Abstract
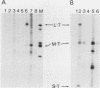
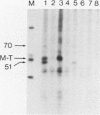
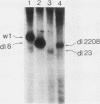
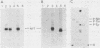
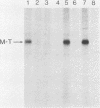
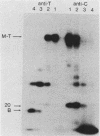
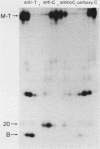
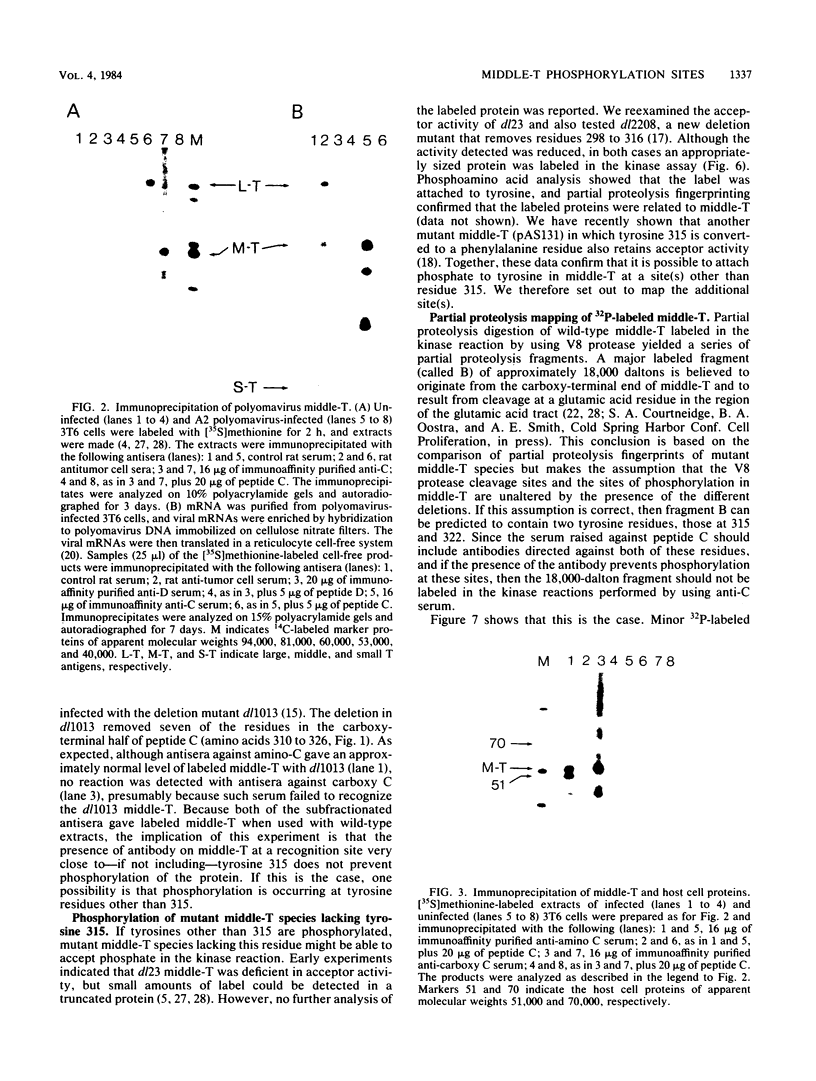
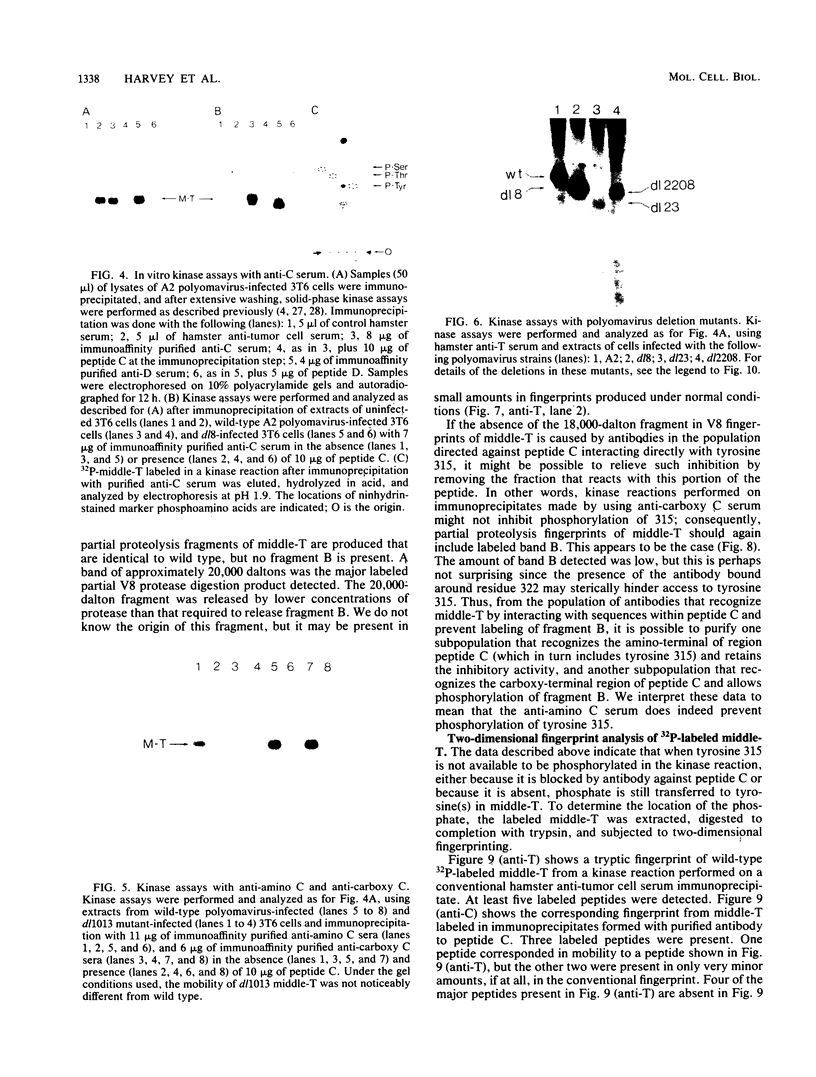
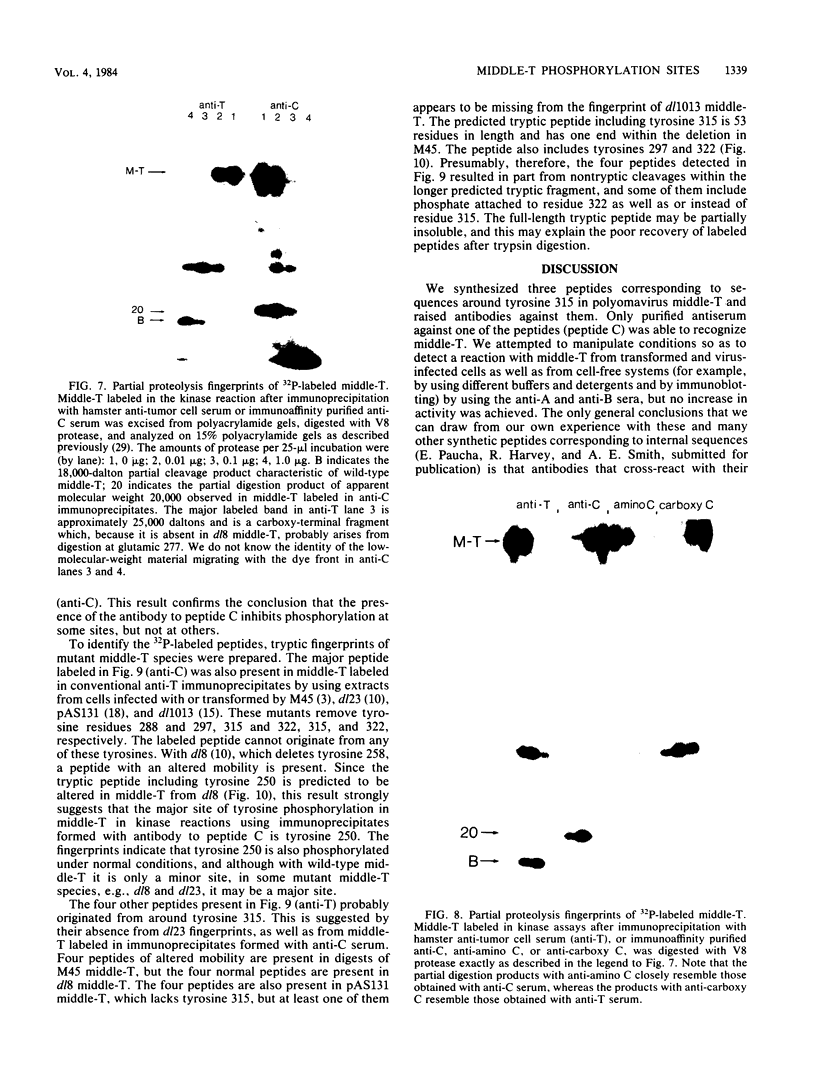
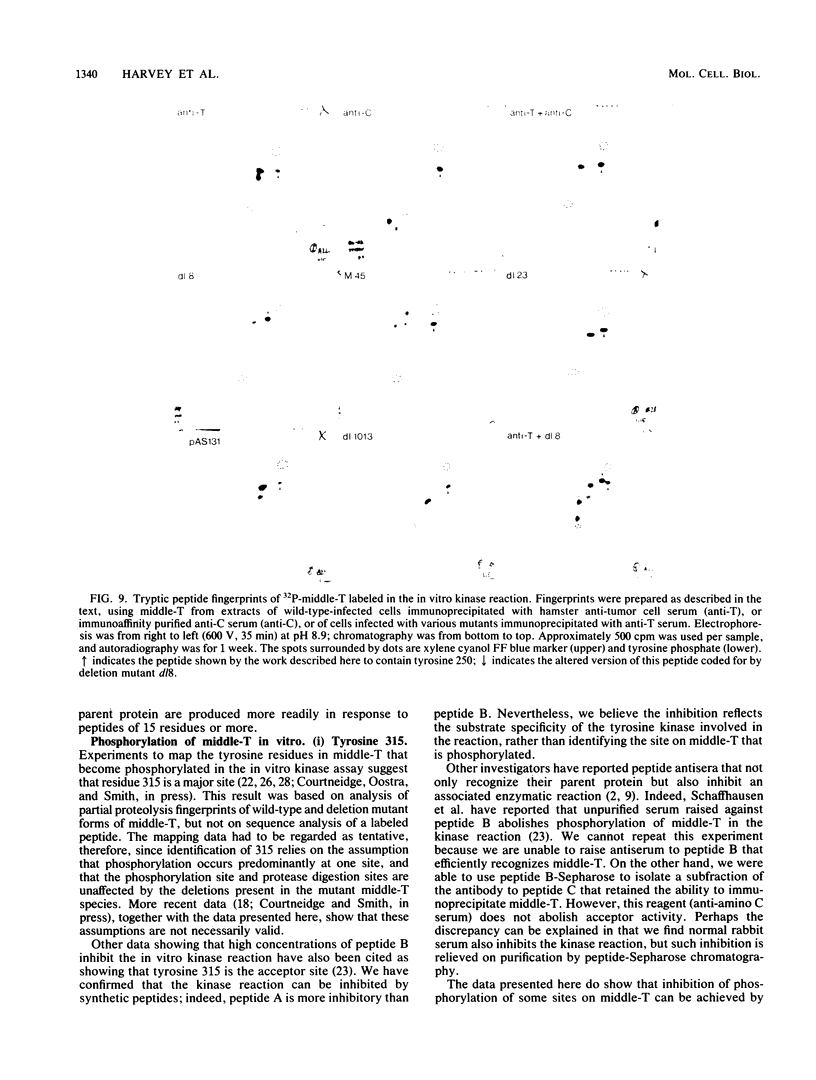
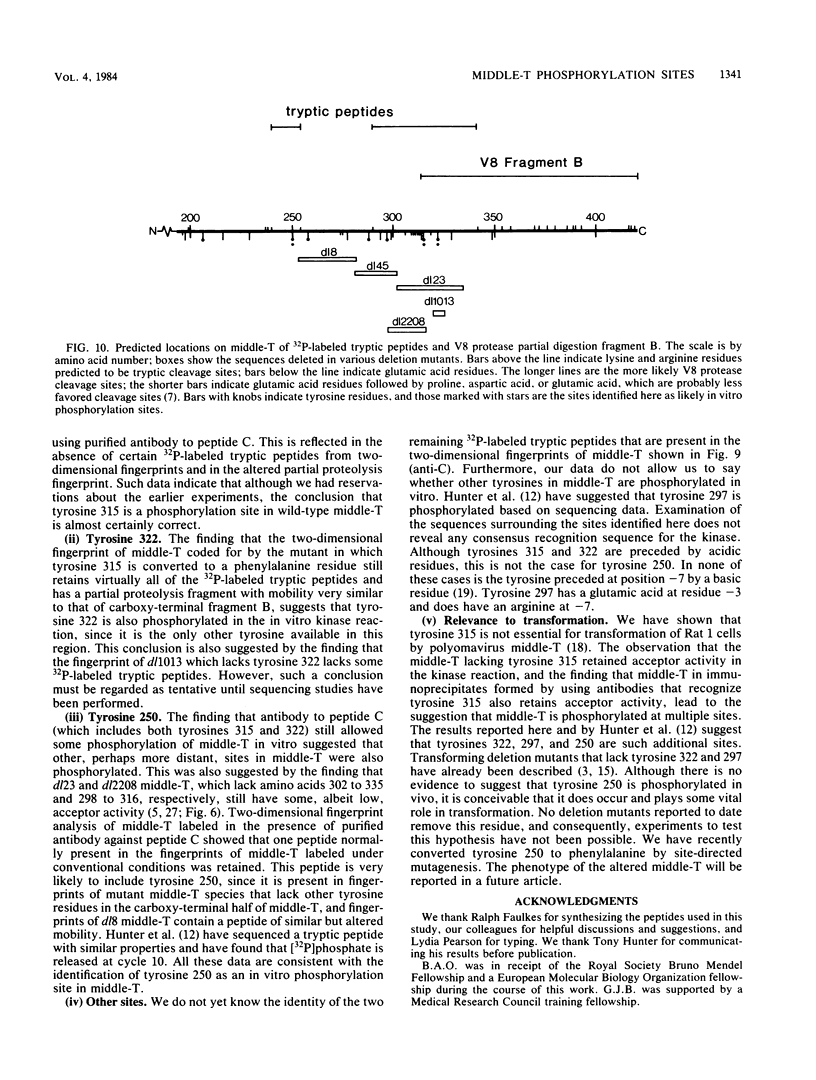
Antibodies were raised against three synthetic peptides corresponding to sequences surrounding tyrosine 315, a putative in vitro phosphorylation site in polyomavirus middle-T antigen. Only one of the peptides (called C and corresponding to residues 311 to 330) elicited antibodies that recognized middle-T efficiently. Middle-T present in immunoprecipitates formed with purified anti-C serum still accepted phosphate on tyrosine in an in vitro kinase reaction. This implies that tyrosines other than 315 and 322 that lie within the antibody binding region are phosphorylated under these conditions. This conclusion was supported by the altered partial V8 proteolysis fingerprint of the labeled middle-T. Two-dimensional tryptic fingerprint analysis of 32P-labeled middle-T showed that several tryptic peptides identified as including tyrosine 315 and 322 were missing from middle-T labeled in anti-C immunoprecipitates compared with middle-T labeled in immunoprecipitates made by using anti-tumor cell serum. However, one major labeled peptide remained. This peptide was also present in fingerprints of 32P-labeled middle-T coded by M45, dl23, pAS131, and dl1013, but a peptide with altered mobility was present in dl8 middle-T. This identified the peptide as including tyrosine 250. We deduce from these data that (i) the presence of the antibody against peptide C inhibits phosphorylation of tyrosines 315 and 322; (ii) middle-T labeled in the kinase reaction after immunoprecipitation with anti-C serum is phosphorylated on tyrosine 250; and (iii) when anti-tumor cell serum is used in the in vitro kinase reaction, middle-T is phosphorylated at multiple sites, including residues 250, 315, and 322.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin G. S. Gastrin and the transforming protein of polyoma virus have evolved from a common ancestor. FEBS Lett. 1982 Jan 11;137(1):1–5. doi: 10.1016/0014-5793(82)80302-5. [DOI] [PubMed] [Google Scholar]
- Baron M. H., Baltimore D. Antibodies against a synthetic peptide of the poliovirus replicase protein: reaction with native, virus-encoded proteins and inhibition of virus-specific polymerase activities in vitro. J Virol. 1982 Sep;43(3):969–978. doi: 10.1128/jvi.43.3.969-978.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendig M. M., Thomas T., Folk W. R. Viable deletion mutant in the medium and large T-antigen-coding sequences of the polyoma virus genome. J Virol. 1980 Mar;33(3):1215–1220. doi: 10.1128/jvi.33.3.1215-1220.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. The complex of polyoma virus middle-T antigen and pp60c-src. EMBO J. 1984 Mar;3(3):585–591. doi: 10.1002/j.1460-2075.1984.tb01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth S. M., Griffin B. E. Monoclonal antibodies against polyoma virus tumor antigens. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1059–1063. doi: 10.1073/pnas.79.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth S. M. Protein kinase activities associated with distinct antigenic forms of polyoma virus middle T-antigen. EMBO J. 1982;1(11):1319–1328. doi: 10.1002/j.1460-2075.1982.tb01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau G. R. Cleavage at glutamic acid with staphylococcal protease. Methods Enzymol. 1977;47:189–191. doi: 10.1016/0076-6879(77)47023-x. [DOI] [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Gentry L. E., Rohrschneider L. R., Casnellie J. E., Krebs E. G. Antibodies to a defined region of pp60src neutralize the tyrosine-specific kinase activity. J Biol Chem. 1983 Sep 25;258(18):11219–11228. [PubMed] [Google Scholar]
- Griffin B. E., Maddock C. New classes of viable deletion mutants in the early region of polyoma virus. J Virol. 1979 Sep;31(3):645–656. doi: 10.1128/jvi.31.3.645-656.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R., Faulkes R., Gillett P., Lindsay N., Paucha E., Bradbury A., Smith A. E. An antibody to a synthetic peptide that recognises SV40 small-t antigen. EMBO J. 1982;1(4):473–477. doi: 10.1002/j.1460-2075.1982.tb01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Hutchinson M. A., Eckhart W. Polyoma middle-sized T antigen can be phosphorylated on tyrosine at multiple sites in vitro. EMBO J. 1984 Jan;3(1):73–79. doi: 10.1002/j.1460-2075.1984.tb01763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson M. A., Hunter T., Eckhart W. Characterization of T antigens in polyoma-infected and transformed cells. Cell. 1978 Sep;15(1):65–77. doi: 10.1016/0092-8674(78)90083-1. [DOI] [PubMed] [Google Scholar]
- Ito Y., Hamagishi Y., Segawa K., Dalianis T., Appella E., Willingham M. Antibodies against a nonapeptide of polyomavirus middle T antigen: cross-reaction with a cellular protein(s). J Virol. 1983 Dec;48(3):709–720. doi: 10.1128/jvi.48.3.709-720.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson G., Nilsson M. G., Dilworth S. M., Smolar N. Characterization of polyoma mutants with altered middle and large T-antigens. J Virol. 1981 Sep;39(3):673–683. doi: 10.1128/jvi.39.3.673-683.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S. V., Tyndall C., Magnusson G. Deletion mapping of a short polyoma virus middle T antigen segment important for transformation. J Virol. 1983 Apr;46(1):284–287. doi: 10.1128/jvi.46.1.284-287.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra B. A., Harvey R., Ely B. K., Markham A. F., Smith A. E. Transforming activity of polyoma virus middle-T antigen probed by site-directed mutagenesis. Nature. 1983 Aug 4;304(5925):456–459. doi: 10.1038/304456a0. [DOI] [PubMed] [Google Scholar]
- Patschinsky T., Hunter T., Esch F. S., Cooper J. A., Sefton B. M. Analysis of the sequence of amino acids surrounding sites of tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1982 Feb;79(4):973–977. doi: 10.1073/pnas.79.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucha E., Harvey R., Smith A. E. Cell-free synthesis of simian virus 40 T-antigens. J Virol. 1978 Oct;28(1):154–170. doi: 10.1128/jvi.28.1.154-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Phosphorylation of polyoma T antigens. Cell. 1979 Dec;18(4):935–946. doi: 10.1016/0092-8674(79)90206-x. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B., Benjamin T. L. Comparison of phosphorylation of two polyoma virus middle T antigens in vivo and in vitro. J Virol. 1981 Oct;40(1):184–196. doi: 10.1128/jvi.40.1.184-196.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B., Benjamin T. L., Pike L., Casnellie J., Krebs E. Antibody to the nonapeptide Glu-Glu-Glu-Glu-Tyr-Met-Pro-Met-Glu is specific for polyoma middle T antigen and inhibits in vitro kinase activity. J Biol Chem. 1982 Nov 10;257(21):12467–12470. [PubMed] [Google Scholar]
- Smart J. E., Oppermann H., Czernilofsky A. P., Purchio A. F., Erikson R. L., Bishop J. M. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src). Proc Natl Acad Sci U S A. 1981 Oct;78(10):6013–6017. doi: 10.1073/pnas.78.10.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Fried M., Ito Y., Spurr N., Smith R. Is polyoma virus middle T antigen a protein kinase? Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):141–147. doi: 10.1101/sqb.1980.044.01.016. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Griffin B., Fried M. Protein kinase activity associated with polyoma virus middle T antigen in vitro. Cell. 1979 Dec;18(4):915–924. doi: 10.1016/0092-8674(79)90204-6. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Paucha E. Extraction and fingerprint analysis of simian virus 40 large and small T-antigens. J Virol. 1978 Oct;28(1):140–153. doi: 10.1128/jvi.28.1.140-153.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G., Shinnick T. M., Green N., Lerner R. A. Antibodies that react with predetermined sites on proteins. Science. 1983 Feb 11;219(4585):660–666. doi: 10.1126/science.6186024. [DOI] [PubMed] [Google Scholar]
- Walter G., Hutchinson M. A., Hunter T., Eckhart W. Antibodies specific for the polyoma virus middle-size tumor antigen. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4882–4886. doi: 10.1073/pnas.78.8.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]