Abstract
The basis for nucleolar dominance in mouse-human cell hybrids which contained all of the mouse chromosomes but an incomplete set of human chromosomes (M greater than H) was examined at the molecular level. S1 mapping data showed that these cells had the expected levels of steady-state rRNA transcribed from mouse ribosomal gene (rDNA) transcription units but undetectable levels of rRNA derived from the human rDNA transcription templates that are also present. RNA polymerase I-dependent, cell-free transcription extracts were made from three hybrid lines and were found to be capable of transcribing cloned rDNA templates of mouse but not human origin. Partially purified human factors required for rDNA transcription in vitro were added to the M greater than H extracts. One fraction with almost no RNA polymerase I activity conferred on these hybrid cell extracts the ability to transcribe a human rDNA template. These rescue experiments suggested that this required human-specific rDNA transcription factor(s) was effectively absent from the lines we examined and could account for nucleolar dominance in M greater than H hybrid cells.
Full text
PDF



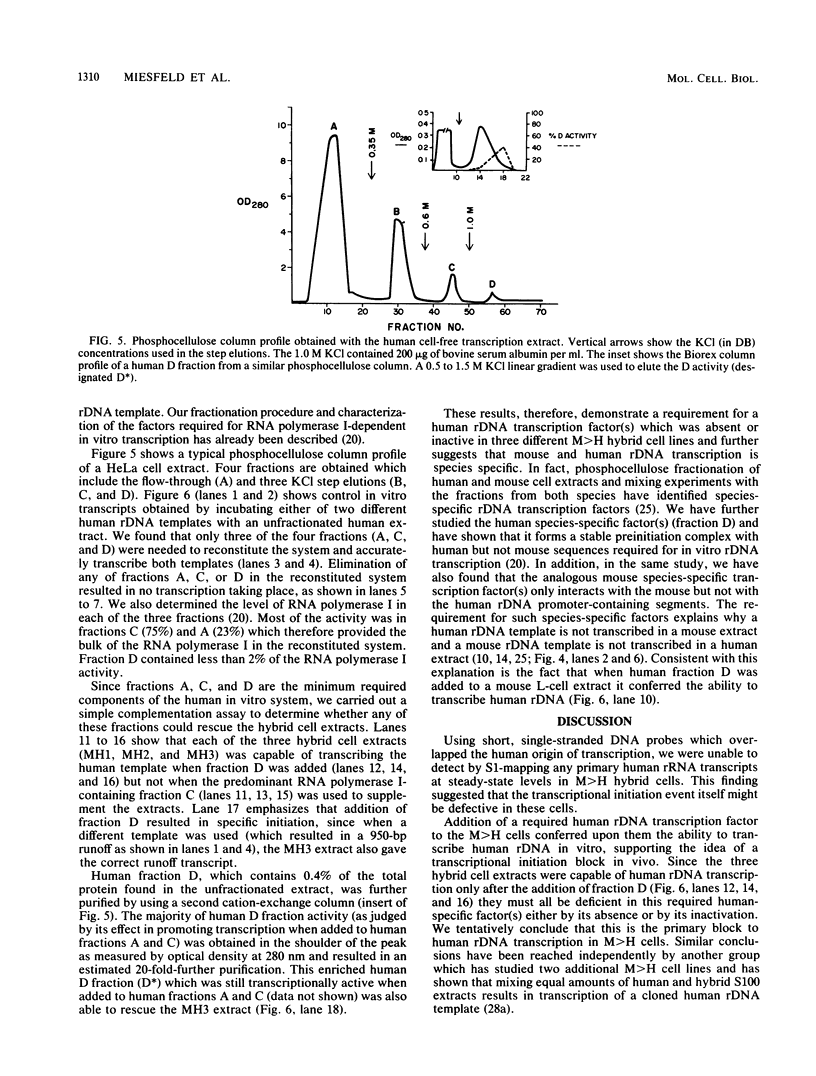


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajiro K., Zweidler A., Borun T., Croce C. M. Species-specific suppression of histone H1 and H2B production in human/mouse hybrids. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5599–5603. doi: 10.1073/pnas.75.11.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnheim N., Seperack P., Banerji J., Lang R. B., Miesfeld R., Marcu K. B. Mouse rDNA nontranscribed spacer sequences are found flanking immunoglobulin CH genes and elsewhere throughout the genome. Cell. 1980 Nov;22(1 Pt 1):179–185. doi: 10.1016/0092-8674(80)90166-x. [DOI] [PubMed] [Google Scholar]
- Arnheim N., Southern E. M. Heterogeneity of the ribosomal genes in mice and men. Cell. 1977 Jun;11(2):363–370. doi: 10.1016/0092-8674(77)90053-8. [DOI] [PubMed] [Google Scholar]
- Blackler A. W., Gecking C. A. Transmission of sex cells of one species through the body of a second species in the genus Xenopus. II. Interspecific matings. Dev Biol. 1972 Mar;27(3):385–394. doi: 10.1016/0012-1606(72)90177-7. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Blackler A. W. Gene amplification proceeds by a chromosome copy mechanism. J Mol Biol. 1972 Jan 14;63(1):75–83. doi: 10.1016/0022-2836(72)90522-0. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Talavera A., Basilico C., Miller O. J. Suppression of production of mouse 28S ribosomal RNA in mouse-human hybrids segregating mouse chromosomes. Proc Natl Acad Sci U S A. 1977 Feb;74(2):694–697. doi: 10.1073/pnas.74.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev V. G., Miller D. A., Rechsteiner M., Miller O. J. Time of suppression of human rRNA genes in mouse-human hybrid cells. Exp Cell Res. 1979 Oct 1;123(1):47–54. doi: 10.1016/0014-4827(79)90419-1. [DOI] [PubMed] [Google Scholar]
- Eliceiri G. L., Green H. Ribosomal RNA synthesis in human-mouse hybrid cells. J Mol Biol. 1969 Apr;41(2):253–260. doi: 10.1016/0022-2836(69)90390-8. [DOI] [PubMed] [Google Scholar]
- Financsek I., Mizumoto K., Mishima Y., Muramatsu M. Human ribosomal RNA gene: nucleotide sequence of the transcription initiation region and comparison of three mammalian genes. Proc Natl Acad Sci U S A. 1982 May;79(10):3092–3096. doi: 10.1073/pnas.79.10.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt I., Roth E., Paule M. R. Ribosomal RNA transcription in vitro is species specific. Nature. 1982 Mar 11;296(5853):173–174. doi: 10.1038/296173a0. [DOI] [PubMed] [Google Scholar]
- Honjo T., Reeder R. H. Preferential transcription of Xenopus laevis ribosomal RNA in interspecies hybrids between Xenopus laevis and Xenopus mulleri. J Mol Biol. 1973 Oct 25;80(2):217–228. doi: 10.1016/0022-2836(73)90168-x. [DOI] [PubMed] [Google Scholar]
- La Volpe A., Taggart M., Macleod D., Bird A. Coupled demethylation of sites in a conserved sequence of Xenopus ribosomal DNA. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):585–592. doi: 10.1101/sqb.1983.047.01.069. [DOI] [PubMed] [Google Scholar]
- Learned R. M., Tjian R. In vitro transcription of human ribosomal RNA genes by RNA polymerase I. J Mol Appl Genet. 1982;1(6):575–584. [PubMed] [Google Scholar]
- Lydersen B. K., Kao F. T., Pettijohn D. Expression of genes coding for non-histone chromosomal proteins in human-Chinese hamster cell hybrids. An electrophoretic analysis. J Biol Chem. 1980 Apr 10;255(7):3002–3007. [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. J. Synthesis of ribosomal RNA in synkaryons and heterokaryons formed between human and rodent cells. J Cell Sci. 1975 Mar;17(3):307–325. doi: 10.1242/jcs.17.3.307. [DOI] [PubMed] [Google Scholar]
- Matsui T., Segall J., Weil P. A., Roeder R. G. Multiple factors required for accurate initiation of transcription by purified RNA polymerase II. J Biol Chem. 1980 Dec 25;255(24):11992–11996. [PubMed] [Google Scholar]
- Miesfeld R., Arnheim N. Identification of the in vivo and in vitro origin of transcription in human rDNA. Nucleic Acids Res. 1982 Jul 10;10(13):3933–3949. doi: 10.1093/nar/10.13.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld R., Arnheim N. Species-specific rDNA transcription is due to promoter-specific binding factors. Mol Cell Biol. 1984 Feb;4(2):221–227. doi: 10.1128/mcb.4.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. A., Dev V. G., Tantravahi R., Miller O. J. Suppression of human nucleolus organizer activity in mouse-human somatic hybrid cells. Exp Cell Res. 1976 Sep;101(2):235–243. doi: 10.1016/0014-4827(76)90373-6. [DOI] [PubMed] [Google Scholar]
- Miller D. A., Tantravahi R., Dev V. G., Miller O. J. Frequency of satellite association of human chromosomes is correlated with amount of Ag-staining of the nucleolus organizer region. Am J Hum Genet. 1977 Sep;29(5):490–502. [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Sollner-Webb B. Transcription of mouse rRNA genes by RNA polymerase I: in vitro and in vivo initiation and processing sites. Cell. 1981 Nov;27(1 Pt 2):165–174. doi: 10.1016/0092-8674(81)90370-6. [DOI] [PubMed] [Google Scholar]
- Miller O. J., Miller D. A., Dev V. G., Tantravahi R., Croce C. M. Expression of human and suppression of mouse nucleolus organizer activity in mouse-human somatic cell hybrids. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4531–4535. doi: 10.1073/pnas.73.12.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y., Financsek I., Kominami R., Muramatsu M. Fractionation and reconstitution of factors required for accurate transcription of mammalian ribosomal RNA genes: identification of a species-dependent initiation factor. Nucleic Acids Res. 1982 Nov 11;10(21):6659–6670. doi: 10.1093/nar/10.21.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan G. T., Bakken A. H., Reeder R. H. Transcription of Xenopus borealis rRNA genes in nuclei of Xenopus laevis oocytes. Dev Biol. 1982 Oct;93(2):471–477. doi: 10.1016/0012-1606(82)90135-x. [DOI] [PubMed] [Google Scholar]
- Moss T. A transcriptional function for the repetitive ribosomal spacer in Xenopus laevis. Nature. 1983 Mar 17;302(5905):223–228. doi: 10.1038/302223a0. [DOI] [PubMed] [Google Scholar]
- Onishi T., Berglund C., Reeder R. H. On the mechanism of nucleolar dominance in mouse-human somatic cell hybrids. Proc Natl Acad Sci U S A. 1984 Jan;81(2):484–487. doi: 10.1073/pnas.81.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Schibler U., Huebner K., Croce C. M. Selective suppression of the transcription of ribosomal genes in mouse-human hybrid cells. J Cell Physiol. 1979 Mar;98(3):553–559. doi: 10.1002/jcp.1040980313. [DOI] [PubMed] [Google Scholar]
- Reeder R. H., Roan J. G., Dunaway M. Spacer regulation of Xenopus ribosomal gene transcription: competition in oocytes. Cell. 1983 Dec;35(2 Pt 1):449–456. doi: 10.1016/0092-8674(83)90178-2. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., McKnight S. L. Accurate transcription of cloned Xenopus rRNA genes by RNA polymerase I: demonstration by S1 nuclease mapping. Nucleic Acids Res. 1982 Jun 11;10(11):3391–3405. doi: 10.1093/nar/10.11.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantravahi R., Miller D. A., D'Ancona G., Croce C. M., Miller O. J. Location of rRNA genes in three inbred strains of rat and suppression of rat rRNA activity in rat-human somatic cell hybrids. Exp Cell Res. 1979 Mar 15;119(2):387–392. doi: 10.1016/0014-4827(79)90368-9. [DOI] [PubMed] [Google Scholar]