Abstract
Exploration missions outside low-Earth orbit are being planned; therefore, it is critical to understand the risk astronauts would be exposed to in the space environment, especially during extravehicular activities (EVAs). Reductions in white blood cell (WBC) numbers can occur as a result of exposure to solar particle event (SPE) radiation. The aim of the present study was to determine the duration of the effects on blood cell numbers from exposure to a single whole-body dose of SPE-like proton radiation or photon radiation as well as to determine the radiation biological effectiveness (RBE) values at those times when radiation exposure causes blood cell numbers to experience the most critical effects when using mice as a model. Our results indicate that both types of radiation cause significant reductions in the numbers of all blood cell types at different times post-irradiation. The RBE values were not significantly different from 1.0. These results indicate that the risk estimations for astronauts from exposure of mice to SPE-like proton radiation are comparable to those previously made for doses of standard reference radiations, suggesting that countermeasures should be developed for the decreases in blood cell counts observed following the exposure of mice to SPE radiation. Key Words: Proton radiation—Gamma radiation—Blood cell counts—Solar particle event. Astrobiology 13, 570–577.
1. Introduction
Human exploration–class missions outside low-Earth orbit are being planned for the near future, and these missions are likely to result in considerably greater radiation doses to astronauts than those received previously. Therefore, it is critical to determine the risks to astronauts from exposure to solar particle event (SPE) radiation during these missions. The National Space Biomedical Research Institute Center of Acute Radiation Research (CARR) is focused on determining the acute radiation risks to astronauts from exposure to SPE radiation, which is produced by solar flares and coronal mass ejections (Harra, 2002; Hellweg and Baumstark-Khan, 2007). The majority of SPE radiation consists of low-energy protons, with a small fraction of helium ions and an even smaller fraction of heavier ions (Hellweg and Baumstark-Khan, 2007). SPEs are difficult to predict and can deliver relatively high doses of radiation in very short periods of time. Portable shelters and spacecrafts with the appropriate shielding (≥10 g/cm2 aluminum) can reduce SPE radiation dose exposure levels (Wilson et al., 1999; Ballarini et al., 2004); however, space suits provide minimal protection against this type of radiation (Cucinotta et al., 2003; Ballarini et al., 2004). Therefore, there is particular concern about the radiation risks for astronauts exposed to SPE radiation during extravehicular activities (EVAs). A dose of 2 Gy during EVAs is expected to be the highest deep dose that could be received by astronauts during an SPE (Dr. Francis Cucinotta, personal communication). This dose could lead to the development of acute radiation syndrome (Townsend, 2005). One of the predicted acute radiation syndromes that astronauts could experience if exposed to the estimated doses of SPE radiation during EVAs, and potentially within the spacecraft, is the hematopoietic syndrome, which involves reductions in the number of circulating white blood cells (WBCs). This prediction is based on results from studies performed in animal model systems and reports concerning survivors of atomic bombs or radiation accidents; in these cases, the exposures involved different types of radiation and often involved radiation doses considerably higher than those expected from SPE radiation (Finch, 1987; Gottlober et al., 2001; Fliedner et al., 2002). These studies did not address the risk to astronauts from reductions in blood cell numbers resulting from radiation exposures at the energies and doses of the types of radiation released during an SPE.
As the energy of most of the protons emitted as part of SPE radiation is <100 MeV (Wilson et al., 1997), many of the CARR studies in mice have used proton energies of approximately 70 MeV (e.g., Maks et al., 2011), given in a manner to result in a homogeneous dose of SPE-like proton radiation. This proton energy is considerably lower than the proton energies normally evaluated in most radiobiology experiments, since most of the published studies in which protons were used employed the range of proton energies used in clinical radiotherapy protocols or even higher energies. The dose rates of SPEs are expected to vary up to a dose rate of 0.5 Gy/h, which is a very low dose rate compared to most studies previously performed with proton radiation. Several dose rates have been evaluated as part of the CARR studies on SPE radiation effects in mice involving peripheral blood cell counts, as well as immunologic and behavioral endpoints. To determine the effects of dose rate on peripheral blood cell count data in mice exposed to SPE-like proton radiation, studies involving low dose rate exposures at 0.5 Gy/h (Maks et al., 2011) and 0.17 Gy/h (Sanzari and Kennedy, unpublished data) compared to high dose rate exposure (0.5 Gy/min) have been performed; the results of these studies indicate very little effect of dose rate on blood cell counts from exposure to SPE radiation at the doses and dose rate ranges evaluated. Thus, the data reported in the present study do not include studies in which different dose rates were used.
Animal models have been used in studies focused on the treatment of radiation injuries (Williams et al., 2010). For the majority of the hematopoietic syndrome studies, mice, dogs, and nonhuman primates have been the models of choice (Williams et al., 2010). In the present study, mice were used as the animal model system. A few other investigators have also evaluated the effects of SPE radiation in mice. Gridley et al. (2008) reported the results of experiments performed to determine the effects of simulated SPE radiation on blood cells in mice at two different time points post-irradiation. We have also reported the effect of SPE-like proton radiation on total WBC counts at 24 h post-irradiation (Maks et al., 2011). Both reports demonstrate statistically significant reductions in the number of circulating blood cells, but they do not determine the kinetics of these effects; therefore, it is not possible to establish the exact time of the nadirs for the different blood cell types and the duration of the effects. A major purpose of the experiments reported in the present paper was to perform a time course study to determine the duration of the effects in mouse blood cell counts after exposure to SPE-like proton radiation, simulated by a single dose of 2 Gy low-energy (∼74 MeV) proton radiation, and compare the results to a similar experiment in which gamma radiation was used as the reference radiation. In these studies, blood cell numbers were measured over a 30-day period, as that is the period of time that peripheral blood cell numbers are reduced (and can exhibit recovery) after animals have been exposed to sufficient doses of radiation to cause the hematopoietic syndrome. One of the most widely used endpoints in mice for acute radiation damage to the hematopoietic system after total-body irradiation is the LD50 (i.e., the lethal dose that kills 50% of the animals), which is determined at 30 days post-irradiation (Williams et al., 2010); thus, blood cell counts over 30 days post-irradiation were measured in these studies. The results demonstrate a significant decrease in all blood cell numbers at different times after exposure to either SPE-like proton or 2 Gy photon radiation.
Radiation biological effectiveness values for different blood cell types were also calculated by using the results from experiments involving exposure of mice to 0.5, 1, and 2 Gy proton and gamma radiation doses so that risk estimates resulting from exposure to SPE radiation could be determined. The results indicate that the radiation biological effectiveness (RBE) values were not significantly different from 1.0 in the proton dose range evaluated, with a modest RBE increase at lower doses. These results indicate that the risk estimations for astronauts from the exposure of mice to SPE-like proton radiation are comparable to those previously made for doses of standard reference radiations. The results suggest that countermeasures should be developed for the decreases in white blood cells following the exposure of mice to SPE-like proton radiation.
2. Materials and Methods
2.1. Mice
Female ICR mice at 6 weeks of age were purchased from Taconic Farms Inc. (Germantown, NY, USA). Animals were acclimated for 7 days in the University of Pennsylvania animal facility. Five animals were housed per cage with ad libitum access to water and food pellets. The animal care and treatment procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
2.2. Gamma irradiation
Mice were restrained in custom-designed Plexiglass chambers and exposed to total-body 137Cs gamma radiation at doses of 0.5, 1, or 2 Gy, which were administered at a dose rate of 0.44 Gy/min in the University of Pennsylvania Gammacell 40 irradiator (Nordion, Ottawa, ON, Canada). Sham irradiated control mice were restrained in Plexiglass chambers and transported to the gamma irradiator, but they were not irradiated. Both irradiated mice and sham irradiated mice were in the Plexiglass chambers for the same period of time.
2.3. Proton irradiation
Mice were restrained in custom-designed Plexiglass chambers and exposed to total-body proton radiation at doses of 0.5, 1, or 2 Gy administered at a dose rate of 0.5 Gy/min. The proton beam was produced by the University of Pennsylvania IBA cyclotron system. The 230 MeV proton beam extracted from the cyclotron was degraded by using the energy selection system to a nominal energy of 151 MeV or range of 16 cm water equivalent thickness (WET). The degraded beam was delivered in double scattering mode with a uniform spread out Bragg peak (SOBP) modulation width of 5 cm. A 23×17 cm opening in the tungsten multi-leaf collimator shaped the beam to a useable field size (>95% of maximum within the flat region) of 20.6×17 cm at the gantry isocenter. Eight mice enclosures with dimensions of 7.2×4.1×4.1 cm were arranged in a 2×4 array forming a 14.2×16.4 cm target area. The center of the enclosure array was placed at the gantry isocenter with an additional 11 cm WET of Solid Water slab (Gammex, Inc., Middleton, WI, USA) placed directly in front of the array, further degrading the proton beam energy to approximately 74 MeV or a range of ∼4.5 cm WET. Five centimeters WET of Solid Water slab was placed directly behind the enclosure array. The mice enclosures are irradiated with a range of proton energies forming the uniformly modulated dose region of the SOBP. The dose-averaged linear energy transfer (LET) of the proton radiation is low (<10 keV/μm) within the mid-SOBP where the mice are located, and rises to higher LET (>10 keV/μm) toward the downstream edge of the SOBP, which lies beyond the mice enclosures (Kantemiris et al., 2011). Dosimetry verification was performed before the irradiations with a 2-D ion chamber array (I'mRT MatriXX, IBA Dosimetry, Bartlett, TN, USA) placed at a depth of 13.3 cm WET. Sham irradiated control mice were also restrained in custom-designated Plexiglass chambers and transported to the proton irradiation facility but were not subjected to irradiation. All mice, irradiated and sham irradiated, were maintained in the Plexiglass chambers for the same period of time.
2.4. Blood sample processing procedures
Blood was collected at the indicated days after proton and gamma irradiation. To compensate for fluctuations in blood cell counts such as those associated with stress, hormones, and/or circadian variations, a group of sham irradiated control mice was included at each time point, and blood samples were obtained at approximately the same time each day. Thus, at each time point, five proton-irradiated mice, five gamma-irradiated mice, and five sham irradiated mice were killed by CO2 inhalation. Blood samples were collected by cardiac puncture, placed into lavender-top blood BD microtainer collection tubes containing EDTA (BD, Franklin Lakes, NJ, USA), and sent to Antech Diagnostics facility (Lake Success, NY, USA) for complete automated blood cell count analyses, which include the absolute numbers of red blood cells (RBCs), platelets, WBCs, as well as the absolute numbers and percent, with respect to the total WBCs, of lymphocytes, monocytes, neutrophils, eosinophils, and basophils. In the present study, we have combined the absolute numbers of neutrophils, eosinophils, and basophils, and the resulting values are presented as granulocytes. The reliability of using Antech Diagnostics has been previously determined (Romero-Weaver and Kennedy, 2012). For each blood cell type, the cell numbers from the irradiated group were divided by the cell numbers of the corresponding sham irradiated control group, and results were expressed as fraction of control.
2.5. RBE calculations
Cell survival curves for the proton and gamma radiation exposures were fitted from the survival data by using the linear-quadratic model: S=e-αD-βD2. The fitted survival values for gamma-ray radiation (Sgamma) were calculated from the gamma-ray survival curve at a dose interval of 0.05 Gy. The dose of gamma-ray radiation (Dgamma) required to produce the same biological effect produced by proton radiation at each radiation dose (Dproton) was calculated by linear approximation in which the fitted survival values for gamma radiation were used. The relatively small dose interval used to calculate the fitted survival values for gamma radiation was selected empirically to assure acceptable precision of the linear approximation. The RBE value for each animal irradiated with protons was determined with the following equation:
 |
The RBE values were plotted against proton radiation dose and analyzed by nonlinear regression analysis with a quadratic model to show the relationship between RBE value and proton radiation dose.
2.6. Statistical analyses
The fraction of control values (cell count of sample/cell count of sham irradiated control) at each time point post-irradiation were analyzed by one-way ANOVA followed by Tukey's test to compare the results from each radiation dose group with those from their corresponding sham irradiated control group with SigmaPlot 12 software. The nonlinear regression analysis showing the relationship between RBE value and proton radiation dose and the associated 95% confidence interval was performed with Minitab statistical software (release 15).
3. Results
3.1. Effects of SPE-like proton and photon radiation on the kinetics of mouse peripheral blood cells
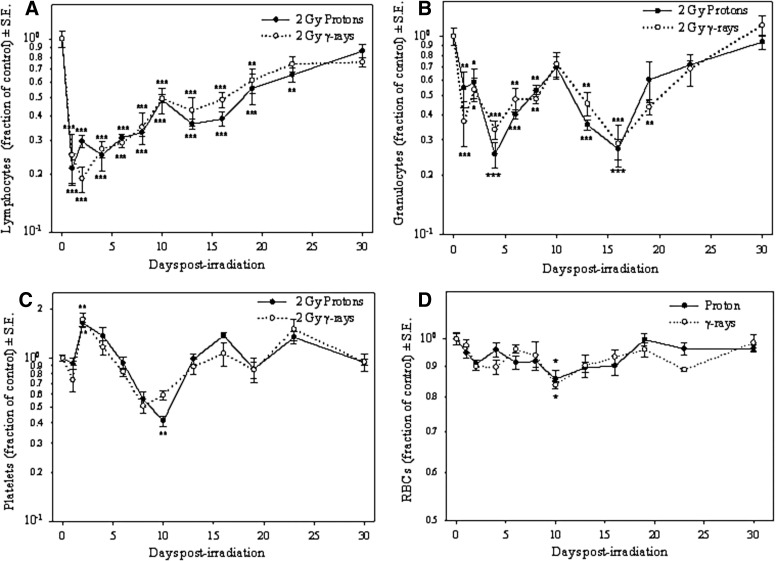
Time course experiments to determine the effects of SPE-like proton or photon radiation on blood cell types following the irradiation of mice for up to 1 month were conducted. The results of these experiments indicate that lymphocyte nadir was reached at 2 days post-irradiation, when values were about 70% and 80% less than the control values after proton and gamma irradiation, respectively; then the numbers gradually increased. By 30 days, the differences between the lymphocyte numbers in the irradiated mice compared to the control mice were not statistically significant (Fig. 1A).
FIG. 1.
Effect of SPE-like proton or 2 Gy gamma radiation as a function of time for lymphocyte counts (A), granulocyte counts (B), platelet counts (C), and RBC counts (D). At each time point, blood cell numbers from the irradiated group were divided by the blood cell numbers of the corresponding sham irradiated control group, and results were expressed as fraction of control. Results are from a representative experiment of two independent experiments that were performed with similar results (*p<0.05, **p<0.01, ***p<0.001 by one-way ANOVA followed by Tukey's test, n=5).
Granulocyte numbers declined in a bimodal fashion during the time period analyzed: the first nadir was observed at 4 days post-irradiation, when the values were as low as 75% of the control values after 2 Gy proton irradiation and 66% less than the control values after 2 Gy gamma irradiation. After 4 days, the granulocyte numbers began to recover, and by 10 days post-irradiation, the differences between the granulocyte counts in the 2 Gy irradiated mice compared to the control mice were not statistically significant. After 10 days post-irradiation, the granulocyte numbers exhibited a second nadir at 16 days post-irradiation when the granulocyte numbers were 73% and 71% less than the control values after 2 Gy proton and 2 Gy gamma irradiation, respectively. The granulocyte numbers then recovered again, and by 23 days post-irradiation, the differences between the granulocyte counts in the 2 Gy irradiated mice compared to the control mice were not statistically significant (Fig. 1B). The granulocyte numbers were reduced in a bimodal fashion in both of the independent experiments performed.
Like the granulocytes, the monocytes also exhibited a bimodal decease: the first nadir was observed at 2 days post-irradiation, when values were as low as 80% of the control value for 2 Gy proton irradiation and lower than 90% of the control value for 2 Gy gamma irradiation; and the second nadir was observed at 13 days post-irradiation, when numbers were about 80% and 70% less than the control values for proton and gamma irradiation, respectively. The differences between the monocyte counts in the 2 Gy irradiated mice compared to the control mice were not statistically significant by Day 19 post-irradiation (data not shown). The monocyte numbers were reduced in a bimodal fashion in both of the independent experiments performed.
Platelets exhibited a statistically significant increase of about 60% above control values with 2 Gy proton irradiation and about 70% above control values with 2 Gy gamma irradiation at 2 days post-irradiation; then the numbers of platelets decreased to their lowest number at 10 days post-irradiation, when the numbers were less than 70% of the control values for both proton and gamma irradiation. The platelet numbers exhibited a fast recovery, and the differences between the platelet counts in the 2 Gy irradiated mice compared to the control mice were not statistically significant by Day 13 post-irradiation (Fig. 1C). The numbers of RBCs did not change significantly during the time course analyzed, except at 10 days post-irradiation, when there was a statistically significant decrease of about 15% of the control values with both 2 Gy proton and 2 Gy gamma irradiation (Fig. 1D). The absolute values of the un-irradiated control samples for all time points are shown in Table 1. The average percent values (±standard deviation) of each of the WBCs in the samples taken from un-irradiated control mice were lymphocytes 77%±5.4; monocytes 2.8%±2.4; neutrophils 17.6%±4.8; eosinophils 2.1%±0.9; and basophils 0.5%±0.4. As described in the Materials and Methods section, the absolute numbers of neutrophils, eosinophils, and basophils have been combined, and the resulting values are presented as granulocytes, which represent about 20% of all WBCs in ICR mice. Also hemoglobin (Hb) and hematocrit (HCT) determinations showed a statistically significant decrease of about 15% of the control values at 10 days post-irradiation with both 2 Gy proton and 2 Gy gamma irradiation (data not shown). Other parameters analyzed, such as mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration, did not show statistically significant changes during the time course analyzed with either 2 Gy proton or 2 Gy gamma irradiation.
Table 1.
Absolute Values±Standard Deviations of Lymphocyte, Granulocyte, and Platelet Counts, Determined from Blood Samples Taken from Un-Irradiated Control Mice (Utilized for the Percent of Control Data Shown in Fig. 1)
| |
Blood cell type |
||
|---|---|---|---|
| Days post-irradiation* | Lymphocytes (cells/μL) | Granulocytes (cells/μL) | Platelets (×103 cells/μL) |
| 0 | 5321±1170 | 1431±466 | 1016±124 |
| 1 | 5649±1185 | 1346±318 | 1388±138 |
| 2 | 7368±1466 | 1689±542 | 817±330 |
| 4 | 7583±1991 | 1792±485 | 805±298 |
| 6 | 8695±1054 | 1876±283 | 1003±100 |
| 8 | 8217±1384 | 1041±461 | 1043±164 |
| 10 | 6729±1025 | 1543±525 | 1077±294 |
| 13 | 6900±1421 | 1392±233 | 1037±202 |
| 16 | 7426±1492 | 1854±748 | 961±238 |
| 19 | 6445±1675 | 1649±806 | 1146±1146 |
| 23 | 6535±1072 | 1707±429 | 727±330 |
| 30 | 7457±1052 | 1317±313 | 1110±228 |
For the un-irradiated control mice, samples were collected at the same time points as those utilized for the irradiated mice.
3.2. RBE calculations
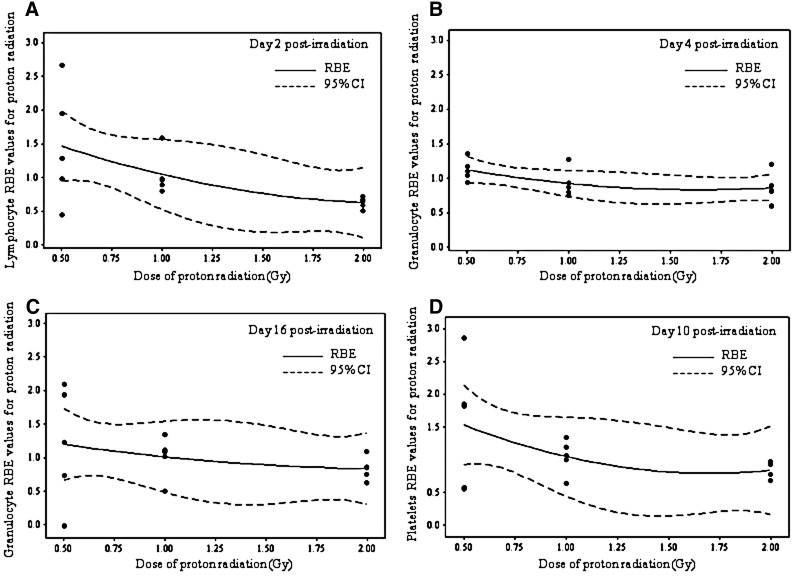
Survival curves were obtained at the nadir time for lymphocytes (2 days post-irradiation), granulocytes (4 and 16 days post-irradiation), and platelets (10 days post-irradiation) (Fig. 2). In the case of RBCs, Hb, and HCT, the survival curves were flat for all time points; therefore, no RBE calculations were determined for these parameters.
FIG. 2.
Survival curves fitted with the linear-quadratic model at the nadir times determined in Fig. 1. For lymphocytes at 2 days post-irradiation (A), granulocytes at 4 and 16 days post-irradiation (B) and (C), respectively, and platelets at 10 days post-irradiation (D). Results are expressed as fraction of control.
The survival curves were obtained by fitting the blood cell counts obtained at doses of 0, 0.5, 1, and 2 Gy with the linear-quadratic model. In a survival curve, the steepness of the initial slope of the curve is determined by α, whereas the quadratic component of cell killing, β, causes the curve to bend at higher radiation doses. In the present study, the β values were almost negligible for all blood cell types analyzed; thus, the α/β ratios were very large, and the survival curves were essentially linear on a log scale (Fig. 2). The data from the survival curves were utilized for RBE calculations (see Material and Methods section for details) and were expressed as a function of dose (Fig. 3). In all cases, the 95% confidence interval for RBE values obtained from these data encompassed 1, indicating that the RBE values were not significantly different from 1.0 in the proton dose range evaluated and the curves exhibited a modest RBE increase at lower doses.
FIG. 3.
RBE values as a function of dose at the nadir times determined in Fig. 1. For lymphocytes at 2 days post-irradiation (A), granulocytes at 4 and 16 days post-irradiation (B) and (C), respectively, and platelets at 10 days post-irradiation (D). CI, confidence interval.
4. Discussion
4.1. Effects of SPE-like proton and photon radiation on the kinetics of mouse peripheral blood cells
The present study was conducted to determine the acute risk to astronauts from the reduction in circulating blood cell numbers as a result of exposure to low-energy protons from an SPE. It has been predicted that exposure to SPE radiation during EVAs can cause reductions in blood cell numbers. However, a detailed time course to determine the exact nadir times and the duration of the radiation effects has not been conducted previously at the energies and doses of the types of radiation released during an SPE. The results of the present study indicate that both SPE-like proton and 2 Gy gamma radiation caused statistically significant reductions in the numbers of all circulating blood cells at different times post-irradiation. These reductions were consistent with results reported previously for studies in which standard reference radiations were used and according to the sensitivity of each blood cell type to radiation exposure (Dainiak, 2002; Fliedner et al., 2002). For instance, in the present study the most pronounced effects of both SPE-like proton and 2 Gy gamma radiation were observed in lymphocytes, which are known to be very sensitive to the cell-killing effects of ionizing radiation (Fliedner et al., 2002). Platelets are very radio-resistant, with a life span in mice of about 5 days, and no significant radiation effects were observed during early time points after irradiation; however, the effects of radiation on bone marrow platelet precursors became apparent at Day 10 post-irradiation. The bone marrow effect leading to a statistically significant reduction in platelets in the circulation had a fast recovery. Circulating RBCs are radio-resistant, with a life span in mice of about 40 days. It has been reported that a drastic decrease in RBC precursors occurs in the bone marrow after exposure to moderate doses of radiation, but it is not reflected in circulating RBCs due to their long life span (Casarett, 1968). In the present study, statistically significant decreases in RBCs, HCT, and Hb were observed at 10 days after exposure of mice to SPE-like proton or 2 Gy gamma irradiation; however, the values of RBCs and HCT were within the normal reference values. Therefore, these changes are not clinically relevant. In the case of Hb, the numbers were below the normal reference values, indicating that exposure to SPE-like proton radiation can cause a transient anemia.
An important and novel finding of the exposure of mice to SPE-like proton radiation or 2 Gy gamma radiation was the statistically significant reduction in granulocytes and monocytes, which occurred in a bimodal fashion during the time period of this study. Granulocytes have a short life span (about 11 h in mice and 7–10 h in humans), which may explain the sharp decline observed as early as 1 day post-irradiation (Casarett, 1968). The bimodal decreases in granulocytes and monocytes were detected in these studies because several closely spaced time points post-irradiation were analyzed. Oscillation in circulating neutrophils (the major cells in granulocytes) has been observed in a condition known as cyclic neutropenia; this condition is inherited as an autosomal-dominant disease and is usually accompanied by oscillations in other circulating blood cells such as monocytes, lymphocytes, and reticulocytes (Dale et al., 2002). Cyclic disorders in other hematological cells have also been reported (Haurie et al., 1998). Cyclic neutropenia can also be induced by chemotherapy and exposure to radiation. Exposure of mice to gamma rays at a dose of 0.5 Gy induced oscillation in granulocytes in a cyclic fashion at 5 weeks post-irradiation, with a 3-week periodicity (Gidali et al., 1985); these oscillations occurred at a much later time frame than the bimodal decrease in granulocytes observed in our study with a dose of 2 Gy of either proton or gamma rays. Whether more oscillations could occur in our model system like those reported in periodic hematological disorders is unlikely. In this study, the last time points were taken at 23 and 30 days post-irradiation; at these points, the numbers of granulocytes returned to values that do not exhibit statistically significant differences when compared to control values. In other independent studies, we have determined the number of circulating granulocytes after exposure to SPE-like proton or 2 Gy gamma radiation at time points between 23 and 30 days and at 35 days post-irradiation; in these studies, the differences between the numbers of granulocytes in the irradiated animals and those of un-irradiated controls were not statistically significant (data not shown). Thus, we believe that by 23 days the bone marrow damage caused by exposure to SPE-like proton radiation or 2 Gy gamma radiation in granulocyte precursors was repaired and no more oscillations would occur.
It is possible that the two fluctuations observed in monocytes resulted from the same bone marrow damage that induced the fluctuations in granulocytes since both cell types arise from the same granulocyte-monocyte progenitors (Iwasaki and Akashi, 2007), and as previously described, fluctuations in monocytes have occurred simultaneously with fluctuations in neutrophils (Dale et al., 2002).
Since about 90% of granulocytes are neutrophils, which constitute the first line of immune defense, the significant bimodal decrease in their circulating numbers may increase the risk to astronauts for developing infections. The development of infections could have potential consequential adverse effects on astronaut health and mission success. In the clinic, treatment is considered for cancer therapy patients when their blood samples indicate that the neutrophil counts are below 500 cells/μL (Dale, 2009). In this study, the neutrophil counts at the granulocyte nadirs of 4 and 16 days post-irradiation were 407 and 392 cells/μL, respectively, after SPE-like proton irradiation, indicating that countermeasures should be developed for the low neutrophil counts observed in the mice exposed to SPE radiation. The decrease in lymphocyte numbers can also adversely affect immune system functions and contribute to the development of infections in astronauts. Therefore, countermeasures should be evaluated for this endpoint as well. The low platelet levels observed between Days 8 and 10 post-irradiation represent a transient risk for astronauts; therefore, efforts to prevent injuries that may increase the risk of hemorrhage could be important after exposure to doses near 2 Gy from SPE radiation due to the reduced numbers of platelets in the circulation. In future studies, the effects on blood cell counts from exposure to SPE radiation with other space stressors, such as microgravity, should be evaluated at the same time points analyzed in this study to determine the combined effects.
4.2. RBE calculations
To determine RBE values at the nadir time of each blood cell type following exposure to SPE-like proton radiation or photon radiation, gamma-ray survival values calculated from the survival curves fitted with the linear-quadratic model were used. The linear-quadratic model is often used to model biological responses to radiation exposures. The quadratic component, β, was negligible for all blood cell counts at the analyzed times; therefore, the linear quadratic model equation (S=e-αD-βD2) was identical to the multi-target model equation (S=e-αD). Thus, the RBE values derived by the linear quadratic model and multi-target model should be comparable. Previously, we reported RBE values for WBCs at 1 day post-irradiation, using the multi-target model (Maks et al., 2011). The RBE value in that study was 1.05 at 1 day post-irradiation. In the present study, the RBE values for lymphocytes, granulocytes, and platelets were not significantly different from 1.0 in the proton dose range evaluated at the nadir time of each blood cell type. These results are in line with other in vivo and in vitro studies of different end points that indicate RBE values close to 1.0 for protons compared to gamma rays or X-rays (Gerweck and Kozin, 1999; Paganetti et al., 2002). These results are also consistent with reported studies that indicate increasing RBE values with decreasing dose, but this effect has been less pronounced in in vivo studies (Paganetti et al., 2002; Paganetti, 2003) like the ones described in the present report. It is planned that, in future studies, the linear-quadratic model will be used to calculate the RBE values for a range of doses representing the expected SPE radiation doses. The calculated RBE values close to 1.0 for SPE-like proton radiation in the studies reported here indicate that risk estimations for astronauts from exposure to SPE proton radiation are comparable to those previously made for doses of standard reference radiations.
5. Conclusions
The present study in which mice were used as the animal model system demonstrated that the low proton energies of SPE radiation have the potential to cause statistically significant reductions in circulating blood cell counts and thus could give rise to acute radiation syndrome symptoms in astronauts. The reduced numbers of neutrophils in mice exposed to SPE radiation suggest that astronauts would be at increased risk of developing infections, which could compromise their health and mission success. The magnitude of the reductions in the number of circulating neutrophils observed in mice suggests that countermeasures should be developed for this adverse biological effect. The RBE results indicate that the risk estimations for astronauts, as discerned from exposure of mice to SPE proton radiation, are comparable to those previously made for doses of standard reference radiations. The combined effect of SPE radiation with other space stressors such as microgravity should be evaluated in future studies on hematopoietic effects of SPE radiation.
Acknowledgments
We thank Drs. Eleanor Blakely and Stephen B. Guetersloh for their helpful discussions on the research investigations presented here. This work was supported by the National Space Biomedical Research Institute (NSBRI)–Center of Acute Radiation Research (CARR) grant and NIH Training Grant 2T32CA009677. The NSBRI is funded through NASA NCC 9-58.
Author Disclosure Statement
No competing financial interest exists.
Abbreviations
CARR, Center of Acute Radiation Research; EVAs, extravehicular activities; Hb, hemoglobin; HCT, hematocrit; LET, linear energy transfer; RBC, red blood cell; RBE, radiation biological effectiveness; SOBP, spread out Bragg peak; SPE, solar particle event; WBC, white blood cell; WET, water equivalent thickness.
References
- Ballarini F. Biaggi M. De Biaggi L. Ferrari A. Ottolenghi A. Panzarasa A. Paretzke H.G. Pelliccioni M. Sala P. Scannicchio D. Zankl M. Role of shielding in modulating the effects of solar particle events: Monte Carlo calculation of absorbed dose and DNA complex lesions in different organs. Adv Space Res. 2004;34:1338–1346. doi: 10.1016/j.asr.2003.08.055. [DOI] [PubMed] [Google Scholar]
- Casarett A.P. Radiation effect on major organ systems of mammals. In: Casarett A.P., editor. Radiation Biology. Prentice-Hall Inc.; Englewood Cliffs, NJ: 1968. pp. 171–216. [Google Scholar]
- Cucinotta F.A. Shavers M.R. Saganti P.B. Miller J. Radiation protection studies of International Space Station extravehicular activity space suits. NASA TP-2003-212051, National Aeronautics and Space Administration, Johnson Space Center; Houston: 2003. [Google Scholar]
- Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30:513–528. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- Dale D.C. Advances in the treatment of neutropenia. Curr Opin Support Palliat Care. 2009;3:207–212. doi: 10.1097/SPC.0b013e32832ea6ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale D.C. Bolyard A.A. Aprikyan A. Cyclic neutropenia. Semin Hematol. 2002;39:89–94. doi: 10.1053/shem.2002.31917. [DOI] [PubMed] [Google Scholar]
- Finch S.C. Landmark perspective: acute radiation syndrome. JAMA. 1987;258:664–667. doi: 10.1001/jama.258.5.664. [DOI] [PubMed] [Google Scholar]
- Fliedner T.M. Graessle D. Paulsen C. Reimers K. Structure and function of bone marrow hemopoiesis: mechanisms of response to ionizing radiation exposure. Cancer Biother Radiopharm. 2002;17:405–426. doi: 10.1089/108497802760363204. [DOI] [PubMed] [Google Scholar]
- Gerweck L.E. Kozin S.V. Relative biological effectiveness of proton beams in clinical therapy. Radiother Oncol. 1999;50:135–142. doi: 10.1016/s0167-8140(98)00092-9. [DOI] [PubMed] [Google Scholar]
- Gidali J. Istvan E. Feher I. Long-term perturbation of hemopoiesis after moderate damage to stem cells. Exp Hematol. 1985;13:647–651. [PubMed] [Google Scholar]
- Gottlober P. Steinert M. Weiss M. Bebeshko V. Belyi D. Nadejina N. Stefani F.H. Wagemaker G. Fliedner T.M. Peter R.U. The outcome of local radiation injuries: 14 years of follow-up after the Chernobyl accident. Radiat Res. 2001;155:409–416. doi: 10.1667/0033-7587(2001)155[0409:toolri]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Gridley D.S. Rizvi A. Luo-Owen X. Makinde A.Y. Coutrakon G.B. Koss P. Slater J.M. Pecaut M.J. Variable hematopoietic responses to acute photons, protons and simulated solar particle event protons. In Vivo. 2008;22:159–169. [PubMed] [Google Scholar]
- Harra L.K. Explosive events on the Sun. Philos Trans A Math Phys Eng Sci. 2002;360:2757–2771. doi: 10.1098/rsta.2002.1057. [DOI] [PubMed] [Google Scholar]
- Haurie C. Dale D.C. Mackey M.C. Cyclical neutropenia and other periodic hematological disorders: a review of mechanisms and mathematical models. Blood. 1998;92:2629–2640. [PubMed] [Google Scholar]
- Hellweg C.E. Baumstark-Khan C. Getting ready for the manned mission to Mars: the astronauts' risk from space radiation. Naturwissenschaften. 2007;94:517–526. doi: 10.1007/s00114-006-0204-0. [DOI] [PubMed] [Google Scholar]
- Iwasaki H. Akashi K. Myeloid lineage commitment from the hematopoietic stem cell. Immunity. 2007;26:726–740. doi: 10.1016/j.immuni.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Kantemiris I. Karaiskos P. Papagiannis P. Angelopoulos A. Dose and dose averaged LET comparison of (1)H, (4)He, (6)Li, (8)Be, (1)(0)B, (1)(2)C, (1)(4)N, and (1)(6)O ion beams forming a spread-out Bragg peak. Med Phys. 2011;38:6585–6591. doi: 10.1118/1.3662911. [DOI] [PubMed] [Google Scholar]
- Maks C.J. Wan X.S. Ware J.H. Romero-Weaver A.L. Sanzari J.K. Wilson J.M. Rightnar S. Wroe A.J. Koss P. Gridley D.S. Slater J.M. Kennedy A.R. Analysis of white blood cell counts in mice after gamma- or proton-radiation exposure. Radiat Res. 2011;176:170–176. doi: 10.1667/RR2413.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganetti H. Significance and implementation of RBE variations in proton beam therapy. Technol Cancer Res Treat. 2003;2:413–426. doi: 10.1177/153303460300200506. [DOI] [PubMed] [Google Scholar]
- Paganetti H. Niemierko A. Ancukiewicz M. Gerweck L.E. Goitein M. Loeffler J.S. Suit H.D. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53:407–421. doi: 10.1016/s0360-3016(02)02754-2. [DOI] [PubMed] [Google Scholar]
- Romero-Weaver A.L. Kennedy A.R. Comparison of two methods for the determination of the effects of ionizing radiation on blood cell counts in mice. Int J Biomed Sci. 2012;8:7–15. [PMC free article] [PubMed] [Google Scholar]
- Townsend L.W. Implications of the space radiation environment for human exploration in deep space. Radiat Prot Dosimetry. 2005;115:44–50. doi: 10.1093/rpd/nci141. [DOI] [PubMed] [Google Scholar]
- Williams J.P. Brown S.L. Georges G.E. Hauer-Jensen M. Hill R.P. Huser A.K. Kirsch D.G. Macvittie T.J. Mason K.A. Medhora M.M. Moulder J.E. Okunieff P. Otterson M.F. Robbins M.E. Smathers J.B. McBride W.H. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173:557–578. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.W. Shinn J.L. Simonsen L.C. Cucinotta F.A. Dubey R.R. Jordan W.R. Jones T.D. Chang C.K. Kim M.Y. NASA Technical Paper 3668, National Aeronautics and Space Administration. Langley Research Center; Hampton, VA: 1997. Exposures to solar particle events in deep space missions. [Google Scholar]
- Wilson J.W. Cucinotta F.A. Shinn J.L. Simonsen L.C. Dubey R.R. Jordan W.R. Jones T.D. Chang C.K. Kim M.Y. Shielding from solar particle event exposures in deep space. Radiat Res. 1999;30:361–382. doi: 10.1016/s1350-4487(99)00063-3. [DOI] [PubMed] [Google Scholar]