Abstract
Aims
Emerging evidence indicates that nuclear receptors play a critical regulatory role in cardiovascular physiology/pathology. Recently, farnesoid-X-receptor (FXR), a member of the metabolic nuclear receptor superfamily, has been demonstrated to be expressed in vascular cells, with important roles in vascular physiology/pathology. However, the potential cardiac function of FXR remains unclear. We investigated the cardiac expression and biological function of FXR.
Methods and results
Farnesoid-X-receptor was detected in both isolated neonatal rat cardiac myocytes and fibroblasts. Natural and synthetic FXR agonists upregulated cardiac FXR expression, stimulated myocyte apoptosis, and reduced myocyte viability dose- and time-dependently. Mechanistic studies demonstrated that FXR agonists disrupted mitochondria, characterized by mitochondrial permeability transition pores activation, mitochondrial potential dissipation, cytochrome c release, and both caspase-9 and -3 activation. Such mitochondrial apoptotic responses were abolished by siRNA-mediated silencing of endogenous FXR or pharmacological inhibition of mitochondrial death signalling. Furthermore, low levels of FXR were detected in the adult mouse heart, with significant (∼2.0-fold) upregulation after myocardial ischaemia/reperfusion (MI/R). Pharmacological inhibition or genetic ablation of FXR significantly reduced myocardial apoptosis by 29.0–53.4%, decreased infarct size by 23.4–49.7%, and improved cardiac function in ischaemic/reperfused myocardium.
Conclusion
These results demonstrate that nuclear receptor FXR acts as a novel functional receptor in cardiac tissue, regulates apoptosis in cardiomyocytes, and contributes to MI/R injury.
Keywords: Nuclear receptors, Apoptosis, Myocytes
Introduction
Apoptosis has previously been shown to play an essential role in the pathogenesis of heart dysfunctions related to ischaemia/reperfusion (I/R), pressure overload, and chronic heart failure.1 Increased cardiomyocyte apoptosis results in contractile tissue loss, compensatory hypertrophy, and reparative fibrosis, all contributive to the development of cardiovascular diseases.1 Therefore, elucidating the mechanisms underlying cardiomyocyte apoptosis may yield novel targets in cardiac disorder treatment, with crucial applications to cardiovascular biology and clinical medicine.
Nuclear hormone receptors represent a family of transcription regulators involved in diverse physiological functions. Several members of this superfamily have been shown to be expressed in the heart and play a pivotal role in regulating cardiac apoptosis.2–5 The farnesoid-X-receptor (FXR; NR1H4) is a recently discovered member of the metabolic nuclear receptor superfamily.6 Farnesoid-X-receptor is expressed in the liver and gastrointestinal tract, where it regulates the homeostasis of cholesterol and bile acid.6 Farnesoid-X-receptor expression has also been reported in several other tissues not classically considered bile acid targets.7,8 Although the roles of FXR in these non-enterohepatic tissues remain largely unknown, several new and unexpected functions of FXR beyond its roles in metabolism were recently identified.6,8 More importantly, recent evidence suggests the presence of FXR in the vasculature,9 with important roles in vascular physiology/pathology.9,10 However, the potential function of FXR in the heart remains largely unknown.
In this study, we have investigated FXR expression in isolated cardiac cells and intact heart tissues. We report our findings that cardiomyocyte-expressed FXR is a novel apoptosis mediator and contributes to myocardial ischaemia/reperfusion (MI/R) injury.
Methods
This investigation conformed to the National Institutes of Health Guidelines on the Use of Laboratory Animals and was approved by the Thomas Jefferson University Committee on Animal Care. An expanded Methods section is available in the Supplementary material online, which includes detailed methods on the following: in vivo murine model of MI/R injury, echocardiography, haemodynamic measurements, infarct size analysis, cell culture and hypoxia/reoxygenation (H/R), in vitro and in vivo apoptosis analysis, western blot analysis, qualitative and quantitative PCR, immunofluorescence analysis, flow cytometry analysis, mitochondrial permeability transition pore (MPTP) and membrane potential (ΔΨm) analysis, cytochrome c ELISA, caspase activities, reactive oxygen species (ROS) assay, and statistical analysis.
Results
Expression profile of the farnesoid-X-receptor in cardiac cells and heart tissues
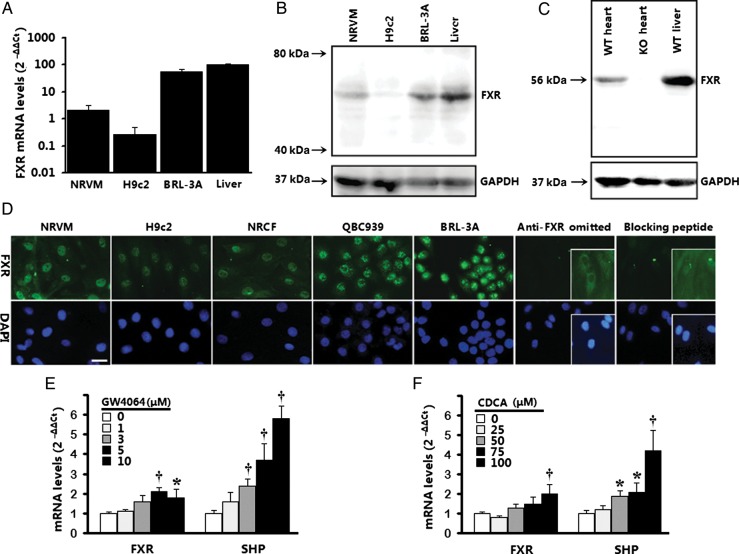
To determine whether FXR, a nuclear receptor originally thought to be primarily expressed in the liver/gastrointestinal tract, is expressed in cardiac cells, FXR expression in various cardiac cells and heart tissues was detected by western blot, real-time PCR, and immunofluorescence analysis. Low level of FXR mRNA was detected by real-time PCR in isolated cardiac myocytes, including neonatal rat ventricular myocytes (NRVMs) and H9c2 cardiac cells. Freshly isolated rat liver and BRL-3A buffalo rat liver cells served as positive controls (Figure 1A). Immunoblotting confirmed the presence of an FXR protein band at ∼56 kDa in positive controls, as well as in NRVMs and H9c2 cells (Figure 1B). To determine that FXR protein is expressed in adult heart tissues, we evaluated FXR expression by western blotting in adult mouse tissues from wild-type (WT) and FXR-deficient mice. Farnesoid-X-receptor protein appeared at the expected molecular weight of ∼56 kDa in the WT heart, but was not detected in the FXR-deficient heart (Figure 1C). In situ immunofluorescence demonstrated the predominantly nuclear location of FXR in NRVMs and H9c2 cells (Figure 1D). The specificity of involved protocol antibodies was confirmed by a pair of negative control experiments; one utilized a specific FXR-blocking peptide (sc-1204 P), and the other omitted the primary antibody. Specific FXR signal was drastically reduced by sc-1204 P, and was absent when primary antibody was omitted. Two positive controls exhibited a distinctive nuclear signal for FXR immunofluorescence, including cultured rat BRL-3A liver cells and human QBC939 cholangiocarcinoma cells (Figure 1D). Together, these data demonstrate constitutive expression of FXR mRNA and protein by cardiomyocytes. Additionally, we detected FXR expression in neonatal rat cardiac fibroblasts (NRCFs), another cell type in the heart (Figure 1D).
Figure 1.
Expression of farnesoid-X-receptor (FXR) in cardiac cells and heart tissues. (A) Farnesoid-X-receptor gene expression by real-time PCR in neonatal rat ventricular myocytes (NRVMs) and H9c2 ventricular cells. Rat liver tissues and BRL-3A rat liver cells served as positive control. Results are representative of three separate cultures. (B) Farnesoid-X-receptor protein expression by western blot in neonatal rat ventricular myocytes and H9c2 ventricular cells. Bands (∼56 kDa) are representative of three separate experiments. (C) Farnesoid-X-receptor protein expression by western blot in heart tissue from wild-type (WT) and farnesoid-X-receptor-knockout (KO) mice. Rat liver lysates from wild-type mice served as positive controls. (D) Immunolocalization of farnesoid-X-receptor in neonatal rat ventricular myocytes and H9c2 cells. Neonatal rat cardiac fibroblasts (NRCFs) are also shown. Primary antibody omission or pre-absorption with blocking peptide served as negative control. Bar = 20 μm. (E and F) Induction of farnesoid-X-receptor and its target gene SHP (small heterodimer partner) by farnesoid-X-receptor agonists (24 h; n= 3–4 per concentration) in neonatal rat ventricular myocytes using real-time PCR. Results were normalized against GAPDH and converted to percentage induction relative to vehicle controls. *P< 0.05, †P< 0.01 vs. vehicle-treated control.
Because activation-induced receptor autoregulation has been reported for FXR in some cell types, we next investigated whether FXR agonist-induced activation would regulate FXR expression in cardiomyocytes. Real-time quantitative PCR indicated that NRVMs activation by either natural (chenodeoxycholic acid, or CDCA) or synthetic (GW4064) FXR activators significantly induced FXR mRNA expression, suggesting the existence of a ligand-mediated auto-activation loop in cardiomyocytes (Figure 1E and F). Furthermore, both agonists significantly induced cardiomyocyte expression of the orphan nuclear receptor small heterodimer partner (SHP; NROB2), a well-known FXR target, in cardiomyocytes (Figure 1E and F).
Farnesoid-X-receptor activation by agonist decreased cardiomyocyte viability by triggering apoptosis
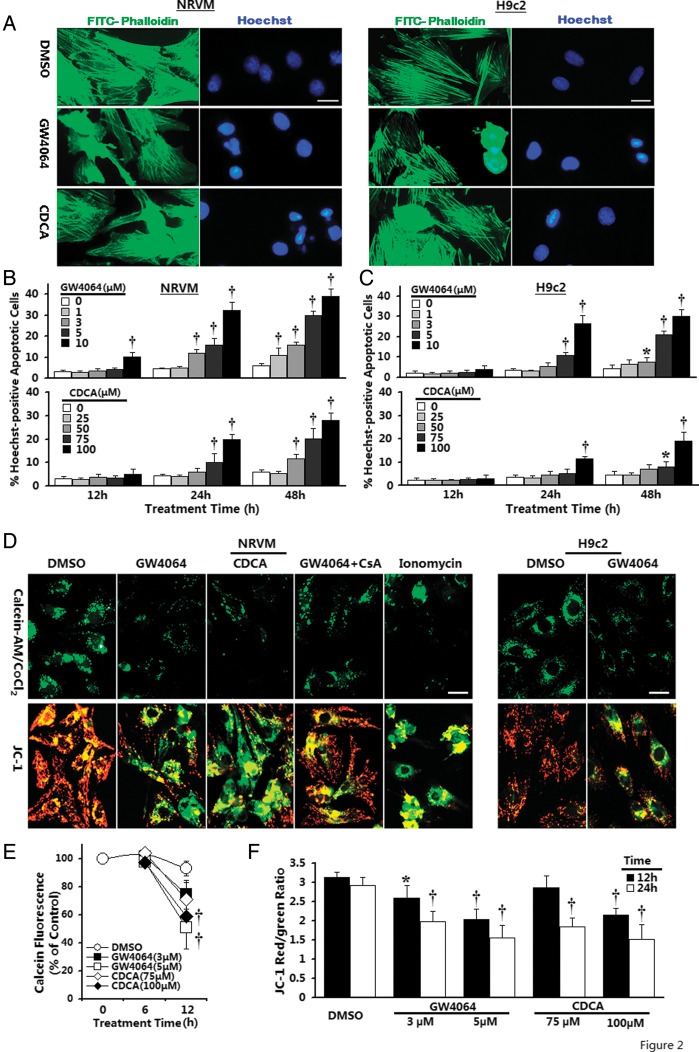
To initially explore the biological function of FXR in cardiomyocytes, we first examined the effect of FXR agonists upon cellular viability. Graded concentrations of GW4064 and CDCA were added to the medium and incubated for 6–72 h. Both GW4064 and CDCA reduced cell viability in a concentration- and time-dependent manner in NRVMs. At 6 h, almost all concentrations of GW4064 tested had no significant effect upon viability (data not shown). However, a 24 h treatment of NRVMs with 3 or 5 μmol/L GW4064 caused ∼15.1 or 26.9% reduction in cell viability, respectively, compared with DMSO-treated controls (Supplementary material online, Figure S1). Chenodeoxycholic acid, although effective, was less efficient than GW4064 in reducing the viability, with significant effects apparent at concentrations of 75 μmol/L after 24 h. To examine whether the FXR agonist-mediated reduction in cell viability resulted from apoptosis, we first employed Hoechst 33258 staining to identify apoptosis. Double-staining with Hoechst for nuclei and phalloidin for sarcomere organization in NRVMs demonstrated GW4064-induced typical apoptosis in a dose- and time-dependent manner (Figure 2A and B). For example, apoptotic nuclei were barely detectable (4.3%) after 12 h exposure to 5 μmol/L GW4064, but were evident (15.8%) in the cells after 24 h, and further elevated (29.8%) after 48 h. At 72 h, however, no further increase in apoptotic cells was visible because of the progression of the apoptotic cells to death, resulting in positive staining for Hoechst and propidium iodide, as well as loss of cells from the cover slip (data not shown). Chenodeoxycholic acid also reproducibly induced a similar although less potent apoptotic response than GW4064 (Figure 2B). Similar apoptotic responses were elicited in H9c2 cardiomyocytes, but relatively higher concentrations of GW4064 and CDCA were required to induce apoptosis in comparison with NRVMs (Figure 2C). To confirm the results of apoptosis from Hoechst 33258 staining, flow cytometric analysis with annexin V–FITC binding and JC-1 labelling was performed, both of which are detectors of early-stage apoptosis. Both GW4064 and CDCA treatment resulted in dose-dependent induction of early apoptosis compared with vehicle-treated NRVMs (Supplementary material online, Figure S2). These data strongly suggest that the activation of FXR induces the apoptotic death of cardiomyocytes at both early and later stages.
Figure 2.
Induction of nuclear condensation and mitochondrial permeability transition pore (MPTP) activation by farnesoid-X-receptor (FXR) activation. (A) Representative confocal images of neonatal rat ventricular myocytes (NRVMs) or H9c2 ventricular cells double-labelled with Hoechst 33258 for nuclei (blue) and phalloidin for cytoskeletal F-actin (green). Typical images shown are from cardiomyocytes after 24 h incubation with GW4064 (5 μmol/L) or chenodeoxycholic acid (CDCA) (100 μmol/L). Bar = 20 μm. (B and C) Time-course of induction of late apoptosis after GW4064 or chenodeoxycholic acid treatment in neonatal rat ventricular myocytes (B) or H9c2 (C) cardiomyocytes. n = 4–6 independent cultures. *P< 0.05, †P< 0.01 vs. vehicle-treated control. (D) Representative confocal images of mitochondrial permeability transition pore activation by calcein AM/CoCl2 labelling (top panel) and mitochondrial depolarization by JC-1 staining (bottom panel). Typical images shown are from cells after incubation with GW4064 (5 μmol/L) or chenodeoxycholic acid (100 μmol/L) for 12 h (neonatal rat ventricular myocytes) or 20 h (H9c2). Bar = 20 μm. (E) Time-courses of changes in mitochondrial calcein signal by flow cytometry after farnesoid-X-receptor agonist treatment (n = 3–4 independent experiments). †P< 0.01 compared with time-matched vehicle-treated control. (F) Quantitative data of mitochondrial ΔΨm by flow cytometry in neonatal rat ventricular myocytes (n = 3–4 independent experiments). *P< 0.05, †P< 0.01 compared with corresponding vehicle-treated control.
Opening of mitochondrial transmembrane pores
Alterations of mitochondrial function have been cited as a vital mechanism for apoptotic cell death in many systems. We investigated the potential activation of mitochondria-mediated signalling pathways by FXR activation. We first assessed MPTP activation by the calcein-AM loading/CoCl2 quenching technique, a method enabling direct visualization of the open/closed status of MPTP by monitoring subcellular distribution of calcein signals within intact living cells. As shown in confocal images (Figure 2D, top), control cells displayed distinct punctate calcein signals in mitochondria, indicative of MPTP in a closed configuration. Exposure to FXR agonists resulted in massive translocation of mitochondrially entrapped calcein into the cytoplasm and loss in punctate calcein fluorescence in mitochondria, indicating MPTP opening. The positive control ionomycin (a potent calcium ionophore, 1 μmol/L) induced a rapid decrease in calcein fluorescence from the preloaded mitochondria (Figure 2D, top). The fluorescence intensity of calcein was further quantified via FACScan. Temporal changes in the intra-mitochondrial calcein signal after FXR agonist treatment are shown in Figure 2E. To ensure that the decrease in intra-mitochondrial calcein staining was related to MPTP changes and not non-specific injury to mitochondrial membranes, cells were treated with cyclosporin A (CsA), a known MPTP inhibitor. Cyclosporin A (1 μmol/L) was efficient at inhibiting FXR agonist-induced calcein leakage from mitochondria, confirming the involvement of MPTP mechanism (Figure 2D, top).
Dissipation of mitochondrial membrane potential
Mitochondrial dysfunction was further studied in living cells by examining mitochondrial ΔΨm, which was determined in situ using JC-1 in both NRVMs and H9C2 cardiomyocytes. Control cells showed bright, thread-like red-orange fluorescent JC-1 aggregates within mitochondria, representing normally hyperpolarized membrane potential (Figure 2D, bottom). In contrast, cells after FXR agonist treatment displayed a shift in JC-1 fluorescence, from red to green signal, presumably due to reduced JC-1 aggregate formation and the diffusion of JC-1 molecules into the cytosol, following the depolarization of mitochondria. The depression of mitochondrial ΔΨm was further confirmed by flow cytometry, which demonstrated decrease in the red/green fluorescence intensity ratio (Figure 2F).
Translocation of cytochrome c
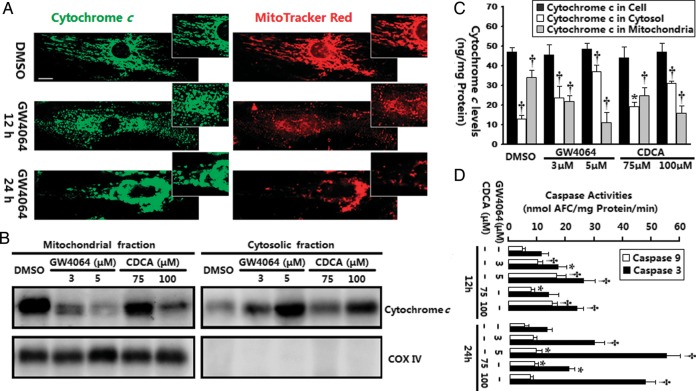
We next investigated whether FXR activation induced the translocation of cytochrome c, using several different approaches. Subcellular cytochrome c localization was first analysed with confocal immunofluorescence. As shown in Figure 3A, cytochrome c staining in control cells revealed a wispy, cigar-shaped subcellular localization pattern, surrounding the nucleus but extending deeply into the cytosol. The subcytoplasmic cytochrome c co-localized with a specific mitochondrial marker, MitoTracker Red, confirming its mitochondrial location. When cells were subjected to FXR agonist treatment, there was a shift in the fluorescence pattern of cytochrome c to a diffuse cytoplasmic state. Interestingly, we also observed that the distribution of MitoTracker Red-labelled mitochondria underwent significant changes during this period. Mitochondria in certain cells did not retain the classical, extended tubular rod-like network, but displayed a spherical dot-shaped distribution, with tendency to aggregate (Figure 3A, inset). Western blot analysis confirmed the mitochondrial release of cytochrome c (Figure 3B). Figure 3C displays quantification data for cytochrome c redistribution by the sandwich enzyme immunoassay. Taken together, three distinct methods confirmed leakage of cytochrome c after FXR ligand stimulation.
Figure 3.
Induction of cytochrome c release and caspases activation by farnesoid-X-receptor (FXR) activation. (A) Confocal images of neonatal rat ventricular myocytes (NRVMs) stained with 2CYTC-199 antibody for cytochrome c (green) and MitoTracker for mitochondria (red). Typical images shown are from neonatal rat ventricular myocytes after incubation with GW4064 (5 μmol/L) for 12 and 24 h (n = 3–5 independent experiments, the same for all other assays). Amplified images show the alteration in mitochondrial organization after treatment with farnesoid-X-receptor agonists. Bar = 10 μm. (B) Western blot of cytochrome c distribution was done on mitochondrial and cytosolic fractions from neonatal rat ventricular myocytes exposed to farnesoid-X-receptor agonists for 12 h. Cytochrome oxidase IV (COX IV) was utilized to verify the quality of the extraction procedure. (C) Quantitative analysis of cytochrome c in mitochondrial and cytosolic fractions by sandwich ELISA. *P< 0.05, †P< 0.01 vs. vehicle-treated control. (D) Caspase-9-like and caspase-3-like activities in neonatal rat ventricular myocytes were assessed by fluorometric assay. *P< 0.05, †P< 0.01 vs. corresponding vehicle-treated control.
Activation of caspase-9/caspase-3
To determine the effects of FXR activation upon caspase cascades, both caspase-9 and caspase-3 activity were tested. As shown in Figure 3D, the activity of caspase-9, an initiator caspase in the mitochondrial pathway, significantly increased with a peak at 12 h after treatment with FXR agonists in NRVMs and declined thereafter. Activation of caspase-3, a critical effector caspase, was strongly induced with a peak at 24 h after treatment with FXR agonists (Figure 3D). Thus, the peak of activation of caspase-9 preceded that of caspase-3, and these two peaks were precedence of the peak of percentage of apoptotic cells (Figure 2B). No significant activation of caspase-8 (an initiator caspase of the extrinsic pathway)11 and caspase-12 (an endoplasmic reticulum-specific stress-activated caspase)12 activities was observed at time points up to 24 h (data not shown). Overall, these observations demonstrated that FXR activation-induced apoptosis is mainly mediated by a mitochondrial-mediated cytochrome c-dependent caspase-9 activation pathway.
Regulation of BCL-2/BAX expression
To gain deeper understanding of the signalling events involved in FXR-induced cardiomyocyte apoptosis and mitochondrial dysfunction, we examined the effect of FXR agonists upon expression of BCL-2 family proteins, which have been implicated as major regulators of mitochondrial homeostasis in many cellular events. Farnesoid-X-receptor activation by agonists inhibited BCL-2 expression, and promoted conformational BAX activation (detailed in Supplementary material online, Figures S3–S5).
Effects of in vitro farnesoid-X-receptor silencing on apoptotic mitochondrial changes in cardiomyocytes
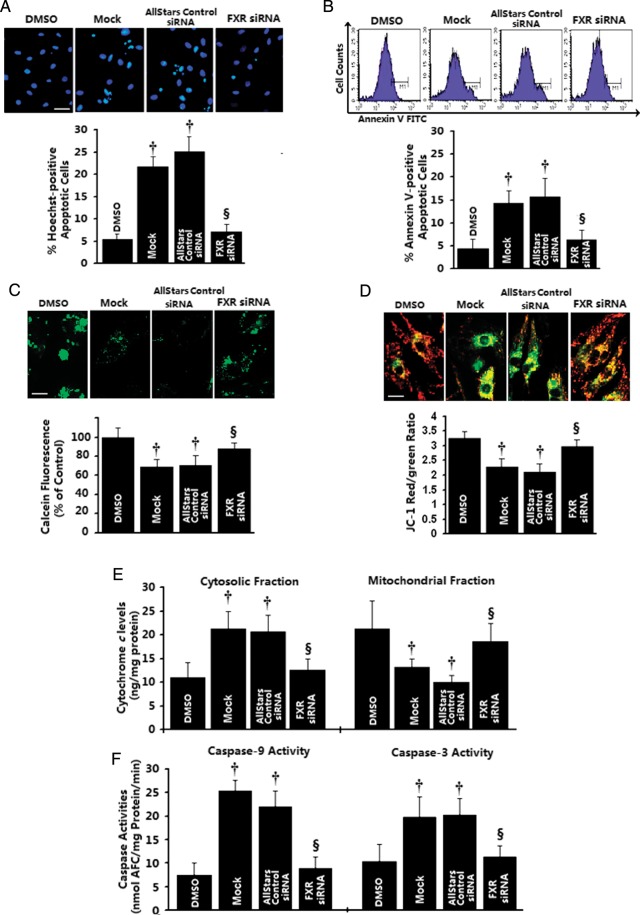
To more firmly establish the direct role of FXR in the regulation of cardiomyocyte apoptosis, we silenced endogenous FXR expression via specific siRNA targeting rat FXR. Cells were transfected with the FXR siRNAs, whereas control wells were mock-transfected or transfected with AllStars Negative Control siRNA. Farnesoid-X-receptor siRNA significantly reduced FXR transcript levels in a concentration-dependent manner, with 20 nmol/L FXR siRNA reducing FXR mRNA levels by 60.5 ± 7.7% in NRVMs and 68.0 ± 9.2% in H9c2 cardiomyocytes compared with mock-transfected, whereas transfection with control siRNA had no effect (Supplementary material online, Figure S6). Next, to explore whether FXR siRNA blocked the induction of apoptosis, cells were stimulated with GW4064 (5 μmol/L) or vehicle for 24 h after siRNA transfection. Without the FXR agonist challenge, FXR-knockdown cells did not differ in cell viability from those treated with scrambled siRNA. Transfection of NRVMs with FXR-specific siRNA, but not scrambled siRNA, blunted GW4064-induced apoptosis in NRVMs, as evidenced by Hoechst staining for the detection of nuclear condensation (7.1% for FXR siRNA vs. 21.8% for mock control, P< 0.01; Figure 4A) as well as by annexin V–FITC staining for phosphatidylserine translocation (6.4% for FXR siRNA vs. 14.3% for mock control, P< 0.01; Figure 4B). Furthermore, suppression of FXR by siRNA attenuated the FXR agonists-induced apoptotic mitochondrial changes in NRVMs, including MPTP opening, mitochondrial ΔΨm collapse, cytochrome c release, and LEHD-specific and DEVD-specific caspase activation (Figure 4C–F). No effects were observed from negative-control siRNA. Taken together, these observations provide further evidence of a direct role for FXR signalling in regulating cardiomyocyte survival and apoptosis.
Figure 4.
Effects of in vitro farnesoid-X-receptor (FXR) silencing on apoptotic mitochondrial changes in cardiomyocytes. Neonatal rat ventricular myocyte (NRVMs) were transfected with 20 nmol/L of farnesoid-X-receptor siRNA, AllStars Negative siRNA, or mock-treated for 24 h, followed by GW4064 (5 μmol/L) treatment for 12 (B–F) or 24 h (A). DMSO-treated cells were utilized as control. (A and B) Late apoptosis assessed by Hoechst staining (A, n= 4 independent experiments; bar= 40 μm) and early apoptotic cells as assessed by annexin V staining (B, n= 3 independent experiments). (C) Representative confocal images of mitochondrial permeability transition pore opening measured by calcein-AM/CoCl2 quenching (upper) and quantitative data by flow cytometry (bottom, n= 3–4 independent experiments). (D) Representative confocal images of mitochondrial depolarization by JC-1 staining (upper) and quantitative data by flow cytometry (bottom, n= 3–4 independent experiments). (E) Mitochondrial and cytosolic cytochrome c levels measured by sandwich ELISA (n= 4 independent experiments). (F) Caspase-9-like and caspase-3-like activities were measured using the colorimetric substrate LEHD-AFC or DEVD-AFC (n= 3–4 independent experiments). *P< 0.05, †P< 0.01 compared with vehicle-treated cells, ‡P< 0.05, §P< 0.01 compared with GW4064-treated cells.
Effect of blocking mitochondrial permeability transition pore or various caspases on farnesoid-X-receptor activation-induced myocyte apoptosis
To gain further evidence for the involvement of mitochondrial signalling pathway in FXR-mediated apoptosis, cardiomyocytes were pre-incubated with 1 μmol/L CsA for 1 h before exposure to 5 μmol/L GW4064 for 24 h. DMSO served as vehicle control. Treatment of myocytes with CsA alone did not affect the apoptotic baseline percentage. However, CsA significantly (all P< 0.01) reversed the pro-apoptotic effect of GW4064 in NRVMs, evidenced by a 41.7% reduction in Hoechst-positive and a 48.7% reduction in annexin V-positive staining cells, a 1.4-fold increase in mitochondrial ΔΨm, a 38.5% reduction in mitochondrial cytochrome c release, a 61.0% reduction in caspase-9 activity, and a 26.0% reduction in caspase-3 activation (Supplementary material online, Figure S7). These data further confirm the functional importance of mitochondrial pathway activation in FXR-mediated apoptosis.
To identify possible involvement of other specific pathways in FXR-mediated apoptosis, we conducted another series of blocking experiments in which cells were pre-incubated with specific caspase-9 inhibitor Z-LEHD-fmk (50 μmol/L), caspase-8 inhibitor Z-IETD-fmk (50 μmol/L), or caspase-12 inhibitor Z-ATAD-fmk (50 μmol/L) for 1 h before exposure to 5 μmol/L GW4064 for 24 h. DMSO was added to cells without inhibitors as negative controls. Treatment of myocytes with various caspase inhibitors at indicated concentrations alone did not affect the baseline percentage of apoptosis (Supplementary material online, Figure S7). However, inhibition of caspase-9 activity with Z-LEHD-fmk abolished the apoptotic effects induced by GW4064 in NRVMs, as evidenced by Hoechst staining (−58.0%) and annexin V staining (−62.5%), without significant effects on the mitochondrial ΔΨm and cytochrome c levels. The specific caspase-8 inhibitor Z-LEHD-fmk and caspase-12 inhibitor Z-ATAD-fmk had no significant effects on these apoptotic response induced by GW4064. These results offered further support for the critical role of mitochondria signalling in FXR-mediated apoptosis.
Expression and activation of farnesoid-X-receptor in ischaemic/reperfused myocardium in vivo
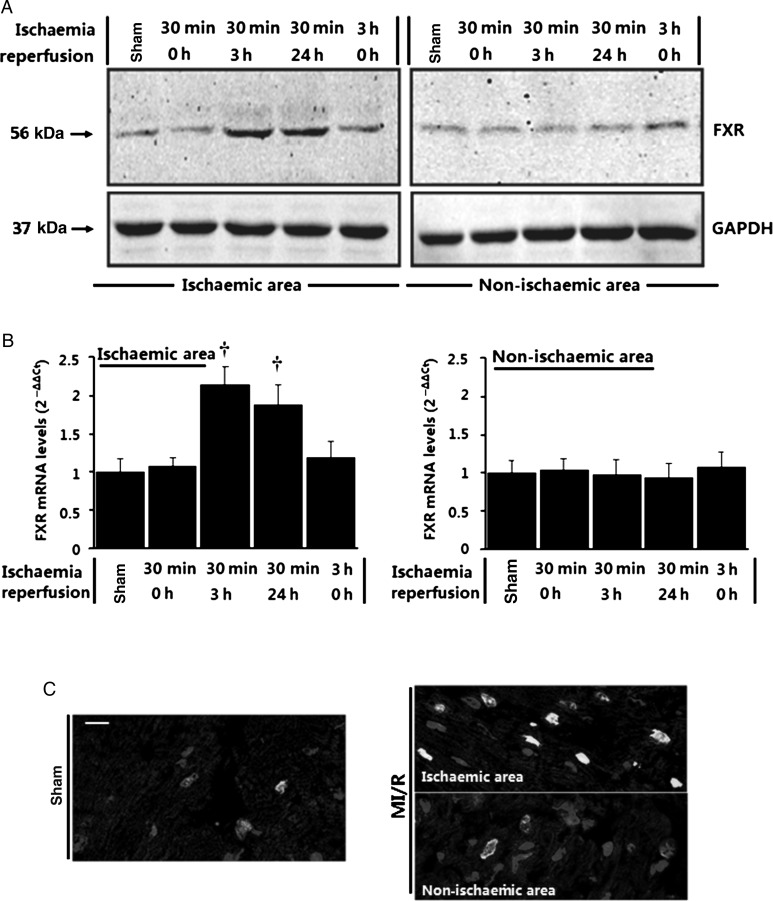
To address the pathophysiological relevance of our in vitro findings from cultured cells, we investigated the expression of FXR in a real pathological condition, i.e. in vivo MI/R. As shown in Figure 5A, low FXR levels were detected in cardiac tissue from normal control mice subjected to sham MI/R. More importantly, a significant increase in FXR was observed in cardiac tissue obtained from ischaemic and reperfused tissue (Figure 5A and B). Time-course studies of FXR expression suggested that FXR levels were not significantly changed during ischaemia, but markedly increased in the ischaemic area at risk by 2.1-fold at 3 h after reperfusion and remained elevated at 24 h after reperfusion (Figure 5A and B). In contrast, FXR levels in the non-ischaemic area were not significantly changed during I/R, suggesting that FXR activation was confined to areas of tissue suffering the reperfusion injury. No significant FXR activation was observed when ischaemia alone continued for up to 3 h (Figure 5A and B). Analysis of the sites of FXR activation was further undertaken by confocal immunofluorescence analysis of left ventricular myocardial sections (Figure 5C). A marked increase in FXR signal was mainly observed in cardiomyocytes in the ischaemic area but not in the non-ischaemic area. Considered together, these data are consistent with FXR activation in the ischaemic risk territory during the reperfusion period.
Figure 5.
Upregulation of farnesoid-X-receptor (FXR) by ischaemia/reperfusion stimuli in the heart. (A and B) Time course of farnesoid-X-receptor activation detected by western blots (A) and real-time quantitative PCR (B) in the heart (n= 5–6 per time point, ischaemic and non-ischaemic areas) subjected to in vivo ischaemia/reperfusion (I/R) for the indicated times. Sham-operated animals were utilized as control. Results were normalized against GAPDH and converted to fold induction relative to sham-operated controls. *P< 0.05, †P< 0.01 compared with sham-operated controls. (C) Confocal immunofluorescence analysis on heart sections from sham-operated and ischaemia/reperfusion mice 24 h after surgery. Sections were subjected to immunofluorescence for farnesoid-X-receptor (green) and α-actinin (red) and to staining for nuclei with DAPI (blue), and overlays are shown (Bar= 20 μm).
Mechanism of farnesoid-X-receptor activation during ischaemia/reperfusion-role of reactive oxygen species
It is well known that ROS play a pivotal role in I/R-induced cardiomyocyte apoptosis by activating numerous signalling pathways.13 Recent studies indicated that activities of some nuclear receptors could be regulated by a redox mechanism.14 To investigate whether ROS are responsible for the activation of FXR by I/R, we utilized an in vitro model of simulated I/R. Cultured NRVMs were subjected to 6 h hypoxia, followed by reoxygenation of the indicated periods. Time-course studies of ROS production suggested that ROS increased significantly during reoxygenation, peaking 1 h after reoxygenation (Supplementary material online, Figure S8A). Hypoxia insult for 6 h alone did not significantly affect FXR expression in myocytes; however, the levels of FXR increased significantly within 3–6 h of reoxygenation (Supplementary material online, Figure S8B). The expression of FXR target gene, SHP, also significantly increased during reoxygenation, with similar kinetics to FXR (Supplementary material online, Figure S8B). To determine whether ROS act as the upstream initiators for the activation of FXR by I/R, cells were pre-incubated with ROS inhibitors, N-t-butyl-α-phenylnitrone (10 mmol/L) or N-acetyl cysteine (10 mmol/L) 1 h before reoxygenation. Both antioxidants abrogated H/R-induced ROS production and the activation of FXR in response to reoxygenation (Supplementary material online, Figure S8C). Direct exposure of myocytes to H2O2 was also found to elicit FXR activation, as evidenced by increased expression of FXR as well as its target gene, SHP, in a concentration-dependent manner (Supplementary material online, Figure S8D). Interestingly, when cardiac fibroblasts obtained from the same cultures were subjected to the same H/R protocol, we did not observe any significant changes of FXR expression in cardiac fibroblasts (Supplementary material online, Figure S8E). Thus, although both myocytes and fibroblasts express FXR, only myocytes exhibit a significant response to hypoxia-reoxygenation with an increase in FXR.
Activation of farnesoid-X-receptor contributed to the myocardial ischaemia/reperfusion injury
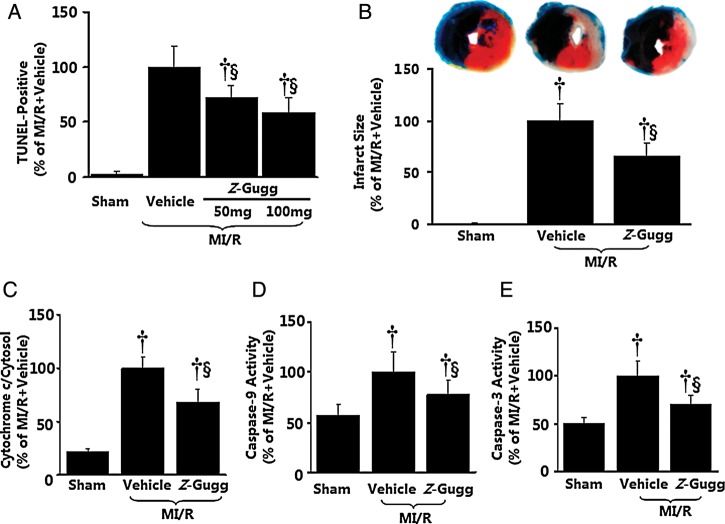
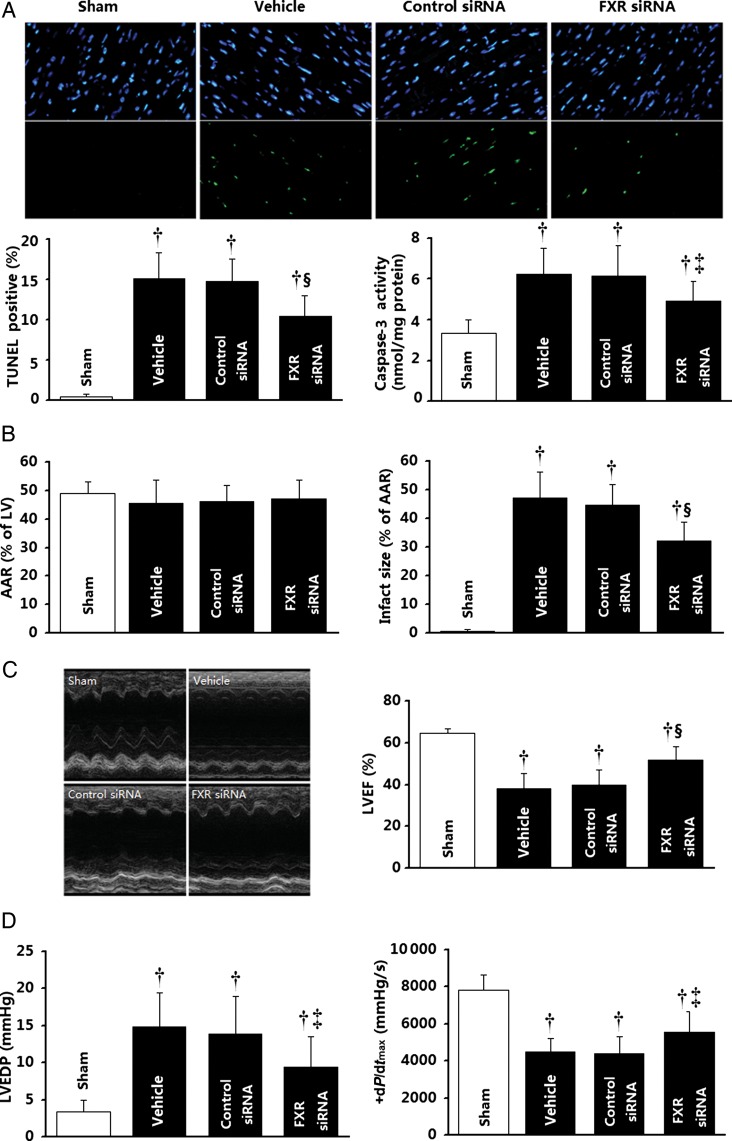
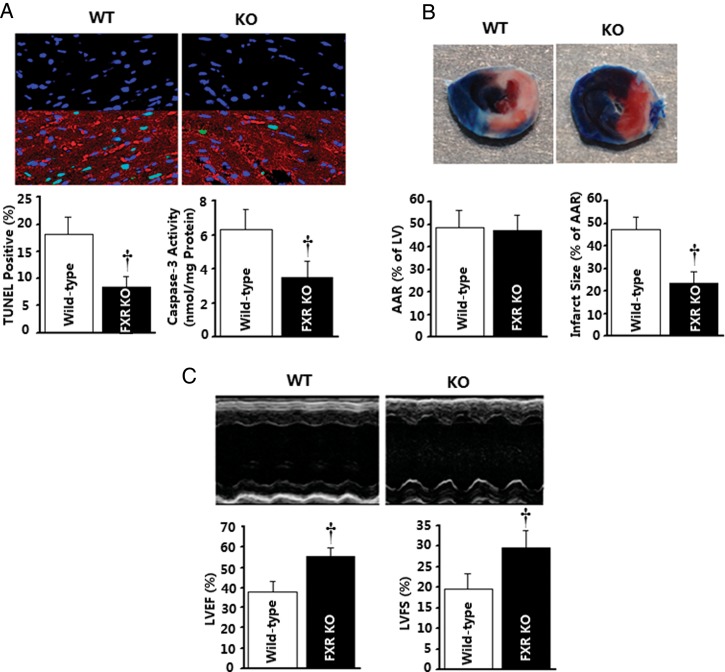
To assess the role of FXR in post-ischaemic myocardial injury, the effect of pharmacological FXR inhibition and siRNA FXR silencing upon MI/R injury was determined. Treatment with Z-guggulsterone (an established FXR antagonist15) 15 min before reperfusion significantly (all P< 0.01) reduced cardiomyocyte apoptosis determined 3 h after reperfusion, as evidenced by reduced TUNEL-positive cells (−41.6%), cytochrome c release (−31.6%), and caspase-3 (−30.4%) activities (Figure 6A, C–E), and decreased myocardial infarct size (−34.2%) determined 24 h after reperfusion (Figure 6B). Direct in vivo intra-myocardial injection of FXR siRNA (cardiac FXR-KD) markedly inhibited mouse cardiac FXR protein expression, with ∼70% inhibition 48 h after siRNA injection (Supplementary material online, Figure S9). Most importantly, this treatment markedly reduced I/R-induced myocardial apoptotic responses as assessed by reduced TUNEL staining (−29.0%) and caspase-3 activity (−20.3%) determined 3 h after reperfusion (Figure 7A) compared with the control siRNA group. Moreover, FXR-KD mice exhibited a 23.4% reduction in infarct size (Figure 7B), as well as enhanced left ventricular ejection fraction (LVEF) by echocardiography (Figure 7C) and improved left ventricular performance (i.e. left ventricular end-diastolic pressure and maximal first derivative of left ventricular pressure) by catheter-based haemodynamic measurement determined 24 h after reperfusion (Figure 7D). To further confirm the role of the FXR in the ischaemia/reperfusion injury, FXR-KO and matched WT C57BL/6 mice were subjected to MI/R. Compared with WT mice, FXR-deficient mice exhibited reduced myocardial apoptosis as determined by TUNEL labelling (−53.4%) and caspase-3 activation (−45.4%) at 3 h after reperfusion (Figure 8A), accompanied by a 49.7% reduction in infarct size (Figure 8B) and a 1.5-fold improved LVEF by echocardiography (Figure 8C) at 24 h after reperfusion. These results provide strong evidence that the nuclear receptor FXR activation plays an important pathogenic role in MI/R injury.
Figure 6.
Effect of pharmacological inhibition of farnesoid-X-receptor (FXR) on myocardial ischaemia/reperfusion injury. (A–E) Farnesoid-X-receptor antagonist Z-guggulsterone (Z-Gugg) reduced post-ischaemic myocardial apoptotic responses determined by TUNEL labelling (A, n= 8–10 hearts per group), cytochrome c release (C, n= 7–8 hearts per group), caspase-9 and -3 activation (D and E, n= 8–12 hearts per group), and myocardial infarct size determined by Evans blue/TTC double-staining (B, n= 10–12 hearts per group). Mean values obtained from vehicle-treated myocardial ischaemia/reperfusion animals were treated as 100%, and individual values from each animal were normalized against the mean values. †P< 0.01 vs. sham; §P< 0.01 vs. myocardial ischaemia/reperfusion + vehicle.
Figure 7.
Effect of genetic inhibition of farnesoid-X-receptor (FXR) by in vivo siRNA transfection upon ischaemia/reperfusion injury. (A) Apoptosis as determined by TUNEL labelling (n= 6–8 hearts per group) and caspase-3 activation (n= 8–11 hearts per group). (B) Myocardial infarction was determined by Evans blue/TTC double-staining (n= 8–10 hearts per group). (C and D) Cardiac function was determined by echocardiography (n= 8–10 animals per group) and catheter-based haemodynamic measurements (n= 8–9 animals per group). Myocardial ischaemia/reperfusion procedures were performed 48 h after intramyocardial siRNA delivery. *P< 0.05, †P< 0.01 vs. the sham group; ‡P< 0.05, §P< 0.01 vs. the control siRNA group. AAR, area at risk; LVEF, left ventricular ejection fraction; LVEDP, left ventricular end-diastolic pressure.
Figure 8.
Myocardial injury caused by ischaemia/reperfusion is reduced in farnesoid-X-receptor (FXR)-deficient mice. Farnesoid-X-receptor-knockout (KO) mice and wild-type (WT) mice were subjected to 30 min of ischaemia and 3 (A) or 24 h (B and C) of reperfusion. (A) Myocardial apoptosis as determined by TUNEL labelling (n= 6 hearts per group) and caspase-3 activation (n= 6 hearts per group). (B) Myocardial infarction was determined by Evans blue/TTC double-staining (n= 5–6 hearts per group). (C) Cardiac function was determined by echocardiography (n= 6 animals per group). †P< 0.01 vs. the wild-type group. AAR, area at risk; LVEF, left ventricular ejection fraction.
Discussion
In the present work, we identify a novel role for the nuclear receptor FXR in cardiomyocytes. The novel contributions include the following: (i) Using cultured cardiomyocytes, we have demonstrated that FXR is expressed in various cardiac cells, and activation of FXR causes significant cardiomyocyte apoptosis. (ii) Mechanistic studies demonstrated that activation of FXR stimulated mitochondrial death signalling, characterized by MPTP activation, mitochondrial potential dissipation, cytochrome c release, and both caspase-9 and -3 activation. (iii) siRNA-mediated silencing of endogenous FXR or pharmacological inhibition of mitochondrial death signal significantly suppressed FXR agonists-mediated apoptotic responses in cardiomyocytes. (iv) The involvement of FXR myocardial apoptosis was further demonstrated in a real pathological condition, i.e. in vivo myocardial ischaemia followed by reperfusion. Pharmacological inhibition or genetic ablation of FXR significantly reduced infarct size and improved cardiac function, indicating that FXR activation in response to MI/R is a novel mechanism contributing to MI/R injury. Taken together, these results demonstrate for the first time that nuclear receptor FXR acts as a novel apoptosis mediator in cardiomyocytes, and contributes to MI/R injury.
Several members of the nuclear hormone receptor superfamily have been reported to regulate cardiac apoptosis, including the peroxisome proliferator-activated receptors (PPARα, β, and γ),2 retinoic acid receptor,3 oestrogen receptor, and androgen receptor.4 Recently, we reported that retinoic-X-receptor (RXR), another nuclear hormone receptor, negatively regulated cardiomyocyte apoptosis via mitochondrial-protective mechanisms.5 The present study further characterized FXR as an endogenous positive regulator in cardiomyocytes. In view of the distinct regulatory roles for these nuclear receptors in cardiomyocyte apoptosis, it is conceivable that a potential regulatory cross-talk among them might serve to maintain the delicate homeostatic balance between cell death and survival. Of particular interest is a recent human HepG2 cell study that reveals that RXR activation may antagonize FXR activity by decreasing FXR DNA binding and preventing co-activator recruitment.16 Additionally, in human HepG2 cells, activation of FXR by agonists was found to induce expression of PPARα gene and its target genes.17 Therefore, the characterization of the molecular cross-talk between FXR and other nuclear receptor (i.e. RXR or PPAR) pathways would be requisite for complete understanding of cardiomyocyte apoptosis processes.
Clinical implications of this study
The current experimental findings are scientifically and clinically important. The identification of FXR as a novel functional receptor in cardiomyocytes may broaden our understanding of the multiple biological functions of FXR beyond the enterohepatic system. Our observations, along with the recent findings regarding the role of FXR in vascular tissue from several research groups,9,10 provide support for FXR as an important regulator of cardiovascular biology. Demonstration of FXR-specific ligands and siRNAs modulating apoptosis implies an important role for FXR in survival signalling and death regulation in cardiomyocytes. Therefore, it is conceivable that dysregulation or malfunction of FXR may play a role in several cardiac diseases related to cardiomyocyte growth and apoptosis, and that FXR represents a potential molecular therapeutic target for the treatment of cardiac disease.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by grants 81070176, 81170192, 30600242, and 30670880 from the National Natural Science Foundation of China (to B.H. and J.P.), and by NIH HL-63828, HL-096686, and American Diabetes Association 7-11-BS-93 (to X.-L.M).
Conflict of interest: none.
Supplementary Material
References
- 1.Movassagh M, Foo RS. Simplified apoptotic cascades. Heart Fail Rev. 2008;13:111–119. doi: 10.1007/s10741-007-9070-x. [DOI] [PubMed] [Google Scholar]
- 2.Son NH, Yu S, Tuinei J, Arai K, Hamai H, Homma S, Shulman GI, Abel ED, Goldberg IJ. PPARγ-induced cardiolipotoxicity in mice is ameliorated by PPARα deficiency despite increases in fatty acid oxidation. J Clin Invest. 2010;120:3443–3454. doi: 10.1172/JCI40905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhary R, Baker KM, Pan J. All-trans retinoic acid prevents angiotensin II- and mechanical stretch-induced reactive oxygen species generation and cardiomyocyte apoptosis. J Cell Physiol. 2008;215:172–181. doi: 10.1002/jcp.21297. [DOI] [PubMed] [Google Scholar]
- 4.Morrissy S, Xu B, Aguilar D, Zhang J, Chen QM. Inhibition of apoptosis by progesterone in cardiomyocytes. Aging Cell. 2010;9:799–809. doi: 10.1111/j.1474-9726.2010.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shan P, Pu J, Yuan A, Shen L, Shen L, Chai D, He B. RXR agonists inhibit oxidative stress-induced apoptosis in H9c2 rat ventricular cells. Biochem Biophys Res Commun. 2008;375:628–633. doi: 10.1016/j.bbrc.2008.08.074. [DOI] [PubMed] [Google Scholar]
- 6.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 7.Higashiyama H, Kinoshita M, Asano S. Immunolocalization of farnesoid X receptor (FXR) in mouse tissues using tissue microarray. Acta Histochem. 2008;110:86–93. doi: 10.1016/j.acthis.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Swales KE, Korbonits M, Carpenter R, Walsh DT, Warner TD, Bishop-Bailey D. The farnesoid X receptor is expressed in breast cancer and regulates apoptosis and aromatase expression. Cancer Res. 2006;66:10120–10126. doi: 10.1158/0008-5472.CAN-06-2399. [DOI] [PubMed] [Google Scholar]
- 9.Bishop-Bailey D, Walsh DT, Warner TD. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci USA. 2004;101:3668–3673. doi: 10.1073/pnas.0400046101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hageman J, Herrema H, Groen AK, Kuipers F. A role of the bile salt receptor FXR in atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:1519–1528. doi: 10.1161/ATVBAHA.109.197897. [DOI] [PubMed] [Google Scholar]
- 11.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J, Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 13.Münzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;31:2741–2748. doi: 10.1093/eurheartj/ehq396. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Z, Völkers M, Din S, Avitabile D, Khan M, Gude N, Mohsin S, Bo T, Truffa S, Alvarez R, Mason M, Fischer KM, Konstandin MH, Zhang XK, Heller Brown J, Sussman MA. Mitochondrial translocation of Nur77 mediates cardiomyocyte apoptosis. Eur Heart J. 2011;32:2179–2188. doi: 10.1093/eurheartj/ehq496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urizar NL, Liverman AB, Dodds DT, Silva FV, Ordentlich P, Yan Y, Gonzalez FJ, Heyman RA, Mangelsdorf DJ, Moore DD. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science. 2002;296:1703–1706. doi: 10.1126/science.1072891. [DOI] [PubMed] [Google Scholar]
- 16.Kassam A, Miao B, Young PR, Mukherjee R. Retinoid X receptor (RXR) agonist-induced antagonism of farnesoid X receptor (FXR) activity due to absence of coactivator recruitment and decreased DNA binding. J Biol Chem. 2003;278:10028–10032. doi: 10.1074/jbc.M208312200. [DOI] [PubMed] [Google Scholar]
- 17.Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor α gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17:259–272. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.