Abstract
Significance: Oxidative stress has been mechanistically linked with aging and chronic diseases, including cancer. In fact, oxidative stress status, chronic disease-related inflammation, and cancer occurred in the aging population are tightly correlated. It is well known that the activation of nuclear factor kappa B (NF-κB) plays important roles in oxidative stress, inflammation, and carcinogenesis. Therefore, targeting NF-κB is an important preventive or therapeutic strategy against oxidative stress, inflammation, and cancer. Recent Advances: A variety of natural compounds has been found to reduce oxidative stress through their antioxidant activity. Among them, isoflavone, indole-3-carbinol (I3C), and its in vivo dimeric compound 3,3′-diindolylmethane (DIM) have shown their promising effects on the inhibition of NF-κB with corresponding reduction of oxidative stress. Critical Issues: It has been found that isoflavone, I3C, and DIM could inhibit cancer development and progression by regulating multiple cellular signaling pathways that are related to oxidative stress and significantly deregulated in cancer. Future Directions: The antioxidative and anticancer effects of these natural agents make them strong candidates for chemoprevention and/or therapy against human malignancies. However, more clinical trials are needed to evaluate the effects of isoflavone and DIM for the prevention of cancer development and also for the treatment of cancer either alone or in combination with conventional cancer therapeutics. Antioxid. Redox Signal. 19, 139–150.
Introduction
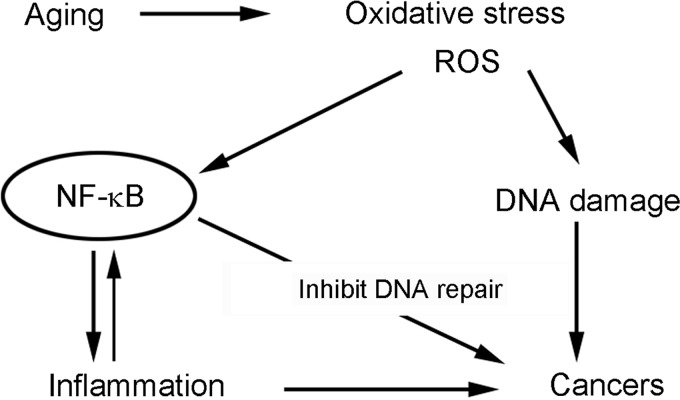
Since humans are living in an aerobic environment, it is obvious that they are continuously and unavoidably exposed to reactive oxygen species (ROS). To overcome ROS, the biological system in the human body interacts with the external environment to maintain a healthy internal environment that maintains homeostasis of cell survival, growth, differentiation, and reproduction. The defense systems in human body have evolved to reduce the accumulation of ROS; however, these defense systems are not always sufficient to decrease the production of ROS, resulting in the accumulation of ROS, causing systemic oxidative stress, especially in older age (40, 126). It is known that oxidative stress is linked to aging and various chronic diseases such as atherosclerosis, neurodegenerative diseases, diabetes, pulmonary fibrosis, and arthritis, which are commonly accompanied by inflammation (12, 35, 102). More importantly, ROS could induce severe DNA damage, which plays an important role in carcinogenesis; therefore, oxidative stress from ROS accumulation could be one of the factors responsible for the development and progression of cancer (43, 47, 97, 98). Once DNA damage occurs, the defense systems in the human body try to repair DNA to prevent mutagenesis. However, under sustained oxidative stress, the repair of DNA damage can be suppressed by several redox-dependent metals, resulting in mutagenesis and carcinogenesis (43, 64) (Fig. 1). Therefore, ROS could induce both inflammation and cancer. Examination of the inflammatory microenvironment in tumor tissues has supported the hypothesis that inflammation is a cofactor in oncogenesis for a variety of cancers (46). Moreover, it is known that ROS under oxidative stress activate nuclear factor kappa B (NF-κB), which also contributes to the inhibition of DNA repair (91). The activation of NF-κB together with deregulation of multiple signaling pathways plays important roles in carcinogenesis. Therefore, antioxidative agents could be effective compounds for the prevention and/or treatment of cancer and inflammation, both of which are associated with oxidative stress.
FIG. 1.
The relationship between oxidative stress, aging, inflammation, and cancer.
To overcome the oxygenic threat, natural antioxidants have evolved in parallel with the natural oxygenic atmosphere. It is known that plants have their own defense system to protect their structures against ROS produced during photosynthesis (14). Therefore, with evolution, plants produce a variety of antioxidant components, which could be beneficial for human health. Indeed, in vitro and in vivo experimental studies have shown that various plant-derived components possess their ability to reduce oxidative stress. Among them, isoflavones, indole-3-carbinol (I3C), and its in vivo dimeric product 3,3′-diindolylmethane (DIM) exhibit a promising effect on the inhibition of ROS accumulation (13, 38, 39, 95, 96). The main sources of isoflavones are soy and other plants in the Legume family. The isoflavones include genistein, daidzein, glycitein, formononetin, biochanin A, desmethylangolensin, and equol. The Brassica family is the main source of I3C and DIM. The isoflavones, I3C and DIM, have shown their beneficial effects on human health. Importantly, these natural compounds inhibit NF-κB activation stimulated by ROS (23, 30), suggesting their potent ability as antioxidants. Moreover, these antioxidants have shown their inhibitory effects on inflammation, oncogenesis, tumor growth, and progression, suggesting that they could be useful as chemopreventive and/or therapeutic agents for the protection against inflammation and cancer.
Oxidative Stress and NF-κB Activation in Cancer
It is well known that the activation of NF-κB is the most important consequence of inflammation associated with all types of cancer (58, 60). In fact, the oxidative stress status, chronic disease-related inflammation, and cancer occurred in the aging population are tightly associated with the activation of NF-κB signaling (58) (Fig. 1). Under the situation of oxidative stress, ROS induce DNA damage and activate the activity of NF-κB (19, 94). The laboratory experiments showed that direct addition of hydrogen peroxide (H2O2) to the culture medium activated the NF-κB DNA-binding activity in many types of cell lines (19). In addition, it was found that ROS accumulated in cells were increased in response to agents that also activated NF-κB (19, 37). These lines of evidence demonstrate that oxidative stress activates NF-κB activity in cells, including inflammatory and cancerous cells.
Once NF-κB is activated, it binds to the NF-κB-specific DNA-binding sites and regulates the transcription of target genes (53, 84). By regulating the transcription of its targets, NF-κB controls the expression of many genes that are involved in the stress response, inflammation, differentiation, cell growth, and apoptosis (61, 83, 84). The alteration in these biological processes has been critically linked with the development and progression of cancer. Since activated NF-κB promotes cell growth and inhibits apoptotic cell death, the uncontrolled cell proliferation leads to the development of cancer. Therefore, the activated NF-κB under oxidative stress has been described as a major culprit in cancers (59).
Indeed, clinical and experimental studies have shown that NF-κB is constitutively activated in Hodgkin's lymphoma (9), multiple myeloma (49), and solid tumors, including lung, pancreatic, ovarian, prostate, breast, and other cancers (20, 51, 68, 77, 88, 105, 114). The activated NF-κB activity is also correlated with drug resistance and poor treatment outcome (20). Therefore, targeting NF-κB signaling activation by specific inhibitors or natural agents with multitargets could be an effective therapeutic strategy for the treatment of cancer by increasing drug sensitivity and inhibiting cancer invasion and metastasis. Inhibition of ROS-mediated activation of NF-κB is now widely accepted as a valid therapeutic strategy for the treatment of inflammation (86, 123) and cancers (15, 44, 57, 93). Thus, plant-derived antioxidants that inhibit NF-κB activity may serve as potential agents for cancer prevention and therapy.
Signaling Pathways that Crosstalk with NF-κB in Cancer
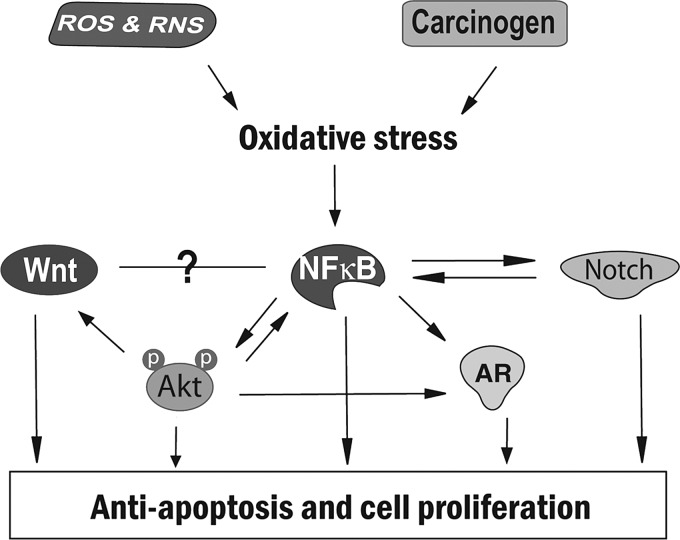
Since cellular signaling is a complex signal network with positive or negative feedback loops, the deregulations of signaling pathways, which crosstalk with NF-κB, often exist in cancer cells. Protein kinase B (Akt) signaling plays important roles in mammalian cell survival, and it is activated in response to various stimuli. It has been shown that H2O2 treatment increased Akt activity in multiple cell lines (119), suggesting the activation of Akt under oxidative stress. Activated Akt promotes cell survival by inhibition of proapoptotic factors, including Bad, Forkhead transcription factors, and caspase-9 (87). It has also been found that NF-κB stimulates Akt activation, while Akt regulates the NF-κB pathway via activation of molecules in the NF-κB signaling pathway (89, 103), suggesting the crosstalk between NF-κB and Akt under oxidative stress (Fig. 2).
FIG. 2.
Crosstalk between NF-κB, Akt, Wnt, Notch, and AR signaling that contributes to cell proliferation and antiapoptosis under oxidative stress. Akt, protein kinase B; AR, androgen receptor; NF-κB, nuclear factor-kappa-B.
Another signaling that crosstalk with NF-κB is Notch signaling. Notch signaling plays a critical role in the regulation and maintenance of stem cells; therefore, appropriate functioning of Notch signaling is required for normal development. It has been shown that Notch is an essential upstream regulator of NF-κB, and that NF-κB activates Notch signaling by inducing the Notch ligand Jagged1 (10, 108), suggesting the existence of an active crosstalk between Notch and NF-κB (Fig. 2). Importantly, activation of Notch receptors and their ligands has been found in various cancers, including lymphomas and cervical, lung, colon, head and neck, renal, and pancreatic cancer (120). In cancer cells, Notch signaling is abnormally activated, leading to the increased proliferation of cancer cells (Fig. 2); therefore, Notch is another important target in cancer therapy, which could indeed be achievable by natural antioxidants, as discussed later in this chapter.
In addition to Notch signaling, Wnt (wingless-type MMTV integration-site family) signaling also plays important roles in the embryonic developmental processes. It is well known that Akt signaling regulates Wnt signaling through glycogen synthase kinase-3-beta (GSK-3β), which phosphorylates β-catenin in Wnt signaling. Wnt and NF-κB could also crosstalk with each other; however, the detailed regulation between these two signaling is still unclear (18, 36) (Fig. 2). The inappropriate expression of Wnt molecules and the inappropriate activation of Wnt signaling have been found in various human tumors. Activation of Wnt signaling promotes β-catenin translocation to the nucleus, resulting in the consequent transcriptional activation of specific target genes and the uncontrolled cell proliferation (Fig. 2).
Androgen receptor (AR) signaling is another cellular signaling that interacts with NF-κB. It is known that AR signaling plays important roles in the normal prostate development as well as in prostate cancer development and progression through the regulation of transcription of androgen-responsive genes (82). Importantly, prostate-specific antigen (PSA), one of the AR-target genes, is a clinically critical marker used to monitor diagnosis, progression, and prognosis of patients with prostate cancer, suggesting the importance of AR signaling, especially in castrate-resistant prostate cancer. It has been shown that Akt could activate AR in a ligand-independent manner in hormone-refractory prostate cancer. Moreover, NF-κB could also upregulate AR, leading to the AR transactivation, PSA expression, and prostate cancer cell growth (33, 125). These findings suggest a complex signal interaction between NF-κB, Akt, and AR under oxidative stress (Fig. 2). Therefore, targeting multiple signaling by natural antioxidants could open a new avenue for cancer therapy.
Isoflavones as Antioxidants Inhibiting Oxidative Stress and Regulating Cellular Signaling in Cancer
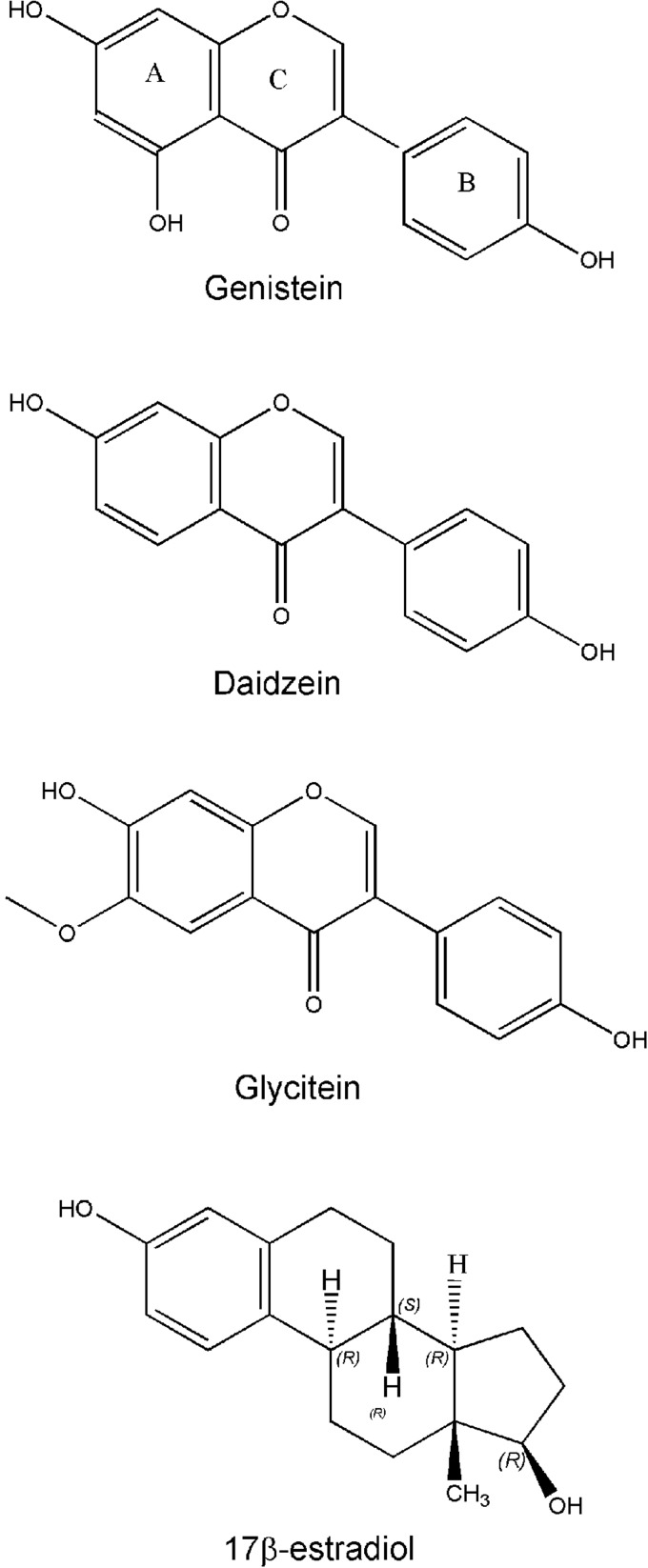
Isoflavones are a subclass of flavonoids and mainly found in soybeans. Genistein, daidzein, and glycitein are three main isoflavones found in soybeans (Fig. 3). Genistein and daidzein are present at a relatively high concentration in soybeans and most soy products, while much lower amount of glycitein exists in soybeans. The basic molecular structure of isoflavones is the flavone nucleus, which consists of 2 benzene rings (A and B) linked through a heterocyclic pyrane C ring (Fig. 3). In the molecular structures, genistein, daidzein, and glycitein have the same flavone nucleus with a some other different side chain. Isoflavones have a close similarity in structure to estradiol (Fig. 3). Because of the similarity, isoflavone can bind to the estrogen receptor (ER). It has been reported that isoflavones exert weak estrogenic activity because of their binding to the ER. Therefore, isoflavone has also been known as phytoestrogens. Since isoflavones bind to the ER and show weak estrogenic activity, the binding of isoflavone to ER prevents more-potent estrogen binding to the ER and thereby inhibits ER signaling, suggesting the estrogen-antagonistic activity of isoflavones. However, it is important to note that under a low-estrogenic environment, isoflavones could function as estrogenic.
FIG. 3.
Molecular structures of isoflavones and estradiol.
It is known that isoflavones exert antioxidant effects on human cells. Genistein has been found to protect cells against ROS by reducing free radicals and downregulating the expression of the stress-response related genes (104, 128). Isoflavones also upregulated gene expression of antioxidant proteins in Caco-2 cells (55). It has been found that isoflavone supplementation reduced H2O2-induced DNA damage in sperm (109), suggesting the antioxidant effects of isoflavone. Importantly, isoflavone inhibited H2O2 production induced by a tumor promoter (12-O-tetradecanoylphorbol-13-acetate) in human polymorphonuclear leukocytes and HL-60 cells, suggesting the inhibitory effect of isoflavone on carcinogenesis mediated through antioxidant activity (122).
The inhibitory effects of isoflavone on cancer
The effects of isoflavone on cancer cells have been widely studied in a variety of cancer cells. The results from our laboratory and other investigators showed that isoflavone inhibited the growth of various cancer cells, including leukemia, lymphoma, neuroblastoma, breast, prostate, lung, gastric, ovarian, and head and neck cancer cells (1, 21, 29, 32, 45, 70, 78, 81, 127). The inhibition of cancer growth by isoflavone could be mediated by the induction of apoptosis and the modulation of expression of the genes related to the cell growth and apoptotic processes (1, 70, 78, 81, 128). By microarray gene expression analysis and mechanistic experiments, we and other investigators have found that isoflavone regulates the molecules in multiple cellular signaling pathways, including NF-κB, Akt, Wnt, Notch, and AR signaling pathways. All of these cellular signaling pathways are critically involved in the control of cell growth, apoptosis, angiogenesis, tumor cell invasion, and metastasis (45, 75–77), suggesting the pleiotropic effects of isoflavone (multitargeting effect) on cancer cells.
Importantly, isoflavones have been found to sensitize cancer cells to conventional therapies, including chemotherapy and radiotherapy (8, 69, 73, 111). Pretreatment of cancer cells with isoflavone before treatment with lower doses of chemotherapeutic agents or radiotherapy caused a significantly greater degree of growth inhibition and apoptosis of cancer cells, suggesting enhanced therapeutic effects of cancer therapy with isoflavone. Moreover, phenoxodiol, one of the isoflavone analogs, showed its promising anticancer effect by sensitizing cancer cells to conventional chemotherapeutics (5). In ovarian cancer cells that became resistant to the conventional chemotherapeutics, treatment with phenoxodiol removed drug resistance and, therefore, made cancer cells susceptible once again to conventional chemotherapeutics, including cisplatin, carboplatin, taxanes, and gemcitabine (56, 106). Phenoxodiol is currently undergoing phase II/III clinical trials to elucidate its effects combined with carboplatin, docetaxel, cisplatin, or paclitaxel in patients with ovarian, fallopian tube, or primary peritoneal cavity cancers (28). Recently, more clinical trials are being conducted using the isoflavone genistein or isoflavone analogs in combination with IL-2, docetaxel, cisplatin, paclitaxel, or other natural agents such as lycopene and vitamin D in the treatment of melanoma, kidney, and ovarian cancers (27, 28). In addition, several clinical trials are being conducted to test the effects of isoflavone treatment combined with radiotherapy in patients with prostate cancer. All of these clinical trials are based on in vitro mechanistic experiments showing that isoflavone inhibits cancer cell growth through the regulation of NF-κB and other signaling pathways.
Inhibition of oxidative stress and NF-κB activation by isoflavone
To investigate the effects of the isoflavone genistein on oxidative stress-induced NF-κB activation, we have conducted a series of in vitro and in vivo experiments to measure the NF-κB DNA-binding activity in lymphocytes from human subjects and in various cancer cells. We found that isoflavone genistein treatment significantly inhibited the NF-κB DNA-binding activity and blocked NF-κB induction stimulated by oxidative stress inducers, H2O2 and tumor necrosis factor (TNF)-α, in cancer cell lines (32). We further investigated the effect of isoflavone supplementation on NF-κB activation in vivo in human volunteers (30). The lymphocytes from healthy volunteers were isolated from peripheral blood and cultured for 24 h in the absence and presence of the isoflavone genistein. The results showed that isoflavone genistein treatment inhibited the NF-κB DNA-binding activity and abrogated TNF-α-induced NF-κB activity (30). Moreover, when human volunteers received soy isoflavone supplements (Novasoy™), TNF-α failed to activate NF-κB activity in lymphocytes harvested from these volunteers (30), suggesting that isoflavone could also inhibit NF-κB activation in vivo.
To test the effects of isoflavone on oxidative stress, we further tested the levels of oxidative DNA damage in the blood of the subjects before and after supplementation with isoflavones. We found that the mean value of 5-OHmdU, a modified DNA base that represents the endogenous status of cellular oxidative stress, was significantly downregulated after 3 weeks of isoflavone supplementation (30), which has also been reported in our published review articles and book chapters. Collectively, these observations clearly demonstrate that isoflavone supplementation reduces the level of 5-OhmdU, decreases oxidative damage, and inhibits NF-κB activation in humans in vivo. Taken together, these in vitro and in vivo studies provide strong evidence showing that isoflavone could function as an antioxidant. Moreover, the antioxidative effects of isoflavone could be responsible for its chemopreventive and chemotherapeutic activity.
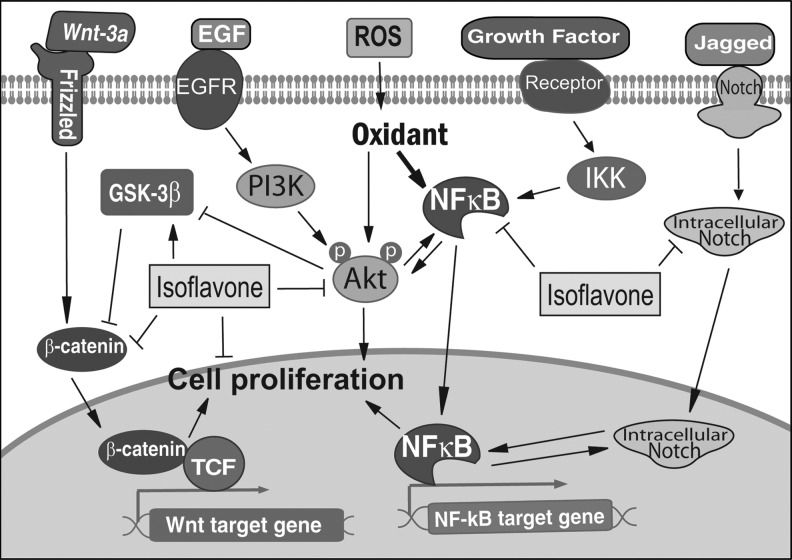
The inhibitory effect of isoflavone on NF-κB activation was also observed in different types of cancer cells. The isoflavone genistein reduced NF-κB activity in T-lymphoma cells via a caspase-mediated cleavage of IκBα (11). The isoflavone genistein also inhibited constitutive and inducible NF-κB activation and decreased IL-8 production in human cystic fibrosis bronchial gland cells (115). It is also reported that isoflavone inhibited NF-κB activation in various solid cancers, including neuroblastoma, breast, prostate, lung, gastric, ovarian, and head and neck cancer cells (1, 32, 45, 78, 81, 127), suggesting that downregulation of NF-κB signaling is an important mechanism by which isoflavone functions as an antioxidant, and thereby inhibits cancer development and progression (Fig. 4).
FIG. 4.
Isoflavone regulates major cellular signaling pathways.
Inhibition of other important signaling pathways by isoflavone
As we have discussed in previous sections, oxidative stress regulates Akt and NF-κB signaling, and there is an active crosstalk between NF-κB and other signaling, including Akt, Wnt, Notch, and AR. In cancer cells, the alterations in multiple signaling have been observed. Among, multiple signaling such as NF-κB, Akt, Wnt, Notch, and AR crosstalk with each other and appears to play important roles in cancer development and progression. Importantly, isoflavone could inhibit the activation of these signaling pathways, suggesting the multitarget effects of isoflavone.
It has been found that there is a crosstalk between NF-κB and Akt signaling as stated earlier (Fig. 4). We found that isoflavone could inhibit phosphorylation of Akt and thereby downregulate Akt kinase activity in prostate cancer cells. Isoflavone pretreatment also abrogated the activation of Akt stimulated by EGF (77). Moreover, we found that isoflavone could exert its inhibitory effects on NF-κB signaling through the downregulation of Akt signaling, resulting in the inhibition of cell growth and induction of apoptosis (77). Isoflavone could also increase the expression of GSK-3β and decrease the phosphorylation of Akt and FOXO3a, leading to the increased apoptosis and decreased cell growth (79). Similar reports by other investigators also showed that isoflavone induced apoptosis by upregulation of p21WAF1 and downregulation of Akt and NF-κB (85, 99).
Wnt signaling has been found to crosstalk with Akt signaling, leading to cancer cell proliferation and cancer progression. We found that isoflavone could induce the expression of GSK-3β, promote GSK-3β binding to β-catenin, and consequently increase the phosphorylation of β-catenin, suggesting the inhibitory effects of isoflavone on both Akt and Wnt signaling (79). Isoflavone also inhibited Wnt-induced cancer cell growth and downregulated the expression of two important Wnt targets, c-Myc and Cyclin D1 (112). Microarray gene expression analysis together with in vivo animal studies have shown that isoflavone could downregulate the expression of upstream and downstream Wnt-signaling molecules, including Wnt-5a (113), Wnt-7a (118), and Cyclin D1 (113), suggesting the inhibitory effects of isoflavone on the Wnt signaling pathway (Fig. 4).
It is well known that NF-κB and Notch form a positive signaling loop that plays important roles in cancer development and progression. The inhibitory effects of isoflavone on both NF-κB and Notch signaling have been observed. We found that isoflavone could suppress Notch signaling, resulting in the subsequent downregulation of NF-κB activity. This downregulation of both Notch and NF-κB signaling by isoflavone increased apoptotic cell death and inhibited cell proliferation of pancreatic cancer cells (121). Isoflavone could also suppress the expression of Notch-2, suggesting that isoflavone inhibits cancer cell proliferation and induces apoptotic cell death through interrupting the crosstalk between Notch and NF-κB signaling (Fig. 4).
Other cellular signaling could also contribute to the isoflavone-induced inhibition of cancer cell growth. It has been known that AR signaling could be activated by Akt or NF-κB signaling in a ligand-independent manner. Isoflavone could downregulate AR, inhibit AR translocation to the nucleus, and subsequently suppress the expression of the AR target PSA in prostate cancer cells (31, 116). Moreover, we found that isoflavone could inhibit cell proliferation and induce apoptotic cell death through the regulation of the Akt/FOXO3a/GSK-3β/AR signaling network (79). Further, we found that isoflavone could enhance the antitumor activity of chemotherapeutics in an experimental animal model of prostate cancer bone metastasis through the regulation of the OPG/RANK/RANKL/MMP-9 signaling network (73). Isoflavone also regulated RANKL/MITF, AR/PSA, and NKX3-1/Akt/p27, leading to the inhibition of osteoclast and osteoblast differentiation, which could suppress bone remodeling and prostate cancer bone metastasis. Therefore, isoflavone could function as an antioxidant affecting multiple targets, which could be responsible for its activity toward prevention and treatment of cancer.
I3C and Its In Vivo Dimeric Product DIM as Antioxidants Inhibiting Oxidative Stress and Regulating Cellular Signaling in Cancer
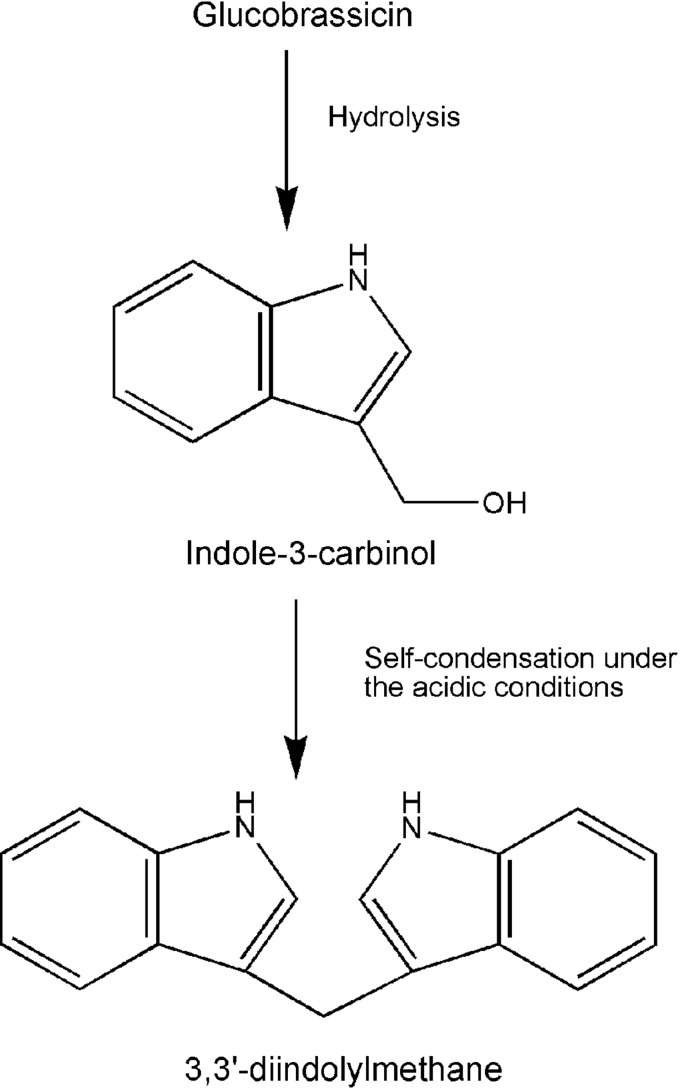
It is well known that diets rich in vegetables are beneficial for the prevention of cancer. In recent years, vegetables of the genus Brassica have received much attention in cancer prevention because of the glucosinolates present in the Brassica. The vegetables of the genus Brassica include all kinds of cabbages, broccoli, cauliflower, and Brussels sprouts. These vegetables contribute most to our intake of glucosinolates. It is known that the hydrolysis products of glucosinolates make a significant contribution to the health benefit of Brassica vegetables (54). Glucosinolates with an indole side chain form indole. The most prevalent glucosinolate with an indole side chain is glucobrassicin, which is predominant in Brassica vegetables. When hydrolysis occurs, glucobrassicin forms an unstable isothiocyanate that degrades to I3C (Fig. 5). Although I3C is biologically active, it is readily converted in vivo to its dimeric product DIM (Fig. 5). Under the acidic conditions of the stomach, I3C undergoes extensive and rapid self-condensation reactions to form several derivatives (117), but DIM is the major derivative and condensation product of I3C. DIM is also biologically active. It is believed that the production of DIM from I3C is a likely prerequisite for the anticarcinogenic activity of I3C.
FIG. 5.
The structures and the formation of I3C and DIM. DIM, 3,3′-diindolylmethane; I3C, indole-3-carbinol.
I3C is capable of acting as a scavenger of free radicals. This scavenging activity of I3C has been implicated in the anticarcinogenic process (6). It has been reported that I3C, DIM, and DIM derivatives could reduce oxidative stress, stimulate the expression of antioxidant response element-driven genes, and protect against DNA damage through its antioxidant activity (13, 17, 92). Moreover, BR-DIM, a formulated DIM with higher bioavailability, at physiologically relevant micromolar and submicromolar concentrations could protect against cell killing upon H2O2 and other oxidant treatment in breast cancer cells and normal cells (39). This protection against oxidative stress was found to be mediated by BR-DIM-induced upregulation of tumor suppressor protein BRCA1 and phosphorylation of BRCA1. BR-DIM also upregulated the antioxidant transcription factor NFE2L2 (NRF2) through the antioxidant-response element in a BRCA1-dependent manner (39). It is worth to note that other pathways such as the Keap1-Nrf2 signaling could also participate in the DIM-reduced oxidative stress. Further, the antioxidative effect of DIM mediated through deregulation of BRCA1 has also been investigated in inflammatory bowel disease and colitis, in which ROS play a key role. It has been shown that DIM could attenuate 2,4,6-trinitrobenzene sulfonic acid-induced colitis in an animal model. The inhibition of ROS-induced colitis by DIM was accompanied by increased expression of BRCA1, by reduced ROS generation, and by decreased expression of vascular cell adhesion molecule 1, which is typically induced by ROS (50). These findings suggest that I3C and DIM could reduce oxidative stress, and thus these agents function as antioxidants.
The inhibitory effects of I3C and DIM on cancer
The effects of DIM in dextran sodium sulfate (DSS)-induced experimental colitis and colitis-associated colon carcinogenesis induced by azoxymethane (AOM)/DSS in animals have been investigated. It has been shown that DIM treatment significantly inhibited the formation of colon tumors in AOM/DSS mice (63). DIM treatment also significantly attenuated severe clinical signs in the colitis model with reduced prostaglandin E2, nitric oxide, and proinflammatory cytokines (63), suggesting that DIM suppresses inflammatory response and further inhibits oncogenesis. I3C treatment also inhibited clonogenic cancer cell growth and induced higher levels of basal caspase-3 and 7 activities (90). We and other investigators have also found that I3C and DIM could inhibit oncogenesis and cancer cell growth and induce apoptosis in various cancer cells (23, 24, 26, 41, 42, 100, 101, 107, 124), suggesting that I3C and DIM may serve as potent agents for the prevention and/or treatment of cancer.
DIM also showed its potent effects on enhancing the antitumor activity of chemotherapy and radiotherapy. We found that DIM significantly increased the sensitivity of cancer cells to erlotinib and cisplatin through the downregulation of epidermal growth factor receptor and NF-κB in pancreatic cancer and squamous cell carcinoma cells (2, 4). ET-743 is an experimental antitumor drug with promising antitumor activity as shown in phase II trials; however, it is hepatotoxic. In an animal study, addition of I3C to the diet before ET-743 administration almost completely abolished the hepatotoxicity without any alterations in the antitumor efficacy of ET-743 (34), suggesting that I3C may counteract the unwanted adverse effects of ET-743, and thus I3C could be used in combination with ET-743 therapy. DIM could also enhance the efficacy of radiotherapy as demonstrated by our recent report showing that DIM and radiation could significantly inhibit primary tumor growth and reduce metastasis to lymph nodes in prostate cancer (110). Collectively, these findings suggest that DIM is a promising agent for cancer therapy in combination with conventional therapy. Currently, more clinical trials are being conducted to test the effects of DIM treatment as a dietary supplement or combined with conventional cancer therapy in patients with prostate, breast, cervical, and laryngeal tumors. The results of these clinical trials will uncover the value of DIM in cancer clinic.
Inhibition of NF-κB activation by I3C and DIM
The anti-inflammatory effect of DIM through NF-κB signaling has been reported. It has been found that DIM inhibited lipopolysaccharide-induced NF-κB transcriptional activity, NF-κB DNA-binding activity, translocation of NF-κB p65 to the nucleus, and degradation of IκBα, leading to the decreased release of inflammatory factors, including nitric oxide, prostaglandin E2, IL-6, and IL-1β (25). Importantly, we and other investigators have shown that I3C and DIM could inhibit NF-κB signaling in a variety of cancers, including prostate, breast, pancreatic, lung, and skin cancer in vitro and in vivo (3, 7, 16, 22, 23, 52, 62, 66, 101). DIM significantly suppressed NF-κB activation and consequently reduced the expression of NF-κB target genes such as VEGF, IL-8, uPA, and MMP-9, leading to the induction of apoptosis and inhibition of cell proliferation, angiogenesis, invasion, and metastasis (16, 66). These reports clearly demonstrate that DIM inhibits tumor growth and progression through downregulation of NF-κB signaling (Fig. 6).
FIG. 6.
I3C and DIM regulate the major cellular signaling pathways.
Inhibition of other important signaling pathways by I3C and DIM
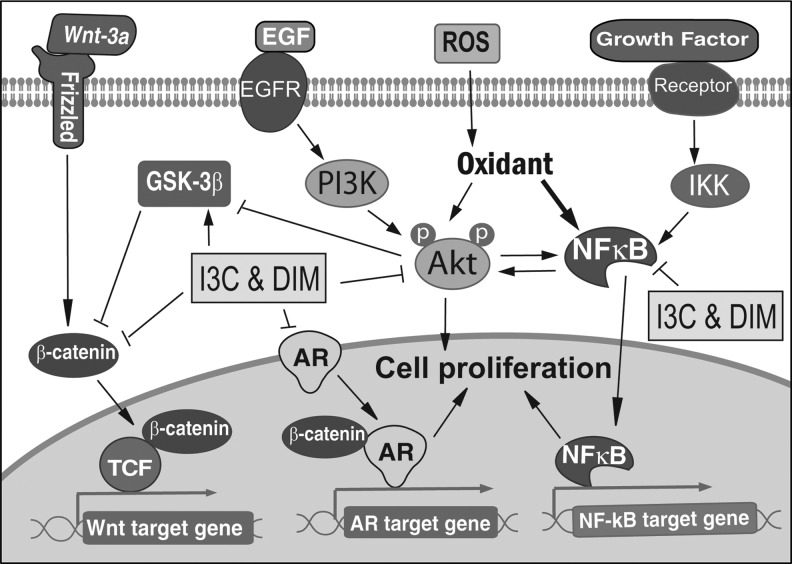
Similar to isoflavone, I3C and DIM exhibit their multitargeting activity in cancer cells. I3C and DIM function as an antioxidant; therefore, they suppress NF-κB and Akt signaling transduction. More importantly, because of the signal crosstalk between NF-κB, Akt, Wnt, and AR signaling, DIM also significantly inhibits AR and Wnt signaling by downregulating NF-κB and Akt activities.
It has been shown that I3C and DIM could regulate Akt signaling in cancer cells. We found that the phosphorylated Akt protein was downregulated in I3C- or DIM-treated prostate cancer cells (24, 71). Moreover, Akt kinase activity was also decreased in I3C- or DIM-treated prostate cancer cells, suggesting the inactivation of Akt upon I3C or DIM treatment. Gene expression profiles also demonstrated the downregulation of PI3K expression in prostate cancer cells treated with I3C (74). Further, DIM suppressed phosphorylation of FOXO3a and abrogated the phosphorylation of Akt and FOXO3a stimulated by IGF-1 (80), suggesting the inhibitory effects of DIM on Akt activation in cancer cells.
DIM has a potent inhibitory effect on AR signaling, which also crosstalk with Akt and NF-κB signaling. DIM has been found to inhibit AR nuclear translocation, PSA expression, and cell proliferation induced by dihydrotestosterone in prostate cancer cells (67). We also found that DIM significantly inhibits NF-κB and Akt activation, AR phosphorylation and nuclear translocation, and the expression of AR and PSA, suggesting that DIM could interrupt the crosstalk between AR, Akt, and NF-κB signaling (16). Further, we found that DIM significantly decreased FOXO3a binding to the promoter of AR, resulting in the downregulation of AR expression, the inhibition of cell proliferation, and the induction of apoptosis in prostate cancer cells (80). These findings demonstrate that DIM inhibits cell proliferation and induces apoptosis through the regulation of Akt/FOXO3a/AR signaling.
DIM has been known to inhibit Wnt signaling through the suppression of Akt/FOXO3a signaling. We found that DIM significantly increased the phosphorylation of β-catenin and inhibited β-catenin nuclear translocation (80), suggesting that DIM could also downregulate the activation of Wnt signaling. Other cellular signaling could also contribute to the DIM-induced inhibition of cancer growth. It is known that more than 50% of human prostate cancer cells overexpress ERG due to the AR-regulated TMPRSS2-ERG fusion gene. Recently, we found that DIM could downregulate ERG and Wnt signaling through the suppression of AR/TMPRSS2-ERG/Wnt signaling, leading to the inhibition of cancer invasion (72). In addition, DIM also upregulated p27 and downregulated RANKL/MITF and AR/PSA signaling, resulting in the inhibition of osteoclast and osteoblast differentiation, which could suppress bone remodeling and prostate cancer bone metastasis. Similar to isoflavone, DIM exerts its pleiotropic (multitargeting) effects on multiple signaling pathways; therefore, it could be a promising agent for the prevention and/or treatment of cancer.
It is important to note that DIM (BR-DIM, which is manufactured by BioResponse, LLC.) has been tested in a phase I clinical trial in patients with prostate cancer by our laboratory, showing that BR-DIM at a oral dose of 225 mg twice daily was safe (48), and thus this dose was chosen for our ongoing phase II clinical trial in preradical prostatectomy patients with prostate cancer. It has been previously shown by our laboratory that DIM could inhibit AR through transcriptional inactivation and nuclear exclusion in cell culture studies (16); however, such observation has not been reported in patients with human prostate cancer. Therefore, we have done an interim analysis of prostate tissue specimens obtained from our currently ongoing phase II clinical trial. We found that the level of expression of AR was reduced after BR-DIM intervention. Most importantly, the nuclear exclusion of AR was quiet dramatic (65), and it appears to be in part due to induction in the expression of miR-34a. These findings are consistent with our previously reported in vitro finding (16). However, much more clinical trials are warranted to fully appreciate the beneficial role of I3C/DIM in cancer prevention and therapy.
Conclusion and Perspectives
Oxidative stress has been linked to aging, inflammation, and carcinogenesis. Moreover, NF-κB signaling plays critical roles in oxidative stress, inflammatory response, and carcinogenesis. Importantly, isoflavones, I3C and DIM, have been shown to reduce oxidative stress via inhibition of NF-κB activation among others. Moreover, these antioxidants also target multiple signaling pathways that are deregulated in cancer, leading to the inhibition of cancer development and progression. More clinical trials are being conducted to evaluate the effects of isoflavone and DIM for the prevention of cancer development and also for the treatment of cancer either alone or in combination with conventional cancer therapeutics. It is our perspectives that further mechanistic studies elucidating the antioxidative and multi-targeting effects of isoflavone and DIM will fully establish the beneficial effects of these important natural agents for the prevention and/or treatment of human malignancies.
Abbreviations Used
- Akt
protein kinase B
- AOM
azoxymethane
- AR
androgen receptor
- DIM
3,3′-diindolylmethane
- DSS
dextran sodium sulfate
- EGFR
epidermal growth factor receptor
- ER
estrogen receptor
- GSK-3β
glycogen synthase kinase 3 beta
- I3C
indole-3-carbinol
- MMP-9
matrix metallopeptidase 9
- NF-κB
nuclear factor-kappa-B
- PSA
prostate-specific antigen
- ROS
reactive oxygen species
- TNF
tumor necrosis factor
- Wnt
wingless-type MMTV integration site family
Acknowledgments
This work was partly funded by the grants from the National Cancer Institute, NIH (5R01CA108535 and 5R01CA083695), to FHS.
References
- 1.Alhasan SA. Pietrasczkiwicz H. Alonso MD. Ensley J. Sarkar FH. Genistein-induced cell cycle arrest and apoptosis in a head and neck squamous cell carcinoma cell line. Nutr Cancer. 1999;34:12–19. doi: 10.1207/S15327914NC340102. [DOI] [PubMed] [Google Scholar]
- 2.Ali S. Banerjee S. Ahmad A. El-Rayes BF. Philip PA. Sarkar FH. Apoptosis-inducing effect of erlotinib is potentiated by 3,3′-diindolylmethane in vitro and in vivo using an orthotopic model of pancreatic cancer. Mol Cancer Ther. 2008;7:1708–1719. doi: 10.1158/1535-7163.MCT-08-0354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Ali S. Banerjee S. Schaffert JM. El-Rayes BF. Philip PA. Sarkar FH. Concurrent inhibition of NF-kappaB, cyclooxygenase-2, and epidermal growth factor receptor leads to greater anti-tumor activity in pancreatic cancer. J Cell Biochem. 2010;110:171–181. doi: 10.1002/jcb.22523. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Ali S. Varghese L. Pereira L. Tulunay-Ugur OE. Kucuk O. Carey TE. Wolf GT. Sarkar FH. Sensitization of squamous cell carcinoma to cisplatin induced killing by natural agents. Cancer Lett. 2009;278:201–209. doi: 10.1016/j.canlet.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Alvero AB. O'Malley D. Brown D. Kelly G. Garg M. Chen W. Rutherford T. Mor G. Molecular mechanism of phenoxodiol-induced apoptosis in ovarian carcinoma cells. Cancer. 2006;106:599–608. doi: 10.1002/cncr.21633. [DOI] [PubMed] [Google Scholar]
- 6.Arnao MB. Sanchez-Bravo J. Acosta M. Indole-3-carbinol as a scavenger of free radicals. Biochem Mol Biol Int. 1996;39:1125–1134. doi: 10.1080/15216549600201302. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee S. Wang Z. Kong D. Sarkar FH. 3,3′-Diindolylmethane enhances chemosensitivity of multiple chemotherapeutic agents in pancreatic cancer. Cancer Res. 2009;69:5592–5600. doi: 10.1158/0008-5472.CAN-09-0838. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Banerjee S. Zhang Y. Ali S. Bhuiyan M. Wang Z. Chiao PJ. Philip PA. Abbruzzese J. Sarkar FH. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005;65:9064–9072. doi: 10.1158/0008-5472.CAN-05-1330. [DOI] [PubMed] [Google Scholar]
- 9.Bargou RC. Emmerich F. Krappmann D. Bommert K. Mapara MY. Arnold W. Royer HD. Grinstein E. Greiner A. Scheidereit C. Dorken B. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. J Clin Invest. 1997;100:2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bash J. Zong WX. Banga S. Rivera A. Ballard DW. Ron Y. Gelinas C. Rel/NF-kappaB can trigger the Notch signaling pathway by inducing the expression of Jagged1, a ligand for Notch receptors. EMBO J. 1999;18:2803–2811. doi: 10.1093/emboj/18.10.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baxa DM. Yoshimura FK. Genistein reduces NF-kappa B in T lymphoma cells via a caspase-mediated cleavage of I kappa B alpha. Biochem Pharmacol. 2003;66:1009–1018. doi: 10.1016/s0006-2952(03)00415-5. [DOI] [PubMed] [Google Scholar]
- 12.Beal MF. Oxidatively modified proteins in aging and disease. Free Radic Biol Med. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 13.Benabadji SH. Wen R. Zheng JB. Dong XC. Yuan SG. Anticarcinogenic and antioxidant activity of diindolylmethane derivatives. Acta Pharmacol Sin. 2004;25:666–671. [PubMed] [Google Scholar]
- 14.Benzie IF. Evolution of dietary antioxidants. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:113–126. doi: 10.1016/s1095-6433(02)00368-9. [DOI] [PubMed] [Google Scholar]
- 15.Bharti AC. Aggarwal BB. Nuclear factor-kappa B and cancer: its role in prevention and therapy. Biochem Pharmacol. 2002;64:883–888. doi: 10.1016/s0006-2952(02)01154-1. [DOI] [PubMed] [Google Scholar]
- 16.Bhuiyan MM. Li Y. Banerjee S. Ahmed F. Wang Z. Ali S. Sarkar FH. Down-regulation of androgen receptor by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in both hormone-sensitive LNCaP and insensitive C4-2B prostate cancer cells. Cancer Res. 2006;66:10064–10072. doi: 10.1158/0008-5472.CAN-06-2011. [DOI] [PubMed] [Google Scholar]
- 17.Bonnesen C. Eggleston IM. Hayes JD. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001;61:6120–6130. [PubMed] [Google Scholar]
- 18.Bournat JC. Brown AM. Soler AP. Wnt-1 dependent activation of the survival factor NF-kappaB in PC12 cells. J Neurosci Res. 2000;61:21–32. doi: 10.1002/1097-4547(20000701)61:1<21::AID-JNR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Bowie A. O'Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 20.Braeuer SJ. Buneker C. Mohr A. Zwacka RM. Constitutively activated nuclear factor-kappaB, but not induced NF-kappaB, leads to TRAIL resistance by up-regulation of X-linked inhibitor of apoptosis protein in human cancer cells. Mol Cancer Res. 2006;4:715–728. doi: 10.1158/1541-7786.MCR-05-0231. [DOI] [PubMed] [Google Scholar]
- 21.Buckley AR. Buckley DJ. Gout PW. Liang H. Rao YP. Blake MJ. Inhibition by genistein of prolactin-induced Nb2 lymphoma cell mitogenesis. Mol Cell Endocrinol. 1993;98:17–25. doi: 10.1016/0303-7207(93)90231-8. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y. Xu J. Jhala N. Pawar P. Zhu ZB. Ma L. Byon CH. McDonald JM. Fas-mediated apoptosis in cholangiocarcinoma cells is enhanced by 3,3′-diindolylmethane through inhibition of AKT signaling and FLICE-like inhibitory protein. Am J Pathol. 2006;169:1833–1842. doi: 10.2353/ajpath.2006.060234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chinni SR. Li Y. Upadhyay S. Koppolu PK. Sarkar FH. Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene. 2001;20:2927–2936. doi: 10.1038/sj.onc.1204365. [DOI] [PubMed] [Google Scholar]
- 24.Chinni SR. Sarkar FH. Akt inactivation is a key event in indole-3-carbinol-induced apoptosis in PC-3 cells. Clin Cancer Res. 2002;8:1228–1236. [PubMed] [Google Scholar]
- 25.Cho HJ. Seon MR. Lee YM. Kim J. Kim JK. Kim SG. Park JH. 3,3′-Diindolylmethane suppresses the inflammatory response to lipopolysaccharide in murine macrophages. J Nutr. 2008;138:17–23. doi: 10.1093/jn/138.1.17. [DOI] [PubMed] [Google Scholar]
- 26.Chung FL. Morse MA. Eklind KI. Xu Y. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis by compounds derived from cruciferous vegetables and green tea. Ann N Y Acad Sci. 1993;686:186–201. doi: 10.1111/j.1749-6632.1993.tb39174.x. [DOI] [PubMed] [Google Scholar]
- 27.Isoflavone combination treatment. ClinicalTrials.org. www.clinicaltrials.gov/ct2/results?term=isoflavone+AND+combination. 2012. ClinicalTrials.orgwww.clinicaltrials.gov/ct2/results?term=isoflavone+AND+combination
- 28.Phenoxodiol clinical trials. ClinicalTrials.org. www.clinicaltrials.gov/ct2/results?term=Phenoxodiol. 2012. ClinicalTrials.orgwww.clinicaltrials.gov/ct2/results?term=Phenoxodiol
- 29.Constantinou A. Kiguchi K. Huberman E. Induction of differentiation and DNA strand breakage in human HL-60 and K-562 leukemia cells by genistein. Cancer Res. 1990;50:2618–2624. [PubMed] [Google Scholar]
- 30.Davis JN. Kucuk O. Djuric Z. Sarkar FH. Soy isoflavone supplementation in healthy men prevents NF-kappa B activation by TNF-alpha in blood lymphocytes. Free Radic Biol Med. 2001;30:1293–1302. doi: 10.1016/s0891-5849(01)00535-4. [DOI] [PubMed] [Google Scholar]
- 31.Davis JN. Kucuk O. Sarkar FH. Expression of prostate-specific antigen is transcriptionally regulated by genistein in prostate cancer cells. Mol Carcinog. 2002;34:91–101. doi: 10.1002/mc.10053. [DOI] [PubMed] [Google Scholar]
- 32.Davis JN. Kucuk O. Sarkar FH. Genistein inhibits NF-kappa B activation in prostate cancer cells. Nutr Cancer. 1999;35:167–174. doi: 10.1207/S15327914NC352_11. [DOI] [PubMed] [Google Scholar]
- 33.Delfino FJ. Boustead JN. Fix C. Walker WH. NF-kappaB and TNF-alpha stimulate androgen receptor expression in Sertoli cells. Mol Cell Endocrinol. 2003;201:1–12. doi: 10.1016/s0303-7207(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 34.Donald S. Verschoyle RD. Greaves P. Colombo T. Zucchetti M. Falcioni C. Zaffaroni M. D'Incalci M. Manson MM. Jimeno J. Steward WP. Gescher AJ. Dietary agent indole-3-carbinol protects female rats against the hepatotoxicity of the antitumor drug ET-743 (trabectidin) without compromising efficacy in a rat mammary carcinoma. Int J Cancer. 2004;111:961–967. doi: 10.1002/ijc.20356. [DOI] [PubMed] [Google Scholar]
- 35.Drummond GR. Selemidis S. Griendling KK. Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du Q. Geller DA. Cross-Regulation Between Wnt and NF-kappaB Signaling Pathways. For Immunopathol Dis Therap. 2010;1:155–181. doi: 10.1615/ForumImmunDisTher.v1.i3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dudek EJ. Shang F. Taylor A. H(2)O(2)-mediated oxidative stress activates NF-kappa B in lens epithelial cells. Free Radic Biol Med. 2001;31:651–658. doi: 10.1016/s0891-5849(01)00634-7. [DOI] [PubMed] [Google Scholar]
- 38.Exner M. Hermann M. Hofbauer R. Kapiotis S. Quehenberger P. Speiser W. Held I. Gmeiner BM. Genistein prevents the glucose autoxidation mediated atherogenic modification of low density lipoprotein. Free Radic Res. 2001;34:101–112. doi: 10.1080/10715760100300101. [DOI] [PubMed] [Google Scholar]
- 39.Fan S. Meng Q. Saha T. Sarkar FH. Rosen EM. Low concentrations of diindolylmethane, a metabolite of indole-3-carbinol, protect against oxidative stress in a BRCA1-dependent manner. Cancer Res. 2009;69:6083–6091. doi: 10.1158/0008-5472.CAN-08-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finkel T. Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 41.Firestone GL. Bjeldanes LF. Indole-3-carbinol and 3-3′-diindolylmethane antiproliferative signaling pathways control cell-cycle gene transcription in human breast cancer cells by regulating promoter-Sp1 transcription factor interactions. J Nutr. 2003;133:2448S–2455S. doi: 10.1093/jn/133.7.2448S. [DOI] [PubMed] [Google Scholar]
- 42.Frydoonfar HR. McGrath DR. Spigelman AD. Inhibition of proliferation of a colon cancer cell line by indole-3-carbinol. Colorectal Dis. 2002;4:205–207. doi: 10.1046/j.1463-1318.2002.00346.x. [DOI] [PubMed] [Google Scholar]
- 43.Galaris D. Evangelou A. The role of oxidative stress in mechanisms of metal-induced carcinogenesis. Crit Rev Oncol Hematol. 2002;42:93–103. doi: 10.1016/s1040-8428(01)00212-8. [DOI] [PubMed] [Google Scholar]
- 44.Garg A. Aggarwal BB. Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia. 2002;16:1053–1068. doi: 10.1038/sj.leu.2402482. [DOI] [PubMed] [Google Scholar]
- 45.Gong L. Li Y. Nedeljkovic-Kurepa A. Sarkar FH. Inactivation of NF-kappaB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene. 2003;22:4702–4709. doi: 10.1038/sj.onc.1206583. [DOI] [PubMed] [Google Scholar]
- 46.Haefner B. NF-kappaB: arresting a major culprit in cancer. Drug Discov Today. 2002;7:653–663. doi: 10.1016/s1359-6446(02)02309-7. [DOI] [PubMed] [Google Scholar]
- 47.Halliwell B. Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: measurement, mechanism and the effects of nutrition. Mutat Res. 1999;443:37–52. doi: 10.1016/s1383-5742(99)00009-5. [DOI] [PubMed] [Google Scholar]
- 48.Heath EI. Heilbrun LK. Li J. Vaishampayan U. Harper F. Pemberton P. Sarkar FH. A phase I dose-escalation study of oral BR-DIM (BioResponse 3,3′- Diindolylmethane) in castrate-resistant, non-metastatic prostate cancer. Am J Transl Res. 2010;2:402–411. [PMC free article] [PubMed] [Google Scholar]
- 49.Hideshima T. Chauhan D. Richardson P. Mitsiades C. Mitsiades N. Hayashi T. Munshi N. Dang L. Castro A. Palombella V. Adams J. Anderson KC. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277:16639–16647. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 50.Huang Z. Zuo L. Zhang Z. Liu J. Chen J. Dong L. Zhang J. 3,3′-Diindolylmethane decreases VCAM-1 expression and alleviates experimental colitis via a BRCA1-dependent antioxidant pathway. Free Radic Biol Med. 2011;50:228–236. doi: 10.1016/j.freeradbiomed.2010.10.703. [DOI] [PubMed] [Google Scholar]
- 51.Hwang JR. Jo K. Lee Y. Sung BJ. Park YW. Lee JH. Upregulation of CD9 in ovarian cancer is related to the induction of TNF-alpha gene expression and constitutive NF-kappaB activation. Carcinogenesis. 2012;33:77–83. doi: 10.1093/carcin/bgr257. [DOI] [PubMed] [Google Scholar]
- 52.Ichite N. Chougule M. Patel AR. Jackson T. Safe S. Singh M. Inhalation delivery of a novel diindolylmethane derivative for the treatment of lung cancer. Mol Cancer Ther. 2010;9:3003–3014. doi: 10.1158/1535-7163.MCT-09-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Israel A. Signal transduction: a regulator branches out. Nature. 2003;423:596–597. doi: 10.1038/423596a. [DOI] [PubMed] [Google Scholar]
- 54.Johnson IT. Glucosinolates: bioavailability and importance to health. Int J Vitam Nutr Res. 2002;72:26–31. doi: 10.1024/0300-9831.72.1.26. [DOI] [PubMed] [Google Scholar]
- 55.Kameoka S. Leavitt P. Chang C. Kuo SM. Expression of antioxidant proteins in human intestinal Caco-2 cells treated with dietary flavonoids. Cancer Lett. 1999;146:161–167. doi: 10.1016/s0304-3835(99)00253-0. [DOI] [PubMed] [Google Scholar]
- 56.Kamsteeg M. Rutherford T. Sapi E. Hanczaruk B. Shahabi S. Flick M. Brown D. Mor G. Phenoxodiol—an isoflavone analog—induces apoptosis in chemoresistant ovarian cancer cells. Oncogene. 2003;22:2611–2620. doi: 10.1038/sj.onc.1206422. [DOI] [PubMed] [Google Scholar]
- 57.Karamouzis MV. Gorgoulis VG. Papavassiliou AG. Transcription factors and neoplasia: vistas in novel drug design. Clin Cancer Res. 2002;8:949–961. [PubMed] [Google Scholar]
- 58.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karin M. Cao Y. Greten FR. Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 60.Karin M. Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 61.Karin M. Takahashi T. Kapahi P. Delhase M. Chen Y. Makris C. Rothwarf D. Baud V. Natoli G. Guido F. Li N. Oxidative stress and gene expression: the AP-1 and NF-kappaB connections. Biofactors. 2001;15:87–89. doi: 10.1002/biof.5520150207. [DOI] [PubMed] [Google Scholar]
- 62.Kim EJ. Park H. Kim J. Park JH. 3,3′-diindolylmethane suppresses 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and tumor promotion in mouse skin via the downregulation of inflammatory mediators. Mol Carcinog. 2010;49:672–683. doi: 10.1002/mc.20640. [DOI] [PubMed] [Google Scholar]
- 63.Kim YH. Kwon HS. Kim DH. Shin EK. Kang YH. Park JH. Shin HK. Kim JK. 3,3′-diindolylmethane attenuates colonic inflammation and tumorigenesis in mice. Inflamm Bowel Dis. 2009;15:1164–1173. doi: 10.1002/ibd.20917. [DOI] [PubMed] [Google Scholar]
- 64.Klaunig JE. Wang Z. Pu X. Zhou S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol Appl Pharmacol. 2011;254:86–99. doi: 10.1016/j.taap.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 65.Kong D. Heath E. Chen W. Cher M. Powell I. Heilbrun L. Li Y. Ali S. Sethi S. Hassan O. Hwang C. Gupta N. Chitale D. Sakr WA. Menon M. Sarkar FH. Epigenetic silencing of miR-34a in human prostate cancer cells and tumor tissue specimens can be reversed by BR-DIM treatment. Am J Transl Res. 2012;4:14–23. [PMC free article] [PubMed] [Google Scholar]
- 66.Kong D. Li Y. Wang Z. Banerjee S. Sarkar FH. Inhibition of angiogenesis and invasion by 3,3′-diindolylmethane is mediated by the nuclear factor-kappaB downstream target genes MMP-9 and uPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res. 2007;67:3310–3319. doi: 10.1158/0008-5472.CAN-06-4277. [DOI] [PubMed] [Google Scholar]
- 67.Le HT. Schaldach CM. Firestone GL. Bjeldanes LF. Plant-derived 3,3′-Diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J Biol Chem. 2003;278:21136–21145. doi: 10.1074/jbc.M300588200. [DOI] [PubMed] [Google Scholar]
- 68.Li L. Aggarwal BB. Shishodia S. Abbruzzese J. Kurzrock R. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101:2351–2362. doi: 10.1002/cncr.20605. [DOI] [PubMed] [Google Scholar]
- 69.Li Y. Ahmed F. Ali S. Philip PA. Kucuk O. Sarkar FH. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005;65:6934–6942. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- 70.Li Y. Bhuiyan M. Sarkar FH. Induction of apoptosis and inhibition of c-erbB-2 in MDA-MB-435 cells by genistein. Int J Oncol. 1999;15:525–533. doi: 10.3892/ijo.15.3.525. [DOI] [PubMed] [Google Scholar]
- 71.Li Y. Chinni SR. Sarkar FH. Selective growth regulatory and pro-apoptotic effects of DIM is mediated by AKT and NF-kappaB pathways in prostate cancer cells. Front Biosci. 2005;10:236–243. doi: 10.2741/1523. [DOI] [PubMed] [Google Scholar]
- 72.Li Y. Kong D. Wang Z. Ahmad A. Bao B. Padhye S. Sarkar FH. Inactivation of AR/TMPRSS2-ERG/Wnt signaling networks attenuates the aggressive behavior of prostate cancer cells. Cancer Prev Res (Phila) 2011;4:1495–1506. doi: 10.1158/1940-6207.CAPR-11-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y. Kucuk O. Hussain M. Abrams J. Cher ML. Sarkar FH. Antitumor and antimetastatic activities of docetaxel are enhanced by genistein through regulation of osteoprotegerin/receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/MMP-9 signaling in prostate cancer. Cancer Res. 2006;66:4816–4825. doi: 10.1158/0008-5472.CAN-05-3752. [DOI] [PubMed] [Google Scholar]
- 74.Li Y. Li X. Sarkar FH. Gene expression profiles of I3C- and DIM-treated PC3 human prostate cancer cells determined by cDNA microarray analysis. J Nutr. 2003;133:1011–1019. doi: 10.1093/jn/133.4.1011. [DOI] [PubMed] [Google Scholar]
- 75.Li Y. Sarkar FH. Down-regulation of invasion and angiogenesis-related genes identified by cDNA microarray analysis of PC3 prostate cancer cells treated with genistein. Cancer Lett. 2002;186:157–164. doi: 10.1016/s0304-3835(02)00349-x. [DOI] [PubMed] [Google Scholar]
- 76.Li Y. Sarkar FH. Gene expression profiles of genistein-treated PC3 prostate cancer cells. J Nutr. 2002;132:3623–3631. doi: 10.1093/jn/132.12.3623. [DOI] [PubMed] [Google Scholar]
- 77.Li Y. Sarkar FH. Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res. 2002;8:2369–2377. [PubMed] [Google Scholar]
- 78.Li Y. Upadhyay S. Bhuiyan M. Sarkar FH. Induction of apoptosis in breast cancer cells MDA-MB-231 by genistein. Oncogene. 1999;18:3166–3172. doi: 10.1038/sj.onc.1202650. [DOI] [PubMed] [Google Scholar]
- 79.Li Y. Wang Z. Kong D. Li R. Sarkar SH. Sarkar FH. Regulation of Akt/FOXO3a/GSK-3beta/AR signaling network by isoflavone in prostate cancer cells. J Biol Chem. 2008;283:27707–27716. doi: 10.1074/jbc.M802759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Y. Wang Z. Kong D. Murthy S. Dou QP. Sheng S. Reddy GP. Sarkar FH. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem. 2007;282:21542–21550. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- 81.Lian F. Bhuiyan M. Li YW. Wall N. Kraut M. Sarkar FH. Genistein-induced G2-M arrest, p21WAF1 upregulation, and apoptosis in a non-small-cell lung cancer cell line. Nutr Cancer. 1998;31:184–191. doi: 10.1080/01635589809514701. [DOI] [PubMed] [Google Scholar]
- 82.Luke MC. Coffey DS. Human androgen receptor binding to the androgen response element of prostate specific antigen. J Androl. 1994;15:41–51. [PubMed] [Google Scholar]
- 83.Luo JL. Kamata H. Karin M. IKK/NF-kappaB signaling: balancing life and death—a new approach to cancer therapy. J Clin Invest. 2005;115:2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luo JL. Kamata H. Karin M. The anti-death machinery in IKK/NF-kappaB signaling. J Clin Immunol. 2005;25:541–550. doi: 10.1007/s10875-005-8217-6. [DOI] [PubMed] [Google Scholar]
- 85.Ma Y. Wang J. Liu L. Zhu H. Chen X. Pan S. Sun X. Jiang H. Genistein potentiates the effect of arsenic trioxide against human hepatocellular carcinoma: role of Akt and nuclear factor-kappaB. Cancer Lett. 2011;301:75–84. doi: 10.1016/j.canlet.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 86.Makarov SS. NF-kappaB as a therapeutic target in chronic inflammation: recent advances. Mol Med Today. 2000;6:441–448. doi: 10.1016/s1357-4310(00)01814-1. [DOI] [PubMed] [Google Scholar]
- 87.Manning BD. Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matsumoto G. Namekawa J. Muta M. Nakamura T. Bando H. Tohyama K. Toi M. Umezawa K. Targeting of nuclear factor kappaB Pathways by dehydroxymethylepoxyquinomicin, a novel inhibitor of breast carcinomas: antitumor and antiangiogenic potential in vivo. Clin Cancer Res. 2005;11:1287–1293. [PubMed] [Google Scholar]
- 89.Meng F. D'Mello SR. NF-kappaB stimulates Akt phosphorylation and gene expression by distinct signaling mechanisms. Biochim Biophys Acta. 2003;1630:35–40. doi: 10.1016/j.bbaexp.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 90.Moiseeva EP. Almeida GM. Jones GD. Manson MM. Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells. Mol Cancer Ther. 2007;6:3071–3079. doi: 10.1158/1535-7163.MCT-07-0117. [DOI] [PubMed] [Google Scholar]
- 91.Morgan MJ. Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nho CW. Jeffery E. Crambene, a bioactive nitrile derived from glucosinolate hydrolysis, acts via the antioxidant response element to upregulate quinone reductase alone or synergistically with indole-3-carbinol. Toxicol Appl Pharmacol. 2004;198:40–48. doi: 10.1016/j.taap.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 93.Orlowski RZ. Baldwin AS. NF-kappaB as a therapeutic target in cancer. Trends Mol Med. 2002;8:385–389. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- 94.Owuor ED. Kong AN. Antioxidants and oxidants regulated signal transduction pathways. Biochem Pharmacol. 2002;64:765–770. doi: 10.1016/s0006-2952(02)01137-1. [DOI] [PubMed] [Google Scholar]
- 95.Park JS. Jung JS. Jeong YH. Hyun JW. Le TK. Kim DH. Choi EC. Kim HS. Antioxidant mechanism of isoflavone metabolites in hydrogen peroxide-stimulated rat primary astrocytes: critical role of hemeoxygenase-1 and NQO1 expression. J Neurochem. 2011;119:909–919. doi: 10.1111/j.1471-4159.2011.07395.x. [DOI] [PubMed] [Google Scholar]
- 96.Patel RP. Boersma BJ. Crawford JH. Hogg N. Kirk M. Kalyanaraman B. Parks DA. Barnes S. Darley-Usmar V. Antioxidant mechanisms of isoflavones in lipid systems: paradoxical effects of peroxyl radical scavenging. Free Radic Biol Med. 2001;31:1570–1581. doi: 10.1016/s0891-5849(01)00737-7. [DOI] [PubMed] [Google Scholar]
- 97.Perl A. Hanczko R. Telarico T. Oaks Z. Landas S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends Mol Med. 2011;17:395–403. doi: 10.1016/j.molmed.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Porta C. Riboldi E. Sica A. Mechanisms linking pathogens-associated inflammation and cancer. Cancer Lett. 2011;305:250–262. doi: 10.1016/j.canlet.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 99.Privat M. Aubel C. Arnould S. Communal Y. Ferrara M. Bignon YJ. AKT and p21 WAF1/CIP1 as potential genistein targets in BRCA1-mutant human breast cancer cell lines. Anticancer Res. 2010;30:2049–2054. [PubMed] [Google Scholar]
- 100.Rahman KM. Aranha O. Sarkar FH. Indole-3-carbinol (I3C) induces apoptosis in tumorigenic but not in nontumorigenic breast epithelial cells. Nutr Cancer. 2003;45:101–112. doi: 10.1207/S15327914NC4501_12. [DOI] [PubMed] [Google Scholar]
- 101.Rahman KW. Li Y. Sarkar FH. Inactivation of Akt and NF-kappaB Play Important Roles During Indole-3-Carbinol-Induced Apoptosis in Breast Cancer Cells. Nutr Cancer. 2004;48:84–94. doi: 10.1207/s15327914nc4801_12. [DOI] [PubMed] [Google Scholar]
- 102.Rath E. Haller D. Inflammation and cellular stress: a mechanistic link between immune-mediated and metabolically driven pathologies. Eur J Nutr. 2011;50:219–233. doi: 10.1007/s00394-011-0197-0. [DOI] [PubMed] [Google Scholar]
- 103.Romashkova JA. Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 104.Ruiz-Larrea MB. Mohan AR. Paganga G. Miller NJ. Bolwell GP. Rice-Evans CA. Antioxidant activity of phytoestrogenic isoflavones. Free Radic Res. 1997;26:63–70. doi: 10.3109/10715769709097785. [DOI] [PubMed] [Google Scholar]
- 105.Saitoh Y. Martinez B. V Uota S. Hasegawa A. Yamamoto N. Imoto I. Inazawa J. Yamaoka S. Overexpression of NF-kappaB inducing kinase underlies constitutive NF-kappaB activation in lung cancer cells. Lung Cancer. 2010;70:263–270. doi: 10.1016/j.lungcan.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 106.Sapi E. Alvero AB. Chen W. O'Malley D. Hao XY. Dwipoyono B. Garg M. Kamsteeg M. Rutherford T. Mor G. Resistance of ovarian carcinoma cells to docetaxel is XIAP dependent and reversible by phenoxodiol. Oncol Res. 2004;14:567–578. doi: 10.3727/0965040042707943. [DOI] [PubMed] [Google Scholar]
- 107.Sarkar FH. Rahman KM. Li Y. Bax translocation to mitochondria is an important event in inducing apoptotic cell death by indole-3-carbinol (I3C) treatment of breast cancer cells. J Nutr. 2003;133:2434S–2439S. doi: 10.1093/jn/133.7.2434S. [DOI] [PubMed] [Google Scholar]
- 108.Schwarzer R. Dorken B. Jundt F. Notch is an essential upstream regulator of NF-kappaB and is relevant for survival of Hodgkin and Reed-Sternberg cells. Leukemia. 2011 doi: 10.1038/leu.2011.265. [DOI] [PubMed] [Google Scholar]
- 109.Sierens J. Hartley JA. Campbell MJ. Leathem AJ. Woodside JV. In vitro isoflavone supplementation reduces hydrogen peroxide-induced DNA damage in sperm. Teratog Carcinog Mutagen. 2002;22:227–234. doi: 10.1002/tcm.10015. [DOI] [PubMed] [Google Scholar]
- 110.Singh-Gupta V. Banerjee S. Yunker CK. Rakowski JT. Joiner MC. Konski AA. Sarkar FH. Hillman GG. B-DIM impairs radiation-induced survival pathways independently of androgen receptor expression and augments radiation efficacy in prostate cancer. Cancer Lett. 2012;318:86–92. doi: 10.1016/j.canlet.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Singh-Gupta V. Joiner MC. Runyan L. Yunker CK. Sarkar FH. Miller S. Gadgeel SM. Konski AA. Hillman GG. Soy isoflavones augment radiation effect by inhibiting APE1/Ref-1 DNA repair activity in non-small cell lung cancer. J Thorac Oncol. 2011;6:688–698. doi: 10.1097/JTO.0b013e31821034ae. [DOI] [PubMed] [Google Scholar]
- 112.Su Y. Simmen RC. Soy isoflavone genistein up-regulates epithelial adhesion molecule E-cadherin expression and attenuates {beta}-catenin signaling in mammary epithelial cells. Carcinogenesis. 2008;30:331–339. doi: 10.1093/carcin/bgn279. [DOI] [PubMed] [Google Scholar]
- 113.Su Y. Simmen FA. Xiao R. Simmen RC. Expression profiling of rat mammary epithelial cells reveals candidate signaling pathways in dietary protection from mammary tumors. Physiol Genomics. 2007;30:8–16. doi: 10.1152/physiolgenomics.00023.2007. [DOI] [PubMed] [Google Scholar]
- 114.Sweeney C. Li L. Shanmugam R. Bhat-Nakshatri P. Jayaprakasan V. Baldridge LA. Gardner T. Smith M. Nakshatri H. Cheng L. Nuclear factor-kappaB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin Cancer Res. 2004;10:5501–5507. doi: 10.1158/1078-0432.CCR-0571-03. [DOI] [PubMed] [Google Scholar]
- 115.Tabary O. Escotte S. Couetil JP. Hubert D. Dusser D. Puchelle E. Jacquot J. Genistein inhibits constitutive and inducible NFkappaB activation and decreases IL-8 production by human cystic fibrosis bronchial gland cells. Am J Pathol. 1999;155:473–481. doi: 10.1016/s0002-9440(10)65143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tepper CG. Vinall RL. Wee CB. Xue L. Shi XB. Burich R. Mack PC. de Vere White RW. GCP-mediated growth inhibition and apoptosis of prostate cancer cells via androgen receptor-dependent and -independent mechanisms. Prostate. 2007;67:521–535. doi: 10.1002/pros.20548. [DOI] [PubMed] [Google Scholar]
- 117.Verhoeven DT. Verhagen H. Goldbohm RA. van den Brandt PA. van Poppel G. A review of mechanisms underlying anticarcinogenicity by brassica vegetables. Chem Biol Interact. 1997;103:79–129. doi: 10.1016/s0009-2797(96)03745-3. [DOI] [PubMed] [Google Scholar]
- 118.Wagner J. Lehmann L. Estrogens modulate the gene expression of Wnt-7a in cultured endometrial adenocarcinoma cells. Mol Nutr Food Res. 2006;50:368–372. doi: 10.1002/mnfr.200500215. [DOI] [PubMed] [Google Scholar]
- 119.Wang X. McCullough KD. Franke TF. Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000;275:14624–14631. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- 120.Wang Z. Li Y. Banerjee S. Sarkar FH. Emerging role of Notch in stem cells and cancer. Cancer Lett. 2009;279:8–12. doi: 10.1016/j.canlet.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Z. Zhang Y. Li Y. Banerjee S. Liao J. Sarkar FH. Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol Cancer Ther. 2006;5:483–493. doi: 10.1158/1535-7163.MCT-05-0299. [DOI] [PubMed] [Google Scholar]
- 122.Wei H. Wei L. Frenkel K. Bowen R. Barnes S. Inhibition of tumor promoter-induced hydrogen peroxide formation in vitro and in vivo by genistein. Nutr Cancer. 1993;20:1–12. doi: 10.1080/01635589309514265. [DOI] [PubMed] [Google Scholar]
- 123.Yamamoto Y. Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang J. Hsu BAJ. Kinseth BAM. Bjeldanes LF. Firestone GL. Indole-3-carbinol induces a G1 cell cycle arrest and inhibits prostate-specific antigen production in human LNCaP prostate carcinoma cells. Cancer. 2003;98:2511–2520. doi: 10.1002/cncr.11844. [DOI] [PubMed] [Google Scholar]
- 125.Zhang L. Altuwaijri S. Deng F. Chen L. Lal P. Bhanot UK. Korets R. Wenske S. Lilja HG. Chang C. Scher HI. Gerald WL. NF-kappaB regulates androgen receptor expression and prostate cancer growth. Am J Pathol. 2009;175:489–499. doi: 10.2353/ajpath.2009.080727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang YB. Zhong ZM. Hou G. Jiang H. Chen JT. Involvement of oxidative stress in age-related bone loss. J Surg Res. 2011;169:e37–e42. doi: 10.1016/j.jss.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 127.Zhou HB. Chen JJ. Wang WX. Cai JT. Du Q. Apoptosis of human primary gastric carcinoma cells induced by genistein. World J Gastroenterol. 2004;10:1822–1825. doi: 10.3748/wjg.v10.i12.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou Y. Lee AS. Mechanism for the suppression of the mammalian stress response by genistein, an anticancer phytoestrogen from soy. J Natl Cancer Inst. 1998;90:381–388. doi: 10.1093/jnci/90.5.381. [DOI] [PubMed] [Google Scholar]