Abstract
Significance: Adequate and supranutritional selenium (Se) intake, maintaining full expression of selenoproteins, has been assumed to be beneficial for human health with respect to prevention of cancer. Strikingly, the effectiveness of dietary Se supplementation depends on many factors: baseline Se status, age, gender, and genetic background of an individual; type of cancer; and time point of intervention in addition to metabolic conversion and dose of applied Se compounds. Recent Advances: Se intake levels for optimization of plasma selenoproteins in humans have been delineated. Regulation, function, and genetic variants of several selenoproteins have been characterized in the intestine, where Se-mediated prevention of colorectal cancer appears to be particularly promising. Critical Issues: Numerous cell culture and animal studies indicate anticarcinogenic capacity of various Se compounds but, at present, the outcome of human studies is inconsistent and, in large part, disappointing. Moreover, supranutritional Se intake may even trigger adverse health effects, possibly increasing the risk for Type 2 diabetes in Se-replete populations. Future Directions: To improve protocols for the use of Se in cancer prevention, knowledge on cellular and systemic actions of Se compounds needs to be broadened and linked to individual-related determinants such as the occurrence of variants in selenoprotein genes and the Se status. Based on better mechanistic insight, populations and individuals that may benefit most from dietary Se supplementation need to be defined and studied in suitably planned intervention trials. Antioxid. Redox Signal. 19, 181–191.
Introduction
The essential trace element and micronutrient selenium (Se) has been appreciated for a plethora of potential health benefits. Se intake from the habitual diet largely depends on the bioavailability and content of Se in crop plants that relies on Se content in soil. Cereals, milk, meat, and fish are suitable sources of Se in human nutrition. Recommended levels for adequate Se intake of adults range between 30 and 85 μg/day, with a “tolerable upper intake level” set at 300–450 μg/day (20, 73). Although overt Se deficiency is rarely observed in humans, the consumption of Se-enriched dietary supplements is common in Europe and more so in the U.S., where one-third of the population regularly ingests multivitamin/mineral supplements (3). Inorganic Se compounds such as sodium selenite and selenate as well as organic Se-compounds, for example, Se-enriched yeast and garlic containing high amounts of selenomethionine and gamma-glutamyl-Se-methylselenocysteine are available (38).
Dietary Se supplementation has been suggested to be useful for the prevention of neurodegenerative and cardiovascular diseases related to chronic oxidative stress and in treatment of patients suffering from inflammatory disorders, viral diseases, and sepsis (20, 35, 84). In recent years, Se has emerged promising to delay the inflammatory process in autoimmune thyroid disease: daily administration of 200 μg selenite to patients with Graves' disease (Morbus Basedow) improved their quality of life and slowed the progression of orbitopathy (56). A potential anticarcinogenic capacity of Se has attracted most public and scientific attention, going back to a large epidemiological study in the 1970s that reported an inverse correlation of Se intake levels with cancer mortality among individuals from 27 countries (80). Notwithstanding the disappointing outcome of recent human intervention studies, Se continues to be considered as a candidate drug for nutritional cancer prevention. Also, synthetic organoselenium compounds have been developed for antitumor activity (64).
In February 2013, a PubMed search for the topic “selenium and human health” yields 672 publications only from the last 5 years, including 158 reviews. For “selenium and cancer,” there are 894 hits from the last 5 years, including 157 reviews. Against this background of many comprehensive reviews, we contribute here a short overview within this Forum Issue “Antioxidants in Cancer Prevention.” The recent Cochrane report by Dennert et al. (17) on the use of Se for human cancer prevention summarized 49 observational and 6 intervention studies. Despite some limitations regarding the design of observational studies and the quality of available data, a higher Se status appeared to be associated with a lower cancer incidence and mortality, particularly in males, whereas the results of intervention studies were inconsistent and raised concern about potential harmful side effects (17). Here, we will discuss results from human studies that highlight determinants influencing the tumor-preventive action of Se. A low Se status has been associated epidemiologically with increased risk to develop colorectal adenoma (41), and an altered expression pattern of selenoproteins is characteristic for colorectal cancer (CRC) tissue (1, 65). Thus, we will summarize advances in understanding regulation and function of selenoproteins in the intestine. Lastly, we will provide a mechanistic approach for understanding epidemiological associations of an increased risk for Type 2 diabetes mellitus (T2DM) when ingesting Se at the supranutritional doses applied for cancer prevention (85, 86).
Optimization of Selenoprotein Biosynthesis Through Se Supplementation
Most of the biological capacity of Se relies on selenoproteins (84). Selenocysteine is co-translationally incorporated into selenoproteins, as distinguished from proteins that contain nonspecifically incorporated selenomethionine. In the human genome, 25 genes encoding selenoproteins have been identified (49). Humans with genetically impaired biosynthesis of selenoproteins develop a multisystem disorder (78). Se availability is the single most important determinant for biosynthesis of many selenoproteins. Thus, optimized expression of selenoproteins through adequate or supranutritional Se intake represents both a key objective and a biomarker of Se-replete status in intervention trials that use Se for cancer prevention (27). When Se supply is inadequate, biosynthesis of individual selenoproteins decreases according to their ranking in the so-called selenoprotein hierarchy. Generally, mRNA and protein expression of glutathione peroxidase 1 (GPx1) are rapidly downregulated under Se-deficient conditions, whereas GPx4 and thioredoxin reductase 1 (TrxR1) levels remain largely unaltered (5, 54). In hepatocytes, Se deficiency causes a substantial decrease in secretion of selenoprotein P (SeP) (31), the selenoprotein with the highest Se content and a primary function as physiological Se transport protein (9, 84). Different tissues retain their Se content and their selenoprotein expression to a different extent under limited Se supply, thus providing a second level in the hierarchy (5, 9). This attribute of Se metabolism may hamper estimates of the Se intake required to optimize expression of individual selenoproteins in selected target tissues.
As surrogate parameter to determine the Se status of an individual, the Se content in hair, toenails, or plasma is routinely measured. However, plasma levels of the two extracellular selenoproteins GPx3 and SeP might be preferable as biomarkers to assess Se status and outcome of dietary Se supplementation. Maximal GPx3 activity in plasma is achieved at a daily intake of ∼70 μg Se and plasma Se levels of 90 ng/ml (18), whereas SeP plasma levels reach a plateau at ∼105 μg Se/day and plasma Se levels of 124 ng/ml (36). This pattern indicates that protection against some cancers is associated rather with optimization of SeP than with optimization of GPx3 in plasma (20). However, changes in plasma Se or selenoprotein levels do not necessarily reflect changes in expression and activity of selenoproteins in tissues at risk of developing cancer. Effects of Se-enriched milk protein and Se-yeast on rectal selenoprotein mRNA levels have been compared in Australians with adequate Se status (basal plasma Se levels of ∼100 ng/ml). While increases in plasma Se levels were similar in both Se-supplemented groups, dairy-Se was more effective to stimulate gene expression of selenoproteins. Dairy-Se increased rectal SeP, GPx1, and GPx2 mRNA levels, whereas Se-yeast only increased rectal SeP mRNA levels (33). First attempts have been undertaken to identify suitable biomarkers of Se status in selected tissues. Feeding mice a marginally Se-deficient diet resulted in decreased gene expression of the selenoproteins GPx1, SelW, SelH, and SelM in colon (44). By gene expression analysis of human lymphocytes, several non-selenoprotein genes encoding ribosomal proteins and translation factors have been found to respond to Se supplementation (66).
Is Se Capable of Preventing Cancer? Lessons from Human Studies
The above-mentioned observations by Schrauzer et al. (80) inspired intervention trials that aimed to test the influence of dietary supplementation with various Se compounds—administered alone or in combination with other antioxidants—on the incidence of different types of cancer and cancer mortality (27, 88). Table 1 lists human intervention studies discussed in this review and the dietary Se compounds and Se doses used therein. If suboptimal Se intake and the resulting low Se status are risk factors to develop cancer, individuals who live in areas with low Se in the soil are expected to benefit most from Se supplements. Se-poor soils are characteristic for some regions in China and several European countries, whereas high Se contents are common for North and South American soils (20).
Table 1.
Dietary Selenium Compounds Applied for Cancer Prevention in Human Intervention Studies Discussed in This Review
| Se compound | Intervention study | Se dose |
|---|---|---|
| Sodium selenite | Qidong study (92) | 15 ppm Se (in table salt) |
| L-selenomethionine | SELECT study (46, 53) | 200 μg Se/day |
| SWOG S9917 study (58) | 200 μg Se/day | |
| Se-enriched yeast | Linxian study (4, 57, 72) | 50 μg Se/day |
| SU.VI.MAX study (61) | 100 μg Se/day | |
| NPC study (13, 19) | 200 μg Se/day | |
| Qidong study (92) | 200 μg Se/day |
Se-enriched yeast is produced by growing yeast in culture medium containing selenomethionine. Please note that Se has been administered in a combination of antioxidants in the Linxian study (Se, vitamin E, and β-carotene) and in the SU.VI.MAX study (Se, vitamin C, vitamin E, β-carotene, and zinc). In SELECT, Se has been used either alone or in combination with vitamin E.
Se, selenium; NPC, Nutritional Prevention of Cancer; SELECT, Selenium and Vitamin E Cancer Prevention Trial; SU.VI.MAX, supplémentation en vitamines et minéraux antioxydants.
Indeed, long-term Se supplementation has been found preventive in two Chinese populations at high risk for cancer. Both selenite and Se-yeast lowered the infection rate with hepatitis B virus and the incidence of primary liver cancer in participants of a study in the Chinese province of Qidong (92). Participants of the Linxian study who received for 5 years a combination of Se-yeast, α-tocopherol and β-carotene showed significantly decreased cancer mortality and a lower incidence of esophageal/gastric cardia cancers (4), and moreover, the risk to develop those cancers was inversely correlated with serum Se levels (57). A recently published follow-up of the Linxian study reported that the beneficial effects of Se, α-tocopherol and β-carotene on overall and gastric cancer mortality were still evident 10 years after termination. Participants who were younger than 55 years at the beginning of the study benefited most from dietary supplementation, suggesting an age-dependent effect of Se (72).
To date, the strongest indication for a tumor-preventive capacity of dietary Se supplements in humans derives from the Nutritional Prevention of Cancer (NPC) trial, carried out with individuals from the Eastern U.S. who received 200 μg Se/day in the form of Se-yeast or a placebo for 4.5 years (13). The NPC trial was designed to examine whether Se supplementation suppresses the recurrence of skin cancer. This primary aim was not achieved. Actually, the risk to develop basal cell and squamous cell carcinoma was nonsignificantly increased for Se-supplemented individuals. However, secondary endpoint analyses revealed decreased cancer mortality and lower incidence of prostate cancer and CRC in male subjects of the Se-treated group. The initial Se status of the volunteers turned out to be a key determining factor for the efficacy of Se-mediated tumor prevention, as only Se-supplemented men with baseline plasma Se levels in the lowest two tertiles (<122 ng/ml) had a decreased cancer incidence, whereas those in the highest tertile even showed an elevated incidence (13, 19). The exciting outcome of the NPC trial entailed several successor studies. Below, we will discuss observations that are suitable for emphasizing both promises and constraints in regard to the use of Se for prevention of prostate cancer and CRC in some detail.
Se in prevention of prostate cancer
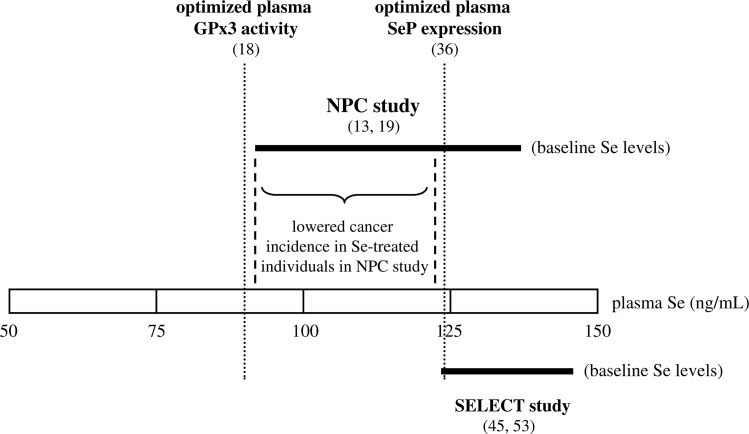
The most severe setback for chemopreventive use of Se resulted from the negative outcome of the U.S. Selenium and Vitamin E Cancer Prevention Trial (SELECT) (53). SELECT intended to test whether daily administration of l-selenomethionine (200 μg), vitamin E (400 IU), or a combination of the two antioxidants protects against prostate cancer. Neither selenomethionine nor vitamin E prevented prostate cancer in healthy men at the age ≥50 years. Moreover, the trial was terminated early, as Se alone increased (nonsignificantly) the risk for Type 2 diabetes and, contrary to expectations, vitamin E alone increased (nonsignificantly) the risk for prostate cancer (53). A follow-up of SELECT revealed a significantly increased risk for participants in the vitamin E group to develop prostate cancer, while confirming that the increased diabetes risk for individuals in the Se group was not significant (46). Limitations in the design of SELECT that may have contributed to its failure and possible strategies to overcome them have been discussed elsewhere (28, 45, 74, 79). With respect to Se, those comments summarized that the most obvious critical factors are the choice of the dietary Se compound and the baseline Se status of trial participants. The chemical nature of the Se compound used for supplements is critical both for in vitro cultivated cells and for dietary intake (8). l-selenomethionine might be less efficient for cancer prevention than Se-enriched yeast or selenite. On the other hand, supplementation of elderly beagle dogs with either selenomethionine or Se-enriched yeast did not yield significant differences in biomarkers of prostate homeostasis such as epithelial cell DNA damage, proliferation, and apoptosis between the groups receiving these two Se compounds (91). Compared with the NPC participants, the baseline plasma Se levels in SELECT volunteers were considerably higher, ranging from 122 to 152 ng/ml (mean 136 ng/ml). In Figure 1, baseline plasma Se levels of the NPC and SELECT participants are depicted in relation to the plasma Se levels associated with optimized activity and expression of the selenoproteins GPx3 and SeP.
FIG. 1.
Baseline plasma selenium (Se) levels of Nutritional Prevention of Cancer (NPC) and Selenium and Vitamin E Cancer Prevention Trial (SELECT) participants. Thick horizontal lines indicate the range between lowest and highest baseline plasma Se levels of NPC and SELECT study participants. As depicted by dashed vertical lines, significantly lowered cancer incidence was observed only among the Se-supplemented NPC participants whose baseline Se levels were in the first and second tertile (91–122 ng/ml Se). For comparison, plasma Se levels associated with optimization of plasma selenoproteins are marked by dotted vertical lines. Se-mediated cancer prevention in the NPC study appears to be associated with optimization of plasma selenoprotein P (SeP) levels.
The key importance of the baseline Se status for the efficacy of Se supplementation in prostate cancer prevention is further highlighted by the recently published outcome of the Phase III trial of Se to prevent prostate cancer in men with high-grade prostatic intraepithelial neoplasia (SWOG S9917). Although there was no difference in prostate cancer incidence between the groups of l-selenomethionine (200 μg/day) and placebo-supplemented patients, the prostate cancer risk was (nonsignificantly) decreased in the Se-treated men within the lowest quartile of baseline plasma Se levels (<106 ng/ml) (58). Mirroring these results, a non-linear U-shaped dose–response relationship between Se status/Se intake and the extent of DNA damage in the aging prostate of elderly beagle dogs has been demonstrated (90).
An intervention study in a Se-poor area, the French supplémentation en vitamines et minéraux antioxydants trial, examined the influence of an Se-containing antioxidant combination (Se-yeast, vitamin C, α-tocopherol, β-carotene, and zinc) on various health parameters of middle-aged volunteers. Antioxidant supplementation for 8 years significantly lowered the incidence of prostate cancer only in men with normal baseline prostate-specific antigen (PSA) levels (PSA<3 μg/L), whereas antioxidant-supplemented men with already elevated PSA levels at the beginning of the study (PSA≥3 μg/L) had even a higher risk to develop prostate cancer than men from the placebo group (61).
A recent meta-analysis of randomized controlled trials, case–control studies and prospective cohort studies included a total of 13254 participants and 5007 cases of prostate cancer (37). Lower prostate cancer risk was associated with plasma/serum Se levels up to 170 ng/ml and with toenail Se levels between 0.85 and 0.94 μg/g (37).
Se in prevention of CRC
The Wheat Bran Fiber Trial, the Polyp Prevention Trial, and the Polyp Prevention Study tested effects of various nutritional interventions in patients with a history of colorectal adenoma, a precursor of CRC. A pooled analysis of data from the three studies corroborated that variations in Se status may affect CRC risk: adenoma recurrences were significantly lower (51% vs. 63%) in the quartile with highest plasma Se levels (150 ng/ml) compared with the quartile with lowest Se (113 ng/ml) (41). Additional determinants have been identified by case–control studies. Data from the North Carolina Colon Cancer Study suggest that high folate intake (>354 μg/day) may decrease the risk to develop CRC in individuals with high serum Se levels (>140 ng/ml) (14). A Spanish study reported significantly lower serum Se levels in two groups of patients with large-size colorectal adenomas or CRC compared with healthy control subjects only for the age group ≤60 years, whereas there was no difference among older individuals (22). In participants of an U.S. cancer screening trial, aged 55 to 74 years, a significant inverse association between serum Se levels and the occurrence of advanced colorectal adenomas was observed only among the high-risk group of recent smokers (68). However, another U.S. case–control study did not find any clear association between serum Se levels and the risk of recurrent CRC (89), and a recent meta-analysis of intervention trials came to the conclusion that oral administration of antioxidants including Se was not effective to prevent colorectal neoplasia in the general population (67).
On the other hand, animal studies provided compelling evidence that Se protects against experimentally-induced carcinogenesis in the colon. The formation of dimethylhydrazine-induced aberrant crypts was significantly increased in rats fed a Se-deficient diet compared with rats fed selenite-supplemented diets; both Se-adequate (0.1 mg Se/kg) and Se-supranutritional (2 mg Se/kg) diets were capable of suppressing preneoplastic lesions (16). The efficacy of dietary Se supplementation might be further enhanced through the use of Se-enriched plant- or animal-derived foods, probably due to better Se bioavailability and/or different metabolic conversion. Selenized proteins isolated from the milk of cows fed a Se-supplemented diet and selenized broccoli protected the large intestine of rodents against chemically-induced carcinogenesis (23, 34). Se-enrichment of broccoli and other cruciferous vegetables might be particularly suitable for cancer prevention, as those plants contain high amounts of glucosinolates that are capable of inducing Nrf2-mediated production of cellular antioxidant enzymes (6). Transgenic mice with a mutated form of the tRNASec gene, resulting in diminished biosynthesis of several selenoproteins, are more susceptible than wild-type mice to azoxymethane-induced aberrant crypt formation. However, this was partly overcome through administration of selenite at a supranutritional dose (2 mg Se/kg diet), indicating that both selenoproteins and constituents of the non-selenoprotein pool of Se metabolites may fulfill anti-carcinogenic functions in the intestine (39).
Advances in Delineating the Role of Selenoproteins in the Intestine
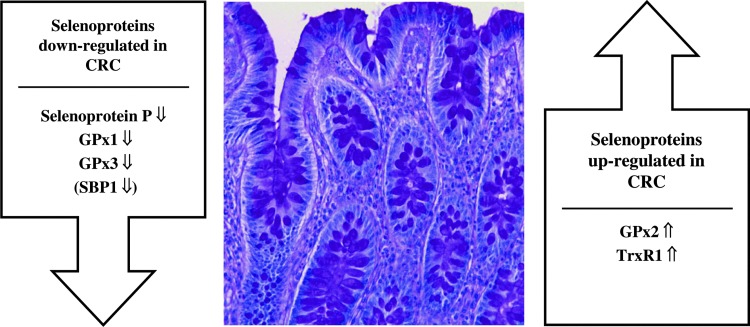
In the following, we summarize advances in understanding the altered expression pattern of selenoproteins in CRC tissue. A summary of the selenoproteins with increased or decreased expression levels in tumorous colonic tissue is given in Figure 2. For more detailed presentations regarding the role of individual selenoproteins in carcinogenesis and prevention of carcinogenesis, we refer to a number of comprehensive reviews (7, 29, 59, 74, 94).
FIG. 2.
Altered expression pattern of selenoproteins in colorectal cancer (CRC). Selenoproteins showing decreased or increased expression in tumorous colonic tissue compared with the corresponding non-neoplastic colonic mucosa are arranged left and right, respectively, of a histological section of healthy human colon that has been stained with hematoxylin and eosin (micrograph, courtesy Dr. H.J. Bidmon). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.
Glutathione peroxidases
All of four major GPx (GPx1–4) are expressed in the intestine. As GPx isoenzymes represent major constituents of the cellular defense system against oxidative stress, their potential role in colorectal carcinogenesis has been addressed (7). Aberrant GPx expression has been reported in sporadic and ulcerative colitis-associated CRC: compared with non-neoplastic tissue, GPx1 and GPx3 were decreased, whereas GPx2 was increased in tumorous colonic tissue (65). GPx2 expression is also retained under Se-deficient conditions, ranking it highest in the selenoprotein hierarchy (5). In colon and rectum, GPx2 is mainly localized at the crypt ground (25). GPx2 knockout mice have been shown to experience an increased apoptosis rate in the crypt ground; however, the loss of GPx2 was partially compensated by upregulation of GPx1 (24). Mice carrying a double knockout (GPx1−/− and GPx2−/−) were highly susceptible to bacteria-induced intestinal inflammation and cancer (12).
GPx3 has been described to be attached to basement membranes of epithelial cells in the gastrointestinal tract (10), where it supposedly protects against hydroperoxide-induced damage. GPx4 is localized in the cytoplasm and nuclei of epithelial cells of the small and large intestine (81). GPx4 might be particularly interesting in regard to Se-dependent chemoprevention, given its nuclear localization and its broad and unique substrate spectrum, including lipid and thymine hydroperoxides. As recently summarized by Méplan and Hesketh (59), there are inconsistent data from studies in different human populations, suggesting a potential influence of a gene variant (rs713041) located in the 3-UTR of GPx4 on CRC risk. Various single nucleotide polymorphisms (SNPs) of the human GPx genes have been identified, but none has been proven to affect the CRC risk, though two-loci interactions were observed for the GPx4 gene with the genes of thioredoxin reductase 2 (TXNRD2) and selenoprotein P (SEPP1) (60).
Selenoprotein P
SeP is primarily produced and secreted by the liver, even though abundant SeP gene expression has been demonstrated in other tissues, including the intestine (9, 84). A significant decline in SeP mRNA levels has been detected in CRC tissue in comparison with the corresponding normal mucosa (1). Expression of SeP substantially decreases during progression of CRC that has originated from ulcerative colitis (65). Association studies have identified four SNPs in the human SeP gene (SEPP1) that are either linked to colorectal adenoma (rs3797310, rs2972994, and rs12055266) or CRC risk (rs7579) (60, 69). The rs7579 variant of the SEPP1 gene has consequences for the function of SeP as a plasma Se transport protein, affecting the pattern of SeP isoforms in plasma and the activities of selenoproteins in lymphocytes (60). Apart from such effects on whole body Se distribution, SEPP1 gene variants and/or downregulated SeP biosynthesis in CRC tissue might result in impaired extracellular antioxidant protection within intestinal epithelia, given the additional enzymatic function of SeP as an (extracellular) hydroperoxide-reducing enzyme (2, 76).
Recently, we have investigated the regulation of intestinal SeP biosynthesis (82), using Caco-2 intestinal epithelial cells as in vitro model. SeP gene expression and secretion were strongly upregulated in the course of enterocytic differentiation of Caco-2 cells through increased binding of the transcription factor hepatocyte nuclear factor-4α to an upstream promoter element (82). Conversely, it is tempting to speculate that SeP might affect the differentiation of enterocytes, as it has been shown for adipocytes. Knockdown of SeP inhibited adipogenic differentiation of 3T3-L1 cells in vitro, probably by concomitant upregulation of proinflammatory cytokines (93). We observed nitric oxide synthase 2-mediated downregulation of SeP biosynthesis upon treatment of Caco-2 cells with proinflammatory cytokines in the colon of mice after induction of chronic experimental colitis (82). It still needs to be figured out whether lowered expression of SeP is merely coincidental or may contribute to the pathogenesis of (ulcerative colitis-related) CRC.
Thioredoxin reductases
Conflicting results have been obtained regarding the levels of TrxR and its substrate Trx in tumorous versus normal colon tissue. Increased expression of TrxR1 and Trx has been reported for a number of cancers including CRC (52), whereas another study did not observe differences in TrxR1 and Trx expression between tumorous and corresponding normal colon tissue (65). Supporting the former study, TrxR1 and GPx1 were reciprocally regulated in liver tumors of mice and in a human colon cancer cell line (26). An association between a variant (rs35009941) of the human gene for TrxR1 (TXNRD1) and advanced colorectal adenoma has been identified (59). A pro-carcinogenic role of the TrxR/Trx system is supported by observations of an increased sensitivity of CRC-derived cell lines toward methylseleninic acid-induced cytotoxicity after knockdown of TrxR (32). Knowledge on modulation of intestinal TrxR by Se intake is still incomplete. Dietary Se supplementation has been reported to increase TrxR activity in ileum and colon of mice (48). Contrarily, rectal TrxR mRNA levels in humans with adequate Se status did not respond to supranutritional Se supply (33).
15-kDa selenoprotein
15-kDa selenoprotein (Sep15) resides in the lumen of the endoplasmic reticulum (ER) in a complex together with uridine diphosphate-glucose:glycoprotein glucosyltransferase, suggesting a role in the quality control of folding of N-glycosylated proteins (47). Expression of Sep15 has been shown to be contrarily regulated by adaptive and acute ER stress (50). Inability to cope with ER stress may trigger intestinal inflammation (42). Two SNPs in the gene encoding Sep15 (rs5845 and rs5859) have been shown to modulate the risk of rectal cancer in a Korean population, though significant associations were only seen for the male gender (87). Knockdown of Sep15 resulted in decreased anchorage-independent growth of CT26 colon cancer cells in vitro and lowered their ability to form tumors and to metastasize when injected into mice (40). Though these observations suggest a pro-carcinogenic role of Sep15 in the intestine, further studies are required to address this question more directly and to uncover the precise function of Sep15 within the ER, for example, through generation of Sep15 knockout mice.
Se-binding protein 1
In contrast to the above-mentioned selenoproteins, Se-binding protein 1 (SBP1) does not contain co-translationally incorporated selenocysteine, binding Se in a mode that remains to be clarified. Its function is not known, but it might be involved in intra-Golgi protein transport (71). SBP1 physically interacts with GPx1 in colonic epithelial cells, and expression of these two Se-containing proteins is regulated reciprocally (21). Expression of SBP1 is frequently downregulated in colorectal carcinoma tissue, and low SBP1 expression levels are correlated with poorer patient survival rates, as shown in two independent studies (43, 51). Similar to the selenoproteins SeP and GPx4 (82, 81), SBP1 levels increase during enterocytic cellular differentiation. Maximal SBP1 expression is observed in terminally differentiated cells of the intestinal epithelium (51). Knockdown of SBP1 in Caco-2 and SW620 cells was paralleled by a downregulation of the epithelial differentiation marker carcinoembryonic antigen (51). So far, it has not been studied whether SBP1 protein levels in the intestine respond to Se supplementation, which would be a prerequisite for being a candidate mediator of the chemopreventive effects of ingested Se.
Attenuation of Akt-Mediated Signaling by Se: Tradeoff Between Cancer Prevention and Increased Diabetes Risk?
Another secondary analysis of the landmark NPC trial revealed that Se-supplemented individuals were more likely to develop Type 2 diabetes mellitus (T2DM) than those assigned to placebo (86). However, only the NPC participants whose baseline plasma Se levels exceeded 122 ng/ml (top tertile) experienced a significantly increased risk of developing T2DM when they were supplemented with Se-enriched yeast (86). Among the selenomethionine-supplemented group of volunteers of the SELECT study, the risk for T2DM was nonsignificantly increased (46, 53). The risk potential of supranutritional Se intake as a diabetogenic factor is subject of ongoing discussion (85). Results of cross-sectional studies are inconsistent so far, as a majority though not all of the studies provided evidence for an association between high plasma Se levels with hyperglycemia and dyslipidemia (20, 63, 85). Recently, plasma adiponectin, a surrogate marker of insulin resistance and T2DM, was measured in elderly (60–74 years) participants of the UK Prevention of Cancer by Intervention with Selenium (PRECISE) trial. There was an inverse cross-sectional association between baseline plasma Se and adiponectin levels. However, Se supplementation for 6 months with 100, 200, or 300 μg Se/day in the form of Se-enriched yeast did not affect the adiponectin level in plasma, arguing against Se-induced development of insulin resistance in this population (75).
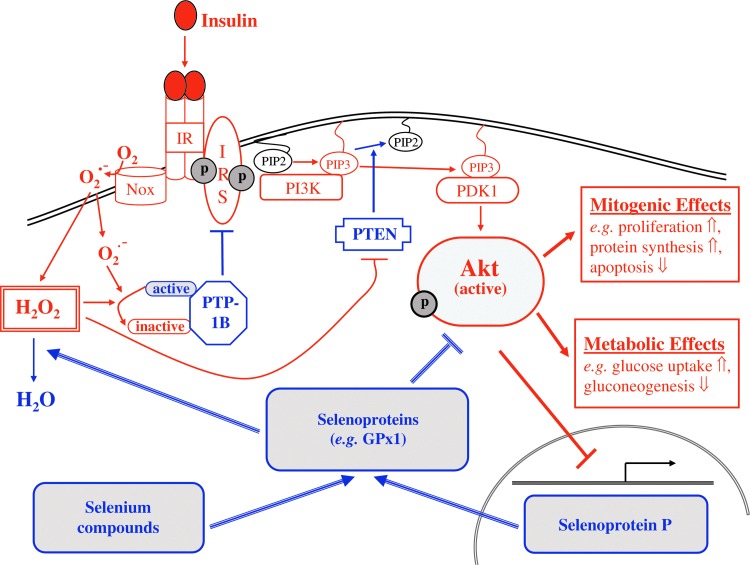
Abundant expression of antioxidant selenoenzymes such as GPx1 may interfere with insulin-regulated cellular pathways through reduction of hydrogen peroxide (H2O2) (85) that otherwise enhances the early insulin signaling cascade and stimulates insulin-induced glucose uptake by transient inhibition of counter-regulatory phosphatases (15, 55). Figure 3 schematically outlines the current concept on Se-mediated attenuation of insulin signaling. Consistent with this hypothesis, patients with genetically impaired biosynthesis of selenoproteins exhibit enhanced systemic and cellular insulin sensitivity (78). Both low-molecular-weight Se compounds (e.g., selenite and methylseleninic acid) and SeP are capable of attenuating insulin-induced phosphorylation (activation) of protein kinase B (Akt) in hepatocytes and in myocytes (62, 70), and conversely, insulin downregulates hepatic biosynthesis of SeP (62, 83), thus providing a potential feedback loop.
FIG. 3.
Scheme depicting attenuation of insulin-induced phosphorylation (activation) of protein kinase B (Akt) by selenium. The components of the insulin signaling cascade, causing activation of Akt and its subsequent metabolic and mitogenic effects, are illustrated in red. Both low-molecular-weight Se compounds and SeP stimulate expression of antioxidant selenoenzymes that reduce hydrogen peroxide, thus counteracting the insulin-induced transient inhibition of protein tyrosine phosphatases (e.g., protein tyrosine phosphatase 1B) and dual-specificity protein phosphatase and tensin homolog (illustrated in blue). Please see text for details. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.
On the one hand, Akt integrates actions of insulin on energy metabolism, and its impaired activation may result in insulin resistance, a key feature of T2DM (77). On the other hand, Akt mediates mitogenic effects of insulin and growth factors (e.g., insulin-like growth factor 1, epidermal growth factor, and platelet-derived growth factor) on angiogenesis, protein translation, cellular proliferation, and survival (30). Dys-regulation of those pathways is implicated in carcinogenesis. In many types of cancer, Akt is hyper-activated and components of Akt-related signaling pathways are mutated (30). Interestingly, a synthetic Akt inhibitor has been demonstrated to block tumor growth in nude mice, while causing transient insulin resistance and reversible hyperglycemia and hyperinsulinemia as side effects (11). It is tempting to speculate that a mild impairment of insulin sensitivity through dietary Se compounds and antioxidant selenoproteins might be the tradeoff for their cancer-preventive action, with Akt being in the focal point. However, such a relation would demand some caution regarding the use of Se-containing nutritional supplements by patients suffering from T2DM.
Concluding Remarks
The landmark studies by Schrauzer et al. (80) and Clark et al. (13) raised the hope that adequate nutritional Se supply might help to decrease cancer incidence and mortality. However, pooled data from present intervention and cross-sectional studies in humans are inconsistent, arguing against the use of Se for cancer prevention in the general population (17, 67). This might be particularly true with respect to Se-replete populations such as in the United States. In our opinion, four points should be considered for better success in the future: (i) the strikingly high number of genetic and metabolic determinants that may affect the individual outcome of dietary Se supplementation, (ii) differences in metabolization and, therefore, biological impact of the different Se compounds, (iii) timing of intervention with regard to the stage of carcinogenesis (effective in prevention) and age of the subject (<60 years), and (iv) potential adverse health effects of supranutritional Se intake.
Abbreviations Used
- Akt
protein kinase B
- CRC
colorectal cancer
- ER
endoplasmic reticulum
- GPx
glutathione peroxidase
- NPC
Nutritional Prevention of Cancer
- PSA
prostate-specific antigen
- SBP1
selenium-binding protein 1
- Se
selenium
- SELECT
Selenium and Vitamin E Cancer Prevention Trial
- SeP
selenoprotein P
- Sep15
15-kDa selenoprotein
- SNP
single nucleotide polymorphism
- SU.VI.MAX
supplémentation en vitamines et minéraux antioxydants
- T2DM
type 2 diabetes mellitus
- Trx
thioredoxin
- TrxR
thioredoxin reductase
Acknowledgments
This work was supported by grants from Deutsche Forschungsgemeinschaft (DFG; Bonn, Germany) to H. Steinbrenner (STE 1782/2-2) and B. Speckmann (SP 1333/1-1). H. Sies is a Fellow of the National Foundation for Cancer Research (NFCR; Bethesda, MD).
References
- 1.Al-Taie OH. Uceyler N. Eubner U. Jakob F. Mörk H. Scheurlen M. Brigelius-Flohé R. Schöttker K. Abel J. Thalheimer A. Katzenberger T. Illert B. Melcher R. Köhrle J. Expression profiling and genetic alterations of the selenoproteins GI-GPx and SePP in colorectal carcinogenesis. Nutr Cancer. 2004;48:6–14. doi: 10.1207/s15327914nc4801_2. [DOI] [PubMed] [Google Scholar]
- 2.Arteel GE. Mostert V. Oubrahim H. Briviba K. Abel J. Sies H. Protection by selenoprotein P in human plasma against peroxynitrite-mediated oxidation and nitration. Biol Chem. 1998;379:1201–1205. [PubMed] [Google Scholar]
- 3.Bailey RL. Gahche JJ. Lentino CV. Dwyer JT. Engel JS. Thomas PR. Betz JM. Sempos CT. Picciano MF. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141:261–266. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blot WJ. Li JY. Taylor PR. Guo W. Dawsey S. Wang GQ. Yang CS. Zheng SF. Gail M. Li GY. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 5.Brigelius-Flohé R. Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med. 1999;27:951–965. doi: 10.1016/s0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- 6.Brigelius-Flohé R. Banning A. Part of the series: from dietary antioxidants to regulators in cellular signaling and gene regulation. Sulforaphane and selenium, partners in adaptive response and prevention of cancer. Free Radic Res. 2006;40:775–787. doi: 10.1080/10715760600722643. [DOI] [PubMed] [Google Scholar]
- 7.Brigelius-Flohé R. Kipp A. Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta. 2009;1790:1555–1568. doi: 10.1016/j.bbagen.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Brigelius-Flohé R. Lötzer K. Maurer S. Schultz M. Leist M. Utilization of selenium from different chemical entities for selenoprotein biosynthesis by mammalian cell lines. Biofactors. 5:125–131. 1995–1996. [PubMed] [Google Scholar]
- 9.Burk RF. Hill KE. Selenoprotein P-expression, functions, and roles in mammals. Biochim Biophys Acta. 2009;1790:1441–1447. doi: 10.1016/j.bbagen.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burk RF. Olson GE. Winfrey VP. Hill KE. Yin D. Glutathione peroxidase-3 produced by the kidney binds to a population of basement membranes in the gastrointestinal tract and in other tissues. Am J Physiol Gastrointest Liver Physiol. 2011;301:G32–G38. doi: 10.1152/ajpgi.00064.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherrin C. Haskell K. Howell B. Jones R. Leander K. Robinson R. Watkins A. Bilodeau M. Hoffman J. Sanderson P. Hartman G. Mahan E. Prueksaritanont T. Jiang G. She QB. Rosen N. Sepp-Lorenzino L. Defeo-Jones D. Huber HE. An allosteric Akt inhibitor effectively blocks Akt signaling and tumor growth with only transient effects on glucose and insulin levels in vivo. Cancer Biol Ther. 2010;9:493–503. doi: 10.4161/cbt.9.7.11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu FF. Esworthy RS. Chu PG. Longmate JA. Huycke MM. Wilczynski S. Doroshow JH. Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer Res. 2004;64:962–968. doi: 10.1158/0008-5472.can-03-2272. [DOI] [PubMed] [Google Scholar]
- 13.Clark LC. Combs GF., Jr. Turnbull BW. Slate EH. Chalker DK. Chow J. Davis LS. Glover RA. Graham GF. Gross EG. Krongrad A. Lesher JL., Jr. Park HK. Sanders BB., Jr. Smith CL. Taylor JR. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 14.Connelly-Frost A. Poole C. Satia JA. Kupper LL. Millikan RC. Sandler RS. Selenium, folate, and colon cancer. Nutr Cancer. 2009;61:165–178. doi: 10.1080/01635580802404188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czech MP. Lawrence JC., Jr. Lynn WS. Evidence for the involvement of sulfhydryl oxidation in the regulation of fat cell hexose transport by insulin. Proc Natl Acad Sci U S A. 1974;71:4173–4177. doi: 10.1073/pnas.71.10.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis CD. Uthus EO. Dietary selenite and azadeoxycytidine treatments affect dimethylhydrazine-induced aberrant crypt formation in rat colon and DNA methylation in HT-29 cells. J Nutr. 2002;132:292–297. doi: 10.1093/jn/132.2.292. [DOI] [PubMed] [Google Scholar]
- 17.Dennert G. Zwahlen M. Brinkman M. Vinceti M. Zeegers MP. Horneber M. Selenium for preventing cancer. Cochrane Database Syst Rev. 2011;5:CD005195. doi: 10.1002/14651858.CD005195.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffield AJ. Thomson CD. Hill KE. Williams S. An estimation of selenium requirements for New Zealanders. Am J Clin Nutr. 1999;70:896–903. doi: 10.1093/ajcn/70.5.896. [DOI] [PubMed] [Google Scholar]
- 19.Duffield-Lillico AJ. Reid ME. Turnbull BW. Combs GF., Jr. Slate EH. Fischbach LA. Marshall JR. Clark LC. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630–639. [PubMed] [Google Scholar]
- 20.Fairweather-Tait SJ. Bao Y. Broadley MR. Collings R. Ford D. Hesketh JE. Hurst R. Selenium in human health and disease. Antioxid Redox Signal. 2011;14:1337–1383. doi: 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- 21.Fang W. Goldberg ML. Pohl NM. Bi X. Tong C. Xiong B. Koh TJ. Diamond AM. Yang W. Functional and physical interaction between the selenium-binding protein 1 (SBP1) and the glutathione peroxidase 1 selenoprotein. Carcinogenesis. 2010;31:1360–1366. doi: 10.1093/carcin/bgq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández-Bañares F. Cabré E. Esteve M. Mingorance MD. Abad-Lacruz A. Lachica M. Gil A. Gassull MA. Serum selenium and risk of large size colorectal adenomas in a geographical area with a low selenium status. Am J Gastroenterol. 2002;97:2103–2108. doi: 10.1111/j.1572-0241.2002.05930.x. [DOI] [PubMed] [Google Scholar]
- 23.Finley JW. Davis CD. Feng Y. Selenium from high selenium broccoli protects rats from colon cancer. J Nutr. 2000;130:2384–2389. doi: 10.1093/jn/130.9.2384. [DOI] [PubMed] [Google Scholar]
- 24.Florian S. Krehl S. Loewinger M. Kipp A. Banning A. Esworthy S. Chu FF. Brigelius-Flohé R. Loss of GPx2 increases apoptosis, mitosis, and GPx1 expression in the intestine of mice. Free Radic Biol Med. 2010;49:1694–1702. doi: 10.1016/j.freeradbiomed.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Florian S. Wingler K. Schmehl K. Jacobasch G. Kreuzer OJ. Meyerhof W. Brigelius-Flohé R. Cellular and subcellular localization of gastrointestinal glutathione peroxidase in normal and malignant human intestinal tissue. Free Radic Res. 2001;35:655–663. doi: 10.1080/10715760100301181. [DOI] [PubMed] [Google Scholar]
- 26.Gladyshev VN. Factor VM. Housseau F. Hatfield DL. Contrasting patterns of regulation of the antioxidant selenoproteins, thioredoxin reductase, and glutathione peroxidase, in cancer cells. Biochem Biophys Res Commun. 1998;251:488–493. doi: 10.1006/bbrc.1998.9495. [DOI] [PubMed] [Google Scholar]
- 27.Gromadzińska J. Reszka E. Bruzelius K. Wasowicz W. Akesson B. Selenium and cancer: biomarkers of selenium status and molecular action of selenium supplements. Eur J Nutr. 2008;47:29–50. doi: 10.1007/s00394-008-2005-z. [DOI] [PubMed] [Google Scholar]
- 28.Hatfield DL. Gladyshev VN. The outcome of Selenium and Vitamin E Cancer Prevention Trial (SELECT) reveals the need for better understanding of selenium biology. Mol Interv. 2009;9:18–21. doi: 10.1124/mi.9.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatfield DL. Yoo MH. Carlson BA. Gladyshev VN. Selenoproteins that function in cancer prevention and promotion. Biochim Biophys Acta. 2009;1790:1541–1545. doi: 10.1016/j.bbagen.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hers I. Vincent EE. Tavaré JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515–1527. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Hill KE. Chittum HS. Lyons PR. Boeglin ME. Burk RF. Effect of selenium on selenoprotein P expression in cultured liver cells. Biochim Biophys Acta. 1996;1313:29–34. doi: 10.1016/0167-4889(96)00047-x. [DOI] [PubMed] [Google Scholar]
- 32.Honeggar M. Beck R. Moos PJ. Thioredoxin reductase 1 ablation sensitizes colon cancer cells to methylseleninate-mediated cytotoxicity. Toxicol Appl Pharmacol. 2009;241:348–355. doi: 10.1016/j.taap.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Y. McIntosh GH. Le Leu RK. Upton JM. Woodman RJ. Young GP. The influence of selenium-enriched milk proteins and selenium yeast on plasma selenium levels and rectal selenoprotein gene expression in human subjects. Br J Nutr. 2011;106:572–582. doi: 10.1017/S0007114511000420. [DOI] [PubMed] [Google Scholar]
- 34.Hu Y. McIntosh GH. Le Leu RK. Woodman R. Young GP. Suppression of colorectal oncogenesis by selenium-enriched milk proteins: apoptosis and K-ras mutations. Cancer Res. 2008;68:4936–4944. doi: 10.1158/0008-5472.CAN-07-6042. [DOI] [PubMed] [Google Scholar]
- 35.Huang Z. Rose AH. Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2012;16:705–743. doi: 10.1089/ars.2011.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurst R. Armah CN. Dainty JR. Hart DJ. Teucher B. Goldson AJ. Broadley MR. Motley AK. Fairweather-Tait SJ. Establishing optimal selenium status: results of a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2010;91:923–931. doi: 10.3945/ajcn.2009.28169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurst R. Hooper L. Norat T. Lau R. Aune D. Greenwood DC. Vieira R. Collings R. Harvey LJ. Sterne JA. Beynon R. Savović J. Fairweather-Tait SJ. Selenium and prostate cancer: systematic review and meta-analysis. Am J Clin Nutr. 2012;96:111–122. doi: 10.3945/ajcn.111.033373. [DOI] [PubMed] [Google Scholar]
- 38.Ip C. Birringer M. Block E. Kotrebai M. Tyson JF. Uden PC. Lisk DJ. Chemical speciation influences comparative activity of selenium-enriched garlic and yeast in mammary cancer prevention. J Agric Food Chem. 2000;48:2062–2070. doi: 10.1021/jf000051f. [DOI] [PubMed] [Google Scholar]
- 39.Irons R. Carlson BA. Hatfield DL. Davis CD. Both selenoproteins and low molecular weight selenocompounds reduce colon cancer risk in mice with genetically impaired selenoprotein expression. J Nutr. 2006;136:1311–1317. doi: 10.1093/jn/136.5.1311. [DOI] [PubMed] [Google Scholar]
- 40.Irons R. Tsuji PA. Carlson BA. Ouyang P. Yoo MH. Xu XM. Hatfield DL. Gladyshev VN. Davis CD. Deficiency in the 15-kDa selenoprotein inhibits tumorigenicity and metastasis of colon cancer cells. Cancer Prev Res (Phila) 2010;3:630–639. doi: 10.1158/1940-6207.CAPR-10-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobs ET. Jiang R. Alberts DS. Greenberg ER. Gunter EW. Karagas MR. Lanza E. Ratnasinghe L. Reid ME. Schatzkin A. Smith-Warner SA. Wallace K. Martínez ME. Selenium and colorectal adenoma: results of a pooled analysis. J Natl Cancer Inst. 2004;96:1669–1675. doi: 10.1093/jnci/djh310. [DOI] [PubMed] [Google Scholar]
- 42.Kaser A. Blumberg RS. Endoplasmic reticulum stress in the intestinal epithelium and inflammatory bowel disease. Semin Immunol. 2009;21:156–163. doi: 10.1016/j.smim.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim H. Kang HJ. You KT. Kim SH. Lee KY. Kim TI. Kim C. Song SY. Kim HJ. Lee C. Suppression of human selenium-binding protein 1 is a late event in colorectal carcinogenesis and is associated with poor survival. Proteomics. 2006;6:3466–3476. doi: 10.1002/pmic.200500629. [DOI] [PubMed] [Google Scholar]
- 44.Kipp A. Banning A. van Schothorst EM. Méplan C. Schomburg L. Evelo C. Coort S. Gaj S. Keijer J. Hesketh J. Brigelius-Flohé R. Four selenoproteins, protein biosynthesis, and Wnt signalling are particularly sensitive to limited selenium intake in mouse colon. Mol Nutr Food Res. 2009;53:1561–1572. doi: 10.1002/mnfr.200900105. [DOI] [PubMed] [Google Scholar]
- 45.Klein EA. Thompson IM. Chemoprevention of prostate cancer: an updated view. World J Urol. 2012;30:189–194. doi: 10.1007/s00345-011-0822-9. [DOI] [PubMed] [Google Scholar]
- 46.Klein EA. Thompson IM., Jr. Tangen CM. Crowley JJ. Lucia MS. Goodman PJ. Minasian LM. Ford LG. Parnes HL. Gaziano JM. Karp DD. Lieber MM. Walther PJ. Klotz L. Parsons JK. Chin JL. Darke AK. Lippman SM. Goodman GE. Meyskens FL., Jr. Baker LH. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korotkov KV. Kumaraswamy E. Zhou Y. Hatfield DL. Gladyshev VN. Association between the 15-kDa selenoprotein and UDP-glucose:glycoprotein glucosyltransferase in the endoplasmic reticulum of mammalian cells. J Biol Chem. 2001;276:15330–15336. doi: 10.1074/jbc.M009861200. [DOI] [PubMed] [Google Scholar]
- 48.Krehl S. Loewinger M. Florian S. Kipp AP. Banning A. Wessjohann LA. Brauer MN. Iori R. Esworthy RS. Chu FF. Brigelius-Flohé R. Glutathione peroxidase-2 and selenium decreased inflammation and tumors in a mouse model of inflammation-associated carcinogenesis whereas sulforaphane effects differed with selenium supply. Carcinogenesis. 2012;33:620–628. doi: 10.1093/carcin/bgr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kryukov GV. Castellano S. Novoselov SV. Lobanov AV. Zehtab O. Guigó R. Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 50.Labunskyy VM. Yoo MH. Hatfield DL. Gladyshev VN. Sep15, a thioredoxin-like selenoprotein, is involved in the unfolded protein response and differentially regulated by adaptive and acute ER stresses. Biochemistry. 2009;48:8458–8465. doi: 10.1021/bi900717p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li T. Yang W. Li M. Byun DS. Tong C. Nasser S. Zhuang M. Arango D. Mariadason JM. Augenlicht LH. Expression of selenium-binding protein 1 characterizes intestinal cell maturation and predicts survival for patients with colorectal cancer. Mol Nutr Food Res. 2008;52:1289–1299. doi: 10.1002/mnfr.200700331. [DOI] [PubMed] [Google Scholar]
- 52.Lincoln DT. Ali Emadi EM. Tonissen KF. Clarke FM. The thioredoxin-thioredoxin reductase system: over-expression in human cancer. Anticancer Res. 2003;23:2425–2433. [PubMed] [Google Scholar]
- 53.Lippman SM. Klein EA. Goodman PJ. Lucia MS. Thompson IM. Ford LG. Parnes HL. Minasian LM. Gaziano JM. Hartline JA. Parsons JK. Bearden JD., 3rd. Crawford ED. Goodman GE. Claudio J. Winquist E. Cook ED. Karp DD. Walther P. Lieber MM. Kristal AR. Darke AK. Arnold KB. Ganz PA. Santella RM. Albanes D. Taylor PR. Probstfield JL. Jagpal TJ. Crowley JJ. Meyskens FL., Jr. Baker LH. Coltman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu J. Holmgren A. Selenoproteins. J Biol Chem. 2009;284:723–727. doi: 10.1074/jbc.R800045200. [DOI] [PubMed] [Google Scholar]
- 55.Mahadev K. Zilbering A. Zhu L. Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem. 2001;276:21938–21942. doi: 10.1074/jbc.C100109200. [DOI] [PubMed] [Google Scholar]
- 56.Marcocci C. Kahaly GJ. Krassas GE. Bartalena L. Prummel M. Stahl M. Altea MA. Nardi M. Pitz S. Boboridis K. Sivelli P. von Arx G. Mourits MP. Baldeschi L. Bencivelli W. Wiersinga W and the European Group on Graves' Orbitopathy. Selenium and the course of mild Graves' orbitopathy. N Engl J Med. 2011;364:1920–1931. doi: 10.1056/NEJMoa1012985. [DOI] [PubMed] [Google Scholar]
- 57.Mark SD. Qiao YL. Dawsey SM. Wu YP. Katki H. Gunter EW. Fraumeni JF., Jr. Blot WJ. Dong ZW. Taylor PR. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst. 2000;92:1753–1763. doi: 10.1093/jnci/92.21.1753. [DOI] [PubMed] [Google Scholar]
- 58.Marshall JR. Tangen CM. Sakr WA. Wood DP., Jr. Berry DL. Klein EA. Lippman SM. Parnes HL. Alberts DS. Jarrard DF. Lee WR. Gaziano JM. Crawford ED. Ely B. Ray M. Davis W. Minasian LM. Thompson IM., Jr Phase III trial of selenium to prevent prostate cancer in men with high-grade prostatic intraepithelial neoplasia: SWOG S9917. Cancer Prev Res (Phila) 2011;4:1761–1769. doi: 10.1158/1940-6207.CAPR-10-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Méplan C. Hesketh J. The influence of selenium and selenoprotein gene variants on colorectal cancer risk. Mutagenesis. 2012;27:177–186. doi: 10.1093/mutage/ger058. [DOI] [PubMed] [Google Scholar]
- 60.Méplan C. Hughes DJ. Pardini B. Naccarati A. Soucek P. Vodickova L. Hlavata I. Vrana D. Vodicka P. Hesketh JE. Genetic variants in selenoprotein genes increase risk of colorectal cancer. Carcinogenesis. 2010;31:1074–1079. doi: 10.1093/carcin/bgq076. [DOI] [PubMed] [Google Scholar]
- 61.Meyer F. Galan P. Douville P. Bairati I. Kegle P. Bertrais S. Estaquio C. Hercberg S. Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU.VI.MAX trial. Int J Cancer. 2005;116:182–186. doi: 10.1002/ijc.21058. [DOI] [PubMed] [Google Scholar]
- 62.Misu H. Takamura T. Takayama H. Hayashi H. Matsuzawa-Nagata N. Kurita S. Ishikura K. Ando H. Takeshita Y. Ota T. Sakurai M. Yamashita T. Mizukoshi E. Yamashita T. Honda M. Miyamoto K. Kubota T. Kubota N. Kadowaki T. Kim HJ. Lee IK. Minokoshi Y. Saito Y. Takahashi K. Yamada Y. Takakura N. Kaneko S. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010;12:483–495. doi: 10.1016/j.cmet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 63.Mueller AS. Mueller K. Wolf NM. Pallauf J. Selenium and diabetes: an enigma? Free Radic Res. 2009;43:1029–1059. doi: 10.1080/10715760903196925. [DOI] [PubMed] [Google Scholar]
- 64.Mugesh G. du Mont WW. Sies H. Chemistry of biologically important synthetic organoselenium compounds. Chem Rev. 2001;101:2125–2179. doi: 10.1021/cr000426w. [DOI] [PubMed] [Google Scholar]
- 65.Murawaki Y. Tsuchiya H. Kanbe T. Harada K. Yashima K. Nozaka K. Tanida O. Kohno M. Mukoyama T. Nishimuki E. Kojo H. Matsura T. Takahashi K. Osaki M. Ito H. Yodoi J. Shiota G. Aberrant expression of selenoproteins in the progression of colorectal cancer. Cancer Lett. 2008;259:218–230. doi: 10.1016/j.canlet.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 66.Pagmantidis V. Méplan C. van Schothorst EM. Keijer J. Hesketh JE. Supplementation of healthy volunteers with nutritionally relevant amounts of selenium increases the expression of lymphocyte protein biosynthesis genes. Am J Clin Nutr. 2008;87:181–189. doi: 10.1093/ajcn/87.1.181. [DOI] [PubMed] [Google Scholar]
- 67.Papaioannou D. Cooper KL. Carroll C. Hind D. Squires H. Tappenden P. Logan RF. Antioxidants in the chemoprevention of colorectal cancer and colorectal adenomas in the general population: a systematic review and meta-analysis. Colorectal Dis. 2011;13:1085–1099. doi: 10.1111/j.1463-1318.2010.02289.x. [DOI] [PubMed] [Google Scholar]
- 68.Peters U. Chatterjee N. Church TR. Mayo C. Sturup S. Foster CB. Schatzkin A. Hayes RB. High serum selenium and reduced risk of advanced colorectal adenoma in a colorectal cancer early detection program. Cancer Epidemiol Biomarkers Prev. 2006;15:315–320. doi: 10.1158/1055-9965.EPI-05-0471. [DOI] [PubMed] [Google Scholar]
- 69.Peters U. Chatterjee N. Hayes RB. Schoen RE. Wang Y. Chanock SJ. Foster CB. Variation in the selenoenzyme genes and risk of advanced distal colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2008;17:1144–1154. doi: 10.1158/1055-9965.EPI-07-2947. [DOI] [PubMed] [Google Scholar]
- 70.Pinto A. Speckmann B. Heisler M. Sies H. Steinbrenner H. Delaying of insulin signal transduction in skeletal muscle cells by selenium compounds. J Inorg Biochem. 2011;105:812–820. doi: 10.1016/j.jinorgbio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 71.Porat A. Sagiv Y. Elazar Z. A 56-kDa selenium-binding protein participates in intra-Golgi protein transport. J Biol Chem. 2000;275:14457–14465. doi: 10.1074/jbc.275.19.14457. [DOI] [PubMed] [Google Scholar]
- 72.Qiao YL. Dawsey SM. Kamangar F. Fan JH. Abnet CC. Sun XD. Johnson LL. Gail MH. Dong ZW. Yu B. Mark SD. Taylor PR. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. 2009;101:507–518. doi: 10.1093/jnci/djp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rayman MP. Food-chain selenium and human health: emphasis on intake. Br J Nutr. 2008;100:254–268. doi: 10.1017/S0007114508939830. [DOI] [PubMed] [Google Scholar]
- 74.Rayman MP. Selenoproteins and human health: insights from epidemiological data. Biochim Biophys Acta. 2009;1790:1533–1540. doi: 10.1016/j.bbagen.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 75.Rayman MP. Blundell-Pound G. Pastor-Barriuso R. Guallar E. Steinbrenner H. Stranges S. A randomized trial of selenium supplementation and risk of type-2 diabetes, as assessed by plasma adiponectin. PLoS One. 2012;7:e45269. doi: 10.1371/journal.pone.0045269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saito Y. Hayashi T. Tanaka A. Watanabe Y. Suzuki M. Saito E. Takahashi K. Selenoprotein P in human plasma as an extracellular phospholipid hydroperoxide glutathione peroxidase. Isolation and enzymatic characterization of human selenoprotein p. J Biol Chem. 1999;274:2866–2871. doi: 10.1074/jbc.274.5.2866. [DOI] [PubMed] [Google Scholar]
- 77.Schinner S. Scherbaum WA. Bornstein SR. Barthel A. Molecular mechanisms of insulin resistance. Diabet Med. 2005;22:674–682. doi: 10.1111/j.1464-5491.2005.01566.x. [DOI] [PubMed] [Google Scholar]
- 78.Schoenmakers E. Agostini M. Mitchell C. Schoenmakers N. Papp L. Rajanayagam O. Padidela R. Ceron-Gutierrez L. Doffinger R. Prevosto C. Luan J. Montano S. Lu J. Castanet M. Clemons N. Groeneveld M. Castets P. Karbaschi M. Aitken S. Dixon A. Williams J. Campi I. Blount M. Burton H. Muntoni F. O'Donovan D. Dean A. Warren A. Brierley C. Baguley D. Guicheney P. Fitzgerald R. Coles A. Gaston H. Todd P. Holmgren A. Khanna KK. Cooke M. Semple R. Halsall D. Wareham N. Schwabe J. Grasso L. Beck-Peccoz P. Ogunko A. Dattani M. Gurnell M. Chatterjee K. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J Clin Invest. 2010;120:4220–4235. doi: 10.1172/JCI43653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schrauzer GN. RE: lessons from the selenium and vitamin E cancer prevention trial (SELECT) Crit Rev Biotechnol. 2009;29:81. doi: 10.1080/07388550902926725. [DOI] [PubMed] [Google Scholar]
- 80.Schrauzer GN. White DA. Schneider CJ. Cancer mortality correlation studies-III: statistical associations with dietary selenium intakes. Bioinorg Chem. 1977;7:23–31. doi: 10.1016/s0006-3061(00)80126-x. [DOI] [PubMed] [Google Scholar]
- 81.Speckmann B. Bidmon HJ. Pinto A. Anlauf M. Sies H. Steinbrenner H. Induction of glutathione peroxidase 4 expression during enterocytic cell differentiation. J Biol Chem. 2011;286:10764–10772. doi: 10.1074/jbc.M110.216028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Speckmann B. Pinto A. Winter M. Forster I. Sies H. Steinbrenner H. Proinflammatory cytokines down-regulate intestinal selenoprotein P biosynthesis via NOS2 induction. Free Radic Biol Med. 2010;49:777–785. doi: 10.1016/j.freeradbiomed.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 83.Speckmann B. Walter PL. Alili L. Reinehr R. Sies H. Klotz LO. Steinbrenner H. Selenoprotein P expression is controlled through interaction of the coactivator PGC-1α with FoxO1a and hepatocyte nuclear factor 4α transcription factors. Hepatology. 2008;48:1998–2006. doi: 10.1002/hep.22526. [DOI] [PubMed] [Google Scholar]
- 84.Steinbrenner H. Sies H. Protection against reactive oxygen species by selenoproteins. Biochim Biophys Acta. 2009;1790:1478–1485. doi: 10.1016/j.bbagen.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 85.Steinbrenner H. Speckmann B. Pinto A. Sies H. High selenium intake and increased diabetes risk: experimental evidence for interplay between selenium and carbohydrate metabolism. J Clin Biochem Nutr. 2011;48:40–45. doi: 10.3164/jcbn.11-002FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stranges S. Marshall JR. Natarajan R. Donahue RP. Trevisan M. Combs GF. Cappuccio FP. Ceriello A. Reid ME. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 87.Sutherland A. Kim DH. Relton C. Ahn YO. Hesketh J. Polymorphisms in the selenoprotein S and 15-kDa selenoprotein genes are associated with altered susceptibility to colorectal cancer. Genes Nutr. 2010;5:215–223. doi: 10.1007/s12263-010-0176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taylor PR. Greenwald P. Nutritional interventions in cancer prevention. J Clin Oncol. 2005;23:333–345. doi: 10.1200/JCO.2005.06.190. [DOI] [PubMed] [Google Scholar]
- 89.Wallace K. Byers T. Morris JS. Cole BF. Greenberg ER. Baron JA. Gudino A. Spate V. Karagas MR. Prediagnostic serum selenium concentration and the risk of recurrent colorectal adenoma: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2003;12:464–467. [PubMed] [Google Scholar]
- 90.Waters DJ. Shen S. Glickman LT. Cooley DM. Bostwick DG. Qian J. Combs GF., Jr. Morris JS. Prostate cancer risk and DNA damage: translational significance of selenium supplementation in a canine model. Carcinogenesis. 2005;26:1256–1262. doi: 10.1093/carcin/bgi077. [DOI] [PubMed] [Google Scholar]
- 91.Waters DJ. Shen S. Kengeri SS. Chiang EC. Combs GF., Jr. Morris JS. Bostwick DG. Prostatic response to supranutritional selenium supplementation: comparison of the target tissue potency of selenomethionine vs. selenium-yeast on markers of prostatic homeostasis. Nutrients. 2012;4:1650–1663. doi: 10.3390/nu4111650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu SY. Zhu YJ. Li WG. Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biol Trace Elem Res. 1997;56:117–124. doi: 10.1007/BF02778987. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y. Chen X. Reducing selenoprotein P expression suppresses adipocyte differentiation as a result of increased preadipocyte inflammation. Am J Physiol Endocrinol Metab. 2011;300:E77–E85. doi: 10.1152/ajpendo.00380.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhuo P. Diamond AM. Molecular mechanisms by which selenoproteins affect cancer risk and progression. Biochim Biophys Acta. 2009;1790:1546–1554. doi: 10.1016/j.bbagen.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]