78)90023-2. [DOI] [PubMed] [Google Scholar]
- Francus T., Birshtein B. K. An IgG2a-producing variant of an IgG2b-producing mouse myeloma cell line. Structural studies on the Fc region of parent and variant heavy chains. Biochemistry. 1978 Oct 3;17(20):4324–4331. doi: 10.1021/bi00613a033. [DOI] [PubMed] [Google Scholar]
- Frangione B., Franklin E. C. Heavy chain diseases: clinical features and molecular significance of the disordered immunoglobulin structure. Semin Hematol. 1973 Jan;10(1):53–64. [PubMed] [Google Scholar]
- Franklin E. C., Frangione B. Structural variants of human and murine immunoglobulins. Contemp Top Mol Immunol. 1975;4:89–126. doi: 10.1007/978-1-4615-8930-3_4. [DOI] [PubMed] [Google Scholar]
- Hoffman L. M., Fritsch M. K., Gorski J. Probable nuclear precursors of preprolactin mRNA in rat pituitary cells. J Biol Chem. 1981 Mar 25;256(6):2597–2600. [PubMed] [Google Scholar]
- Kantor J. A., Turner P. H., Nienhuis A. W. Beta Thalassemia: mutations which affect processing of the beta-Globin mRNA precursor. Cell. 1980 Aug;21(1):149–157. doi: 10.1016/0092-8674(80)90122-1. [DOI] [PubMed] [Google Scholar]
- Kenter A. L., Birshtein B. K. Genetic mechanism accounting for precise immunoglobulin domain deletion in a variant of MPC 11 myeloma cells. Science. 1979 Dec 14;206(4424):1307–1309. doi: 10.1126/science.117550. [DOI] [PubMed] [Google Scholar]
- Kuehl W. M., Scharff M. D. Synthesis of a carboxyl-terminal (constant region) fragment of the immunoglobulin light chain by a mouse myeloma cell line. J Mol Biol. 1974 Nov 5;89(3):409–421. doi: 10.1016/0022-2836(74)90472-0. [DOI] [PubMed] [Google Scholar]
- Lang R. B., Stanton L. W., Marcu K. B. On immunoglobulin heavy chain gene switching: two gamma 2b genes are rearranged via switch sequences in MPC-11 cells but only one is expressed. Nucleic Acids Res. 1982 Jan 22;10(2):611–630. doi: 10.1093/nar/10.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskov R., Scharff M. D. Synthesis, assembly, and secretion of gamma globulin by mouse myeloma cells. I. Adaptation of the Merwin plasma cell tumor-11 to culture, cloning, and characterization of gamma globulin subunits. J Exp Med. 1970 Mar 1;131(3):515–541. doi: 10.1084/jem.131.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Maquat L. E., Kinniburgh A. J., Beach L. R., Honig G. R., Lazerson J., Ershler W. B., Ross J. Processing of human beta-globin mRNA precursor to mRNA is defective in three patients with beta+-thalassemia. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4287–4291. doi: 10.1073/pnas.77.7.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Milcarek C., Price R., Penman S. The metabolism of a poly(A) minus mRNA fraction in HeLa cells. Cell. 1974 Sep;3(1):1–10. doi: 10.1016/0092-8674(74)90030-0. [DOI] [PubMed] [Google Scholar]
- Monk R. J., Morrison S. L., Milcarek C. Heavy-chain mutants derived from gamma 2b mouse myeloma: characterization of heavy-chain messenger ribonucleic acid, proteins, and secretion in delection mutants and messenger ribonucleic acid in gamma2a mutant progeny. Biochemistry. 1981 Apr 14;20(8):2330–2339. doi: 10.1021/bi00511a040. [DOI] [PubMed] [Google Scholar]
- Morrison S. L. Murine heavy chain disease. Eur J Immunol. 1978 Mar;8(3):194–199. doi: 10.1002/eji.1830080311. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Goff S. C., Hechtman R. L. Mutation in an intervening sequence splice junction in man. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5041–5045. doi: 10.1073/pnas.78.8.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Sexton J. P., Goff S. C., Kazazian H. H., Jr Inactivation of an acceptor RNA splice site by a short deletion in beta-thalassemia. J Biol Chem. 1983 Jun 25;258(12):7249–7251. [PubMed] [Google Scholar]
- Polsky F., Edgell M. H., Seidman J. G., Leder P. High capacity gel preparative electrophoresis for purification of fragments of genomic DNA. Anal Biochem. 1978 Jul 1;87(2):397–410. doi: 10.1016/0003-2697(78)90689-9. [DOI] [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Marcu K. B., Perry R. P. The synthesis and processing of the messenger RNAs specifying heavy and light chain immunoglobulins in MPC-11 cells. Cell. 1978 Dec;15(4):1495–1509. doi: 10.1016/0092-8674(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Schnell H., Steinmetz M., Zachau H. G., Schechter I. An unusual translocation of immunoglobulin gene segments in variants of the mouse myeloma MPC11. Nature. 1980 Jul 10;286(5769):170–173. doi: 10.1038/286170a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. A mutant immunoglobulin light chain is formed by aberrant DNA- and RNA-splicing events. Nature. 1980 Aug 21;286(5775):779–783. doi: 10.1038/286779a0. [DOI] [PubMed] [Google Scholar]
- Sinclair R. M. Solar eclipses and ancient artistic motifs. Science. 1977 May 13;196(4291):715–717. doi: 10.1126/science.196.4291.715. [DOI] [PubMed] [Google Scholar]
- Staden R. Further procedures for sequence analysis by computer. Nucleic Acids Res. 1978 Mar;5(3):1013–1016. doi: 10.1093/nar/5.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P. W., Marcu K. B., Newell N., Richards J., Blattner F. R. Sequence of the cloned gene for the constant region of murine gamma 2b immunoglobulin heavy chain. Science. 1979 Dec 14;206(4424):1303–1306. doi: 10.1126/science.117549. [DOI] [PubMed] [Google Scholar]
- Tucker P. W., Marcu K. B., Slightom J. L., Blattner F. R. Structure of the constant and 3' untranslated regions of the murine gamma 2b heavy chain messenger RNA. Science. 1979 Dec 14;206(4424):1299–1303. doi: 10.1126/science.117548. [DOI] [PubMed] [Google Scholar]
- Yamawaki-Kataoka Y., Kataoka T., Takahashi N., Obata M., Honjo T. Complete nucleotide sequence of immunoglobulin gamma2b chain gene cloned from newborn nouse DNA. Nature. 1980 Feb 21;283(5749):786–789. doi: 10.1038/283786a0. [DOI] [PubMed] [Google Scholar]
- Zack D. J., Morrison S. L., Cook W. D., Dackowski W., Scharff M. D. Somatically generated mouse myeloma variants synthesizing IgA half-molecules. J Exp Med. 1981 Nov 1;154(5):1554–1569. doi: 10.1084/jem.154.5.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]



