Abstract
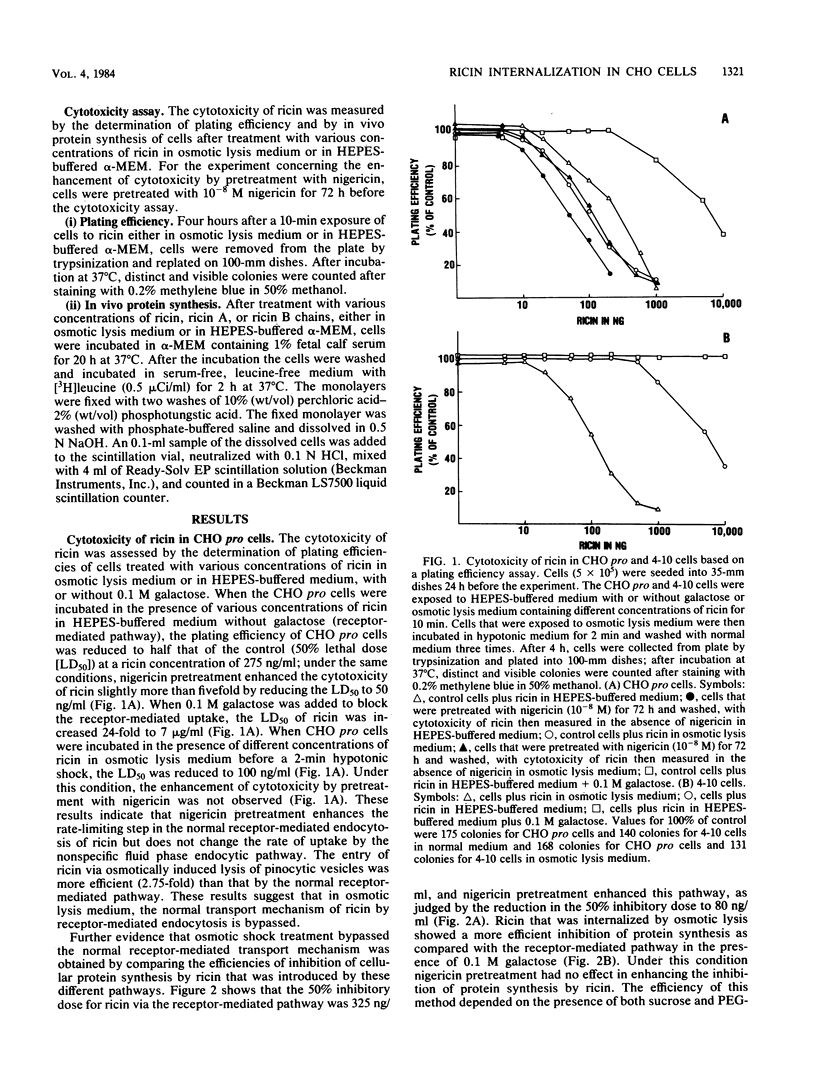
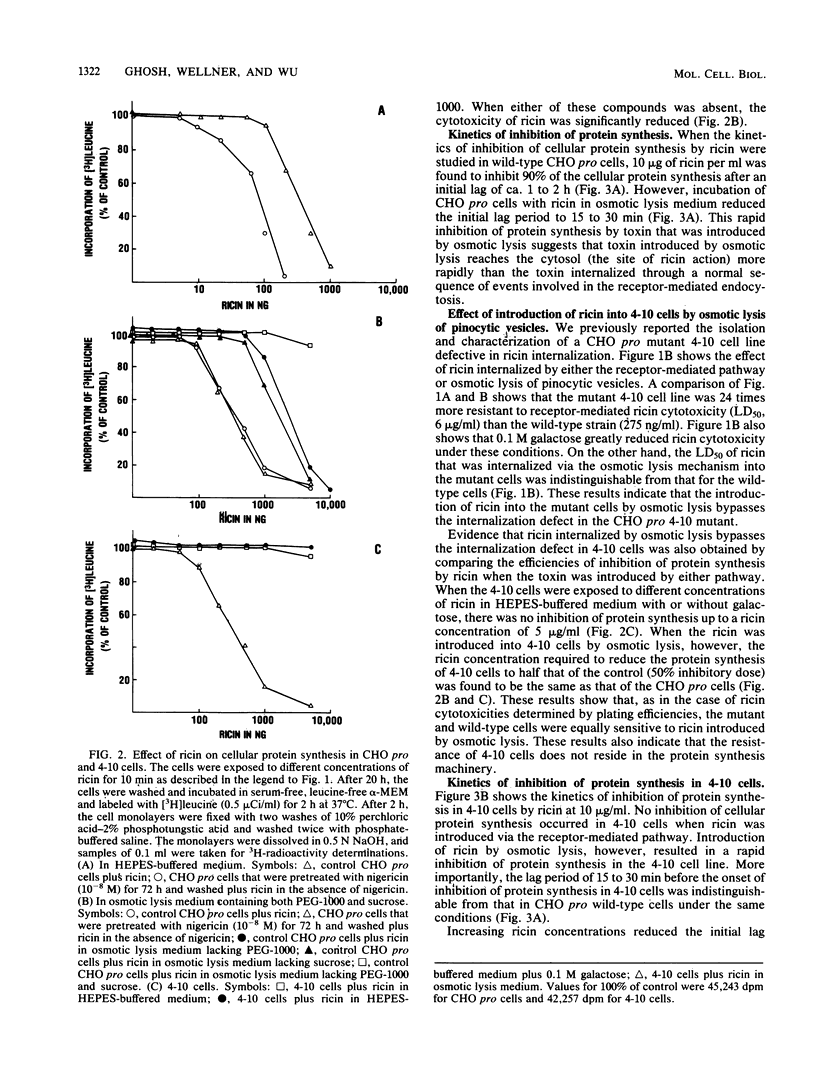
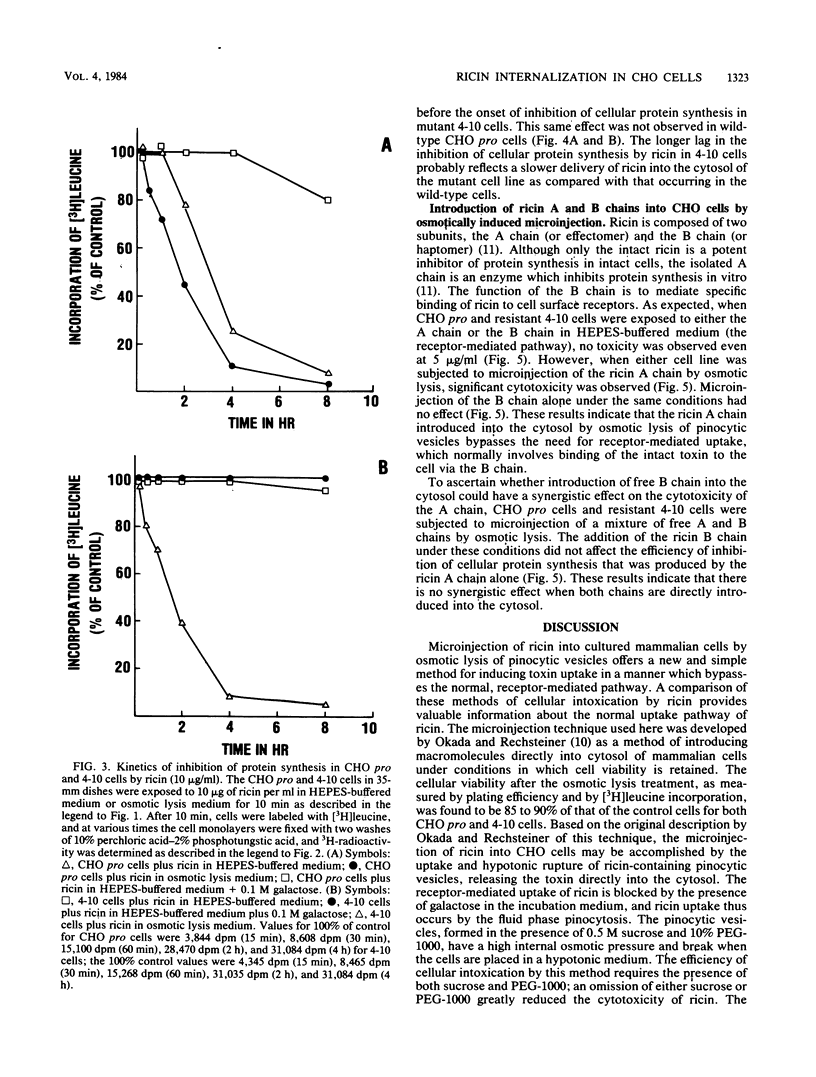
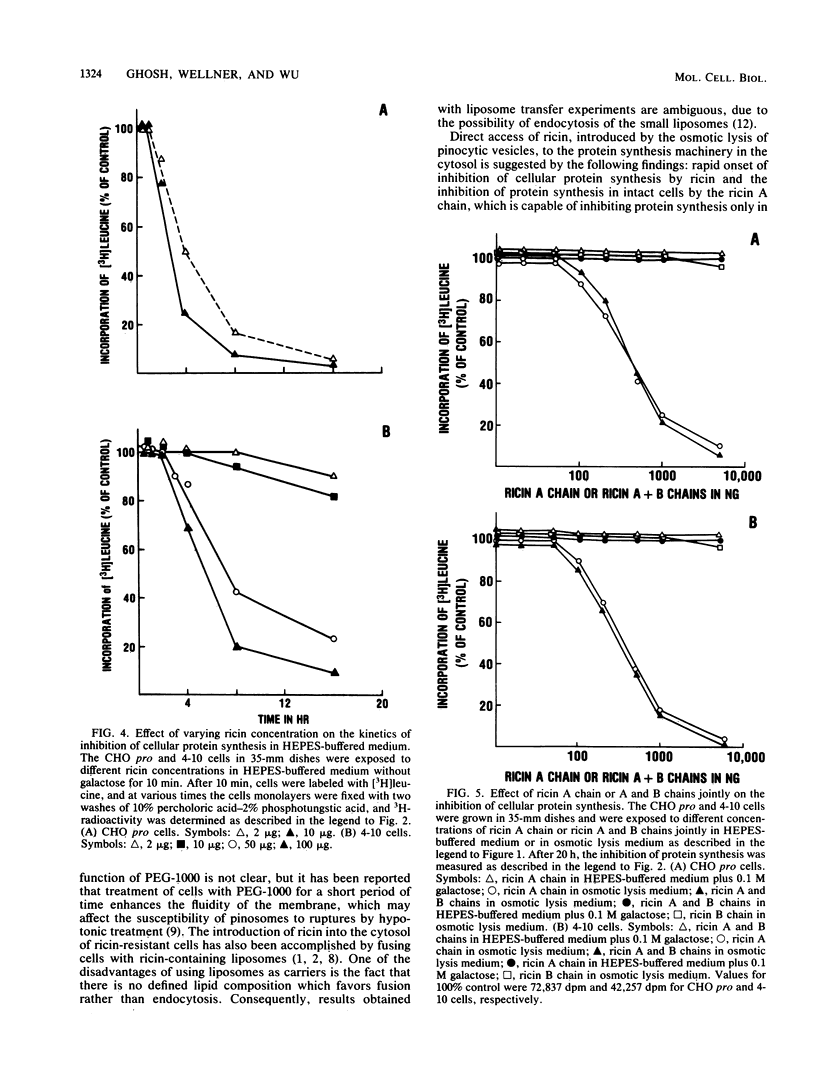
By osmotic lysis of pinocytic vesicles we were able to inject ricin or ricin A chain directly into the cytosol of Chinese hamster ovary cells. The lag time of 1 to 2 h before the onset of the inhibition of protein synthesis by ricin in intact cells was reduced to 15 to 30 min by this method. Preincubation of cells with a low concentration of nigericin, which was shown earlier to enhance the cytotoxicity of ricin, had no effect under this condition. Direct transfer of either intact ricin or the ricin A subunit by osmotic lysis of pinocytic vesicles into the cytosol of the ricin-resistant CHO mutant cell line 4-10 rendered the mutant 4-10 cells as sensitive to ricin as the CHO pro wild-type cells. Both the lag time and the rate of inhibition of protein synthesis in the wild-type and mutant cell lines after the introduction of ricin by osmotic lysis of pinocytic vesicles were the same. These results indicate that injection of ricin into the cytosol by osmotic lysis of pinosomes bypasses the internalization defect in the mutant cell line.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dimitriadis G. J., Butters T. D. Liposome-mediated ricin toxicity in ricin-resistant cells. FEBS Lett. 1979 Feb 1;98(1):33–36. doi: 10.1016/0014-5793(79)80145-3. [DOI] [PubMed] [Google Scholar]
- Gardas A., Macpherson I. Microinjection of ricin entrapped in unilamellar liposomes into a ricin-resistant mutant of baby hamster kidney cells. Biochim Biophys Acta. 1979 May 16;584(3):538–541. doi: 10.1016/0304-4165(79)90126-0. [DOI] [PubMed] [Google Scholar]
- Gottlieb C., Baenziger J., Kornfeld S. Deficient uridine diphosphate-N-acetylglucosamine:glycoprotein N-acetylglucosaminyltransferase activity in a clone of Chinese hamster ovary cells with altered surface glycoproteins. J Biol Chem. 1975 May 10;250(9):3303–3309. [PubMed] [Google Scholar]
- Houston L. L. Transport of ricin A chain after prior treatment of mouse leukemia cells with ricin B chain. J Biol Chem. 1982 Feb 10;257(3):1532–1539. [PubMed] [Google Scholar]
- Hyman R., Lacorbiere M., Stavarek S., Nicolson G. Derivation of lymphoma variants with reduced sensitivity to plant lectins. J Natl Cancer Inst. 1974 Mar;52(3):963–969. doi: 10.1093/jnci/52.3.963. [DOI] [PubMed] [Google Scholar]
- Meager A., Ungkitchanukit A., Nairn R., Hughes R. C. Ricin resistance in baby hamster kidney cells. Nature. 1975 Sep 11;257(5522):137–139. doi: 10.1038/257137a0. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr, Youle R. J. Monoclonal antibody-ricin or ricin A chain hybrids: kinetic analysis of cell killing for tumor therapy. Immunol Rev. 1982;62:75–91. doi: 10.1111/j.1600-065x.1982.tb00390.x. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Poste G. Mechanism of resistance to ricin toxin in selected mouse lymphoma cell lines. J Supramol Struct. 1978;8(3):235–245. doi: 10.1002/jss.400080303. [DOI] [PubMed] [Google Scholar]
- Ohno H., Sakai T., Tsuchida E., Honda K., Sasakawa S. Interaction of human erythrocyte ghosts or liposomes with polyethylene glycol detected by fluorescence polarization. Biochem Biophys Res Commun. 1981 Sep 16;102(1):426–431. doi: 10.1016/0006-291x(81)91538-2. [DOI] [PubMed] [Google Scholar]
- Okada C. Y., Rechsteiner M. Introduction of macromolecules into cultured mammalian cells by osmotic lysis of pinocytic vesicles. Cell. 1982 May;29(1):33–41. doi: 10.1016/0092-8674(82)90087-3. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Weinstein J. N. Interactions of liposomes with mammalian cells. Annu Rev Biophys Bioeng. 1978;7:435–468. doi: 10.1146/annurev.bb.07.060178.002251. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Gill D. M. Diphtheria. Science. 1973 Oct 26;182(4110):353–358. doi: 10.1126/science.182.4110.353. [DOI] [PubMed] [Google Scholar]
- Ray B., Wu H. C. Chinese hamster ovary cell mutants defective in the internalization of ricin. Mol Cell Biol. 1982 May;2(5):535–544. doi: 10.1128/mcb.2.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B., Wu H. C. Enhanced internalization of ricin in nigericin-pretreated Chinese hamster ovary cells. Mol Cell Biol. 1981 Jun;1(6):560–567. doi: 10.1128/mcb.1.6.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B., Wu H. C. Enhancement of cytotoxicities of ricin and Pseudomonas toxin in Chinese hamster ovary cells by nigericin. Mol Cell Biol. 1981 Jun;1(6):552–559. doi: 10.1128/mcb.1.6.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. C., Hyman R., Stallings V., Nicolson G. L. Cell-surface changes in Ricinus communis toxin (ricin)-resistant variant of a murine lymphoma. J Natl Cancer Inst. 1977 Apr;58(4):1027–1033. doi: 10.1093/jnci/58.4.1027. [DOI] [PubMed] [Google Scholar]
- Stanley P., Caillibot V., Siminovitch L. Selection and characterization of eight phenotypically distinct lines of lectin-resistant Chinese hamster ovary cell. Cell. 1975 Oct;6(2):121–128. doi: 10.1016/0092-8674(75)90002-1. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Cushley W., Uhr J. W. Synergy of ricin A chain-containing immunotoxins and ricin B chain-containing immunotoxins in in vitro killing of neoplastic human B cells. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6332–6335. doi: 10.1073/pnas.80.20.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]


