Abstract
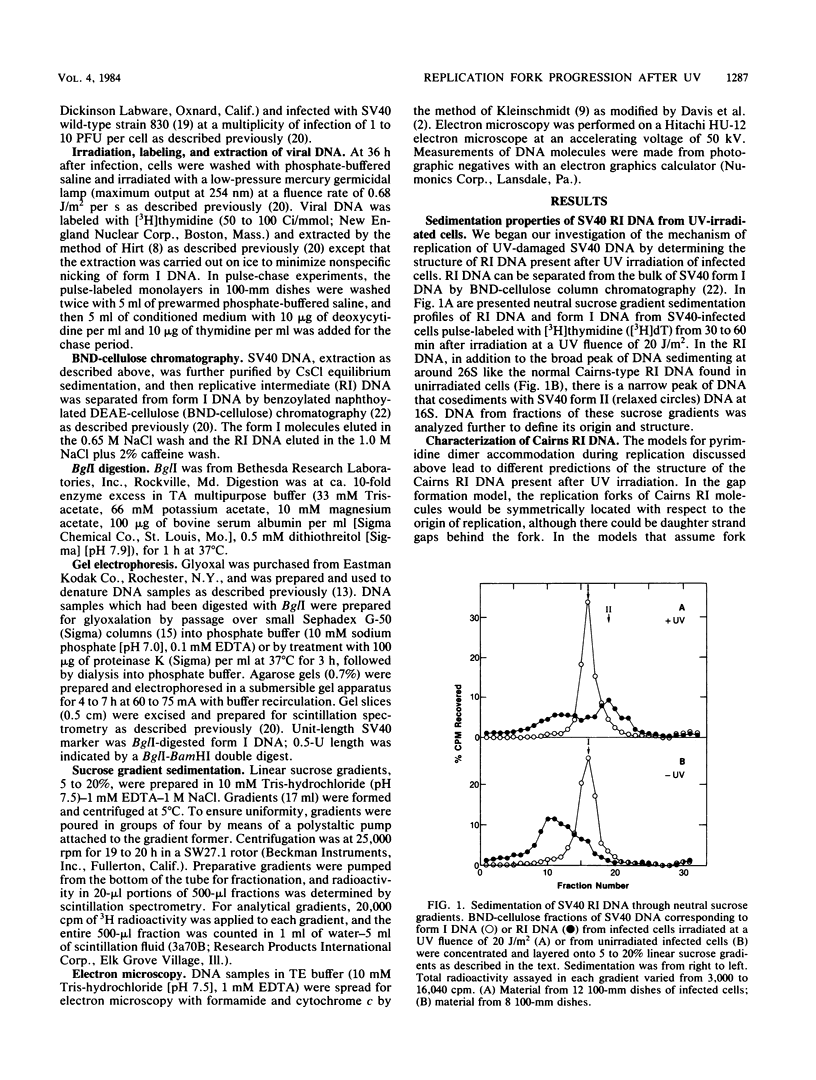
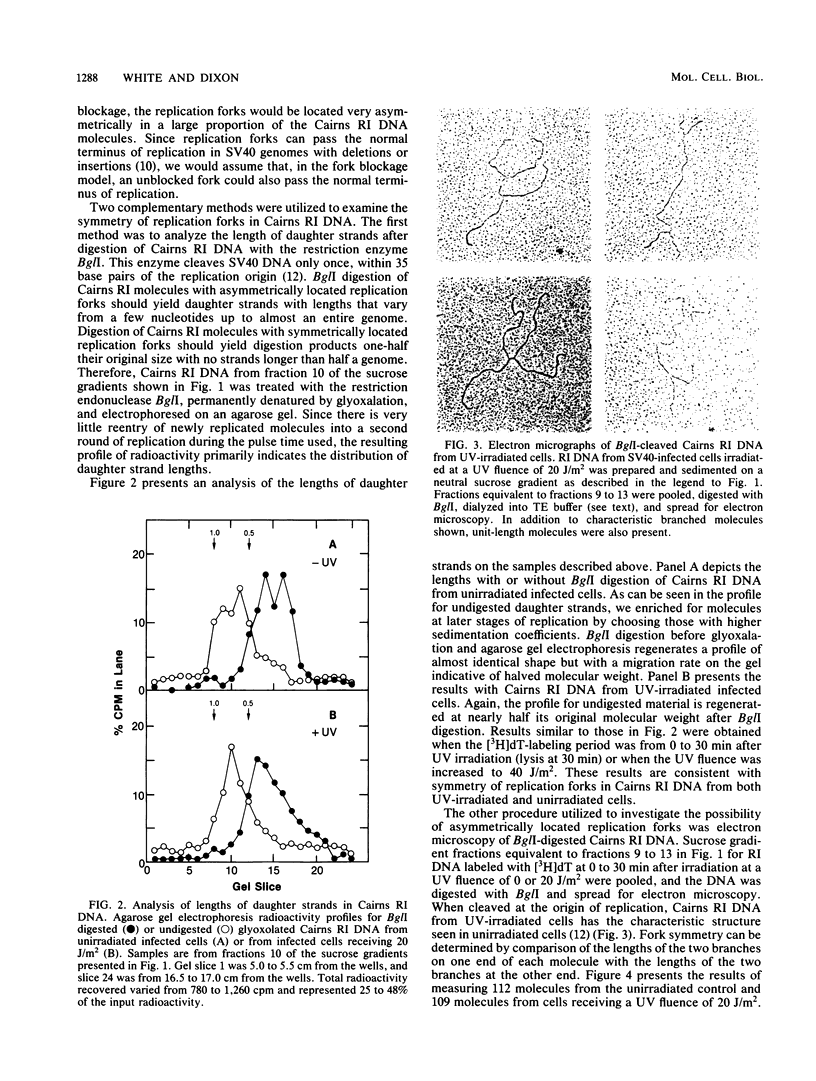
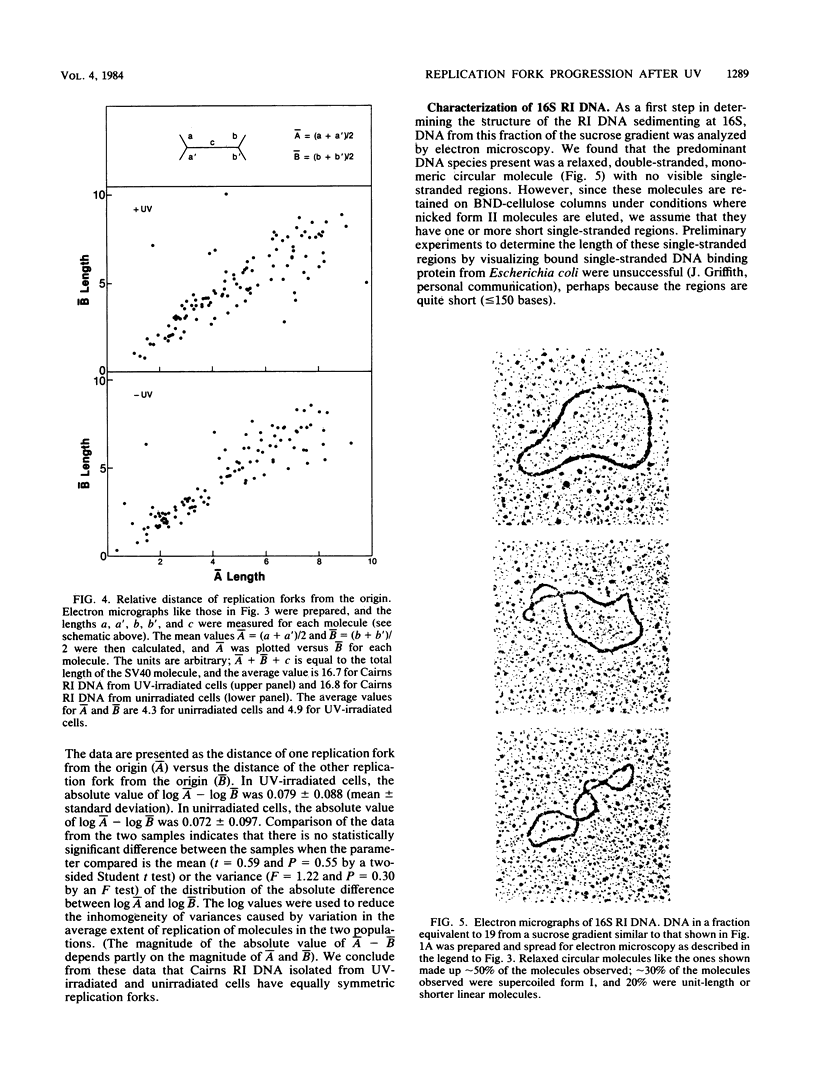
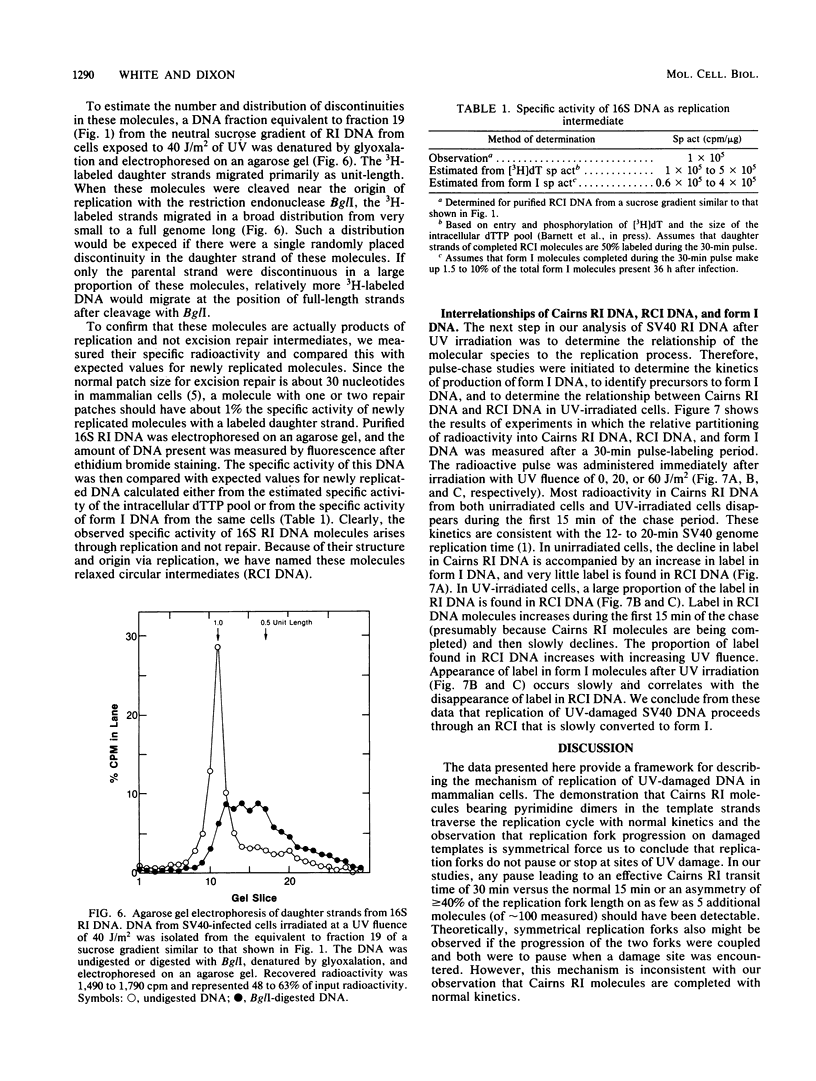
We have analyzed the structural characteristics of simian virus 40 replicative intermediate DNA produced after UV irradiation and the kinetics of conversion of this intermediate DNA into form I DNA. Replicative intermediate DNA isolated at 30 or 60 min after UV irradiation consists primarily of two species of molecules that sediment in neutral sucrose gradients as either Cairns theta structures or relaxed monomeric circles. Replication forks on the Cairns intermediate DNA are symmetrically located with respect to the origin of replication, ruling out the possibility of asymmetric pauses or blocks to replication fork progression at damage sites. The relaxed circles contain at least one randomly located discontinuity in the daughter strand. Pulse-chase experiments demonstrated that a UV fluence-dependent fraction of the Cairns intermediate DNA progresses through the relaxed circular intermediate before being converted to completed form I molecules. Disappearance of Cairns intermediate DNA occurs at the same rate in irradiated and unirradiated cells, whereas completion of the relaxed circular intermediate DNA occurs at a slow rate, relatively independent of UV fluence. These data support a model for replication of UV-damaged DNA in which replication rapidly continues past damage sites via a gap formation event.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Danna K. J., Nathans D. Bidirectional replication of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3097–3100. doi: 10.1073/pnas.69.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J. Inhibition of DNA replication by ultraviolet light. Biophys J. 1976 Aug;16(8):849–860. doi: 10.1016/S0006-3495(76)85735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J. Inhibition of simian virus 40 DNA replication by ultraviolet light. Virology. 1983 Jul 30;128(2):298–309. doi: 10.1016/0042-6822(83)90257-x. [DOI] [PubMed] [Google Scholar]
- Edenberg H., Hanawalt P. Size of repair patches in the DNA of ultraviolet-irradiated HeLa cells. Biochim Biophys Acta. 1972 Jul 20;272(3):361–372. doi: 10.1016/0005-2787(72)90389-9. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K. Persistence of pyrimidine dimers during post-replication repair in ultraviolet light-irradiated Escherichia coli K12. J Mol Biol. 1974 Jul 25;87(1):103–119. doi: 10.1016/0022-2836(74)90563-4. [DOI] [PubMed] [Google Scholar]
- Hall J. D., Mount D. W. Mechanisms of DNA replication and mutagenesis in ultraviolet-irradiated bacteria and mammalian cells. Prog Nucleic Acid Res Mol Biol. 1981;25:53–126. doi: 10.1016/s0079-6603(08)60483-3. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Non-specific termination of simian virus 40 DNA replication. J Mol Biol. 1975 Sep 5;97(1):113–118. doi: 10.1016/s0022-2836(75)80026-x. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972 May 28;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P. The initiation of SV40 DNA synthesis is not unique to the replication origin. Cell. 1980 Jun;20(2):381–391. doi: 10.1016/0092-8674(80)90624-8. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. D., Cleaver J. E. Postreplication repair: questions of its definition and possible alteration in xeroderma pigmentosum cell strains. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3927–3931. doi: 10.1073/pnas.76.8.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Robb J. A., Huebner K. Effect of cell chromosome number on simian virus 40 replication. Exp Cell Res. 1973 Sep;81(1):120–126. doi: 10.1016/0014-4827(73)90118-3. [DOI] [PubMed] [Google Scholar]
- Sarasin A. R., Hanawalt P. C. Replication of ultraviolet-irradiated simian virus 40 in monkey kidney cells. J Mol Biol. 1980 Apr;138(2):299–319. doi: 10.1016/0022-2836(80)90288-0. [DOI] [PubMed] [Google Scholar]
- Sarasin A., Gaillard C., Feunteun J. Induced mutagenesis of Simian virus 40 in carcinogen-treated monkey cells. Basic Life Sci. 1983;23:311–334. doi: 10.1007/978-1-4684-4382-0_13. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacks P. C., White J. H., Dixon K. Accommodation of pyrimidine dimers during replication of UV-damaged simian virus 40 DNA. Mol Cell Biol. 1983 Aug;3(8):1403–1411. doi: 10.1128/mcb.3.8.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Tapper D. P., DePamphilis M. L. Discontinuous DNA replication: accumulation of Simian virus 40 DNA at specific stages in its replication. J Mol Biol. 1978 Apr 15;120(3):401–422. doi: 10.1016/0022-2836(78)90427-8. [DOI] [PubMed] [Google Scholar]
- Williams J. I., Cleaver J. E. Perturbations in simian virus 40 DNA synthesis by ultraviolet light. Mutat Res. 1978 Dec;52(3):301–311. doi: 10.1016/0027-5107(78)90169-0. [DOI] [PubMed] [Google Scholar]
- Yunis J. J. The chromosomal basis of human neoplasia. Science. 1983 Jul 15;221(4607):227–236. doi: 10.1126/science.6336310. [DOI] [PubMed] [Google Scholar]





